Abstract
Acute lung injury (ALI) is characterized by severe lung edema and an increase in the inflammatory reaction. Considerable evidence has indicated that microRNAs (miRNAs or miRs) are involved in various human diseases; however, the expression profile and function of miRNAs in ALI have been rarely reported. The present study used miRNA microarray and reverse transcription-quantitative polymerase chain reaction to demonstrate that miR-181b is the one of the most significantly upregulated miRNA after lipopolysaccharide (LPS) stimulation in human bronchial epithelial cells, BEAS-2B. To elaborate the role of miR-181b in ALI, an assay was performed to investigate the overexpression of miR-181b in BEAS-2B cells, and the expression of inflammatory factors was then analyzed. The overexpression of miR-181b resulted in the induction of an increment in interleukin (IL)-6 levels. p65 was identified to be a primary component of NF-κB, since it was upregulated in the miR-181b overexpression in the BEAS-2B cells, while pyrrolidine dithiocarbamate, a specific inhibitor of NF-κB, was found to be able to abrogate the upregulation of the expression of p65. In conclusion, the findings of the present study suggested that miR-181b may be involved in the process of LPS-induced inflammation in BEAS-2B cells by activating the NF-κB signaling pathway, which implies that it may serve as a potential therapeutic target for ALI.
Keywords: acute lung injury, microRNA-181b, interleukin-6, lipopolysaccharide, human bronchial epithelial cells
Introduction
Acute lung injury (ALI), with acute respiratory distress syndrome (ARDS) being its most severe manifestation, is a common disease with hazardous effects on human health. Despite the positive survival trends that have been reported in the past two decades in patients with ALI/ARDS, the mortality rate remains at 30–50%, particularly among older patients who exhibit sepsis, which is the most common predisposing factor (1). ALI/ARDS is partly characterized by persistent, uncontrolled pulmonary inflammation, which occurs in response to a variety of insults, including pneumonia, sepsis and trauma (2). Epithelial cells and macrophages comprise the primary line of defense, upon exogenous insults in the lung. A cascade of events is triggered by the injured cells, including acute inflammatory response, recruitment of immune cells, including monocytes and macrophages, and release of the cytokines, interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) (3). IL-6 and TNF-α are transcriptional activators that is crucial to the activation of several proinflammatory genes in human respiratory epithelial cells (4,5). The NF-κB protein family consists of homodimeric or heterodimeric subunits of members of the Rel family, including p50 and p65. Functional NF-κB dimers always contain a p65 subunit and possess marked proinflammatory activity (6). Despite considerable research and an increased understanding of the pathophysiological processes involved in the pathogenesis of ALI (2,3), the mechanism of the disease remains to be elucidated.
MicroRNA (miRNA or miR) molecules are small, single-stranded, non-coding RNAs that typically bind to the 3′ untranslated region (3′UTR) of target mRNA sequences, which results in reduced protein expression, mainly by destabilizing target mRNAs and/or through the inhibition of translation (7,8). MiRNAs have been found to play an important role in various biological processes (9). Previous studies have demonstrated that miRNAs are dynamically regulated in various human diseases, including cardiovascular diseases (10,11) and tumorigenesis (12,13). In addition, certain studies have indicated that miRNAs are extensively involved in inflammation (14–16). Changes in the expression levels of certain miRNAs may be involved in the regulation of the inflammatory process and tissue repair in ALI/ARDS (17). Cai et al revealed that the levels of miR-16 were reduced in lipopolysaccharide (LPS)-induced experimental ALI (18). In addition, miR-16 treatment reduced the expression levels of the TNF-α and IL-6 proinflammatory cytokines following exposure of macrophages to LPS. Furthermore, Xie et al identified a decrease in the pulmonary miR-127 expression in alveolar macrophages exposed to LPS and in an animal model of noninfectious ALI (19). miR-127 treatment was also demonstrated to reduce the IL-1β, TNF-α and IL-6 production in macrophages that had been exposed to LPS, as well as to reduce the lesion degree in an experimental ALI model in vivo. Iliopoulos et al (20) reported that, in ER-Src cells, miR-181b targets CYLD directly, which results in an increased NF-κB activity and maintenance of the inflammatory feedback loop underlying the epigenetic switch that links inflammation to cancer. Therefore, the therapeutic targeting of these miRNAs may be used as a way to suppress the inflammatory response following ALI. However, the role of miRNAs in the mediation of ALI has only recently been examined (18–20), and requires further investigation.
The aim of the present study was to characterize the regulation of the miRNA expression using LPS challenge. Through an miRNA array-based screen, miR-181b was identified as a regulator of BEAS-2B human bronchial epithelial cells using LPS challenge. The study investigated the effect of LPS treatment on the expression of miR-181b, as well as the association of miR-181b with the expression levels of p65 and inflammation-associated cell factors, such as IL-6, which are closely associated with ALI ARDS. In addition, the role of miR-181b in ALI and its potential application as a diagnostic and prognostic marker of the disease were investigated (21).
Materials and methods
Reagents
Fetal calf serum was obtained from Gibco-BRL (Grand Island NY, USA). The following materials were obtained from Qiagen (Hilden, Germany): miScript miRNA Mimic syn-hsa-miR-181b; Pre-miR miRNA negative control; QuantiTect primer assays; miScript II RT kit; miScript SYBR Green PCR kit; and HiPerFect transfection reagent. Pyrrolidine dithiocarbamate (PDTC), a specific inhibitor of NF-κB, was purchased from Sigma-Aldrich (St. Louis, MO, USA). The TNF-α and IL-6 ELISA kits were from MultiSciences Biotech Co., Ltd. (Hangzhou, China; cat. nos. 70-E-EK1061 and 70-E-EK1821, respectively). The NE-PER extraction reagent and BCA protein assay kit were from Pierce Chemical Co. (Rockford, IL, USA). Monoclonal rabbit anti-human p65 antibodies (cat. no. 1546-s) were obtained from Epitomics (Burlingame, CA, USA). Anti-β-actin monoclonal antibodies (cat. no. sc-8432) were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Cell culture
BEAS-2B cells were obtained from the Second Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China) and cultured in RPMI 1640 supplemented with 10% fetal calf serum and penicillin-streptomycin (100X; Gino Biomedical Technology Co., Ltd., Hangzhou, China) in a humidified CO2 incubator at 37°C. When the cells reached >85% confluence, they were trypsinized (Gino Biomedical Technology Co., Ltd.) and subcultured. The cells were generally used between passages 20–30 to avoid the generation of variation.
miRNA extraction and microarray analysis
BEAS-2B cells were seeded into 6-well plates at a density of 5×105 cells/well for a 24-h incubation prior to LPS treatment. Duplicate wells were used as the controls (medium only). The remaining wells were stimulated with 10 µg/ml LPS (the LPS concentration was determined according to the pre-test and references) (22) for 24 h. Subsequently, the cells were harvested for various assays, including RNA extraction, small RNA separation, quality control, labeling, hybridization and scanning, which were performed by LC Sciences LLC (Houston, TX, USA) using a Chip01_H16_070802 miRNA array chip, based on Sanger miRBase release 16.0 (23,24) (http://www.mirbase.org). Preliminary statistical analysis was also performed by LC Sciences LLC on raw data normalized using the locally-weighted regression method on the background-subtracted data. The microarray used for the experiments contained three probe replicates for each miRNA. A value of P<0.01 between pixels was considered to indicate a statistically significant difference.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA extraction and small RNA separation were performed using the miRNeasy Mini kit (cat. no. 217004; Qiagen). Cells and total RNA were treated as previously described. RT was performed with 200 ng RNA using the miScript II RT kit for miRNA transcription. RT-qPCR was performed using the miScript SYBR Green PCR kit, according to the manufacturer's instruction. Amplification and data analysis were performed using the 7500 Real Time PCR system (Applied Biosystems Life Technologies, Foster City, CA, USA). PCR cycling conditions were as follows: Inital activation at 95°C for 15 min, followed by 40 cycles of annealing at 94°C for 15 sec, annealing at 55°C for 30 sec and extension at 70°C for 35 sec. QuantiTect primer assays were used for the determination of miR-181b, miR-23c, miR-26b and Rnu6B expression. Values were normalized to the level of Rnu6B expression (Qiagen).
PDTC treatment
BEAS-2B cells were seeded in 6-well plates (3×105 cells/well). After 24 h, the cells were treated with 50, 100 or 200 µM PDTC for 1.5 h.
Transfection
BEAS-2B cells, with or without PDTC treatment, were transfected with miScript miRNA Mimic syn-hsa-miR-181b at 10 nmol/l using HiPerFect transfection reagent. Negative control, LPS (10 µg/ml) were used as positive control for miR-181b overexpression. The extent of miR-181b overexpression was measured by RT-qPCR. The supernatants, as well as the adherent cells, were collected at 48 h post-transfection for further analysis.
ELISA
Following transfection, the supernatants were collected at various time points (0, 12, 24 and 48 h) by centrifuged at 1000 × g for 10 min and then stored in −80°C. The expression levels of TNF-α and IL-6 were measured using the aforementioned commercial kits, according to the manufacturers' instructions. All absorbance results were normalized according to the standard curves.
Western blotting
For nuclear protein extraction, cells were lysed in NE-PER extraction reagent according to the manufacturer's instructions. Protein concentrations were determined using a BCA protein assay kit. A total of 30 µg protein was then loaded and electrophoresed on a 12% SDS-polyacrylamide gel, and transferred to the nitrocellulose membrane. The membranes were subsequently probed with anti-p65 (1:1,000 dilution) and anti-β-actin (1:1,000 dilution) monoclonal antibodies, respectively. The secondary antibody used for detection was linked with horseradish peroxidase. Next, an enhanced chemiluminescence method was used to detect the conjugated horseradish peroxidase (EMD Millipore, Billerica, MD, USA) and Image J (version 1.49) was used to analyze the immunoblots.
Statistical analysis
Differences between groups were compared using the Student's t-test for continuous variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Elevated levels of IL-6 in BEAS-2B cells following LPS stimulation
LPS pretreatment has been previously used to model inflammation in animal inhalation experiments (25). In addition, LPS is known to induce the expression of proinflammatory cytokines, such as IL-6, in BEAS-2B and A549 cells (22). In the present study, the expression of IL-6 was initially examined (Fig. 1). Compared with the negative control, IL-6 expression was significantly increased in cells treated with LPS at each time interval (P<0.01). Elevated levels of this cytokine have been detected in patients with ARDS and shown to be directly associated with the severity of lung inflammation and mortality (26). In the current study, TNF-α secretion was not detected in culture supernatants from the negative control and syn-hsa-miR-181b groups. The lowest TNF-α standards showed good reproducibility (3.8% coefficient of variation), thus the limit of detection was <2.048 pg/ml.
Figure 1.
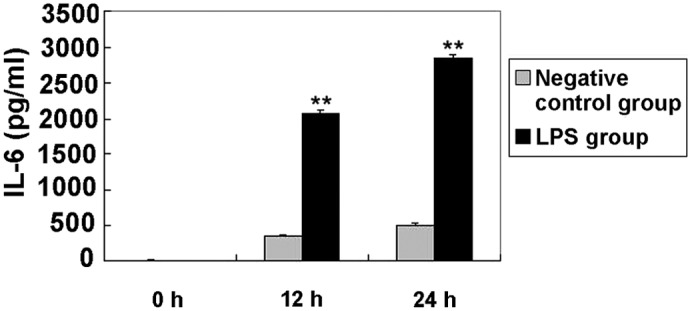
LPS-induced time-dependent release of IL-6 in BEAS-2B human bronchial epithelial cells. Cells were cultured with the negative control and LPS for 0, 12 and 24 h. Conditioned media were aspirated at each time interval and assayed for cytokine release using ELISA. The data are expressed as pg/ml of the conditioned media, presented as the mean ± standard error (n=3). **P<0.01, vs. negative control group. LPS, lipopolysaccharide; IL, interleukin
Activation of miR-181b in response to LPS stimulation in BEAS-2B cells
Although there is a poor understanding of the underlying mechanisms of ALI, an enhanced inflammatory response is known to be involved in this process and, at least partly, contribute to the development of the disease (2,3). Notably, miRNAs are emerging as new regulators of inflammatory and immune responses (14). In order to investigate the potential role of miRNAs in LPS-challenged BEAS-2B cells, the miRNA expression profile was analyzed. Cells were treated with or without 10 µg/ml LPS for 24 h and then the RNA extraction was submitted for an miRNA chip assay (miRHuman_16.0_070802 miRNA array). The profile analysis revealed that the expression of 41 miRNAs, particularly that of miR-181b, miR-23c and miR-26b, presented significant alterations in LPS-treated cells (P<0.01; Table I). The array results were further verified using qPCR, which revealed that the expression of miR-181b, miR-23c and miR-26b was a bona fide target of certain signaling pathways (Fig. 2). Of these potential candidates, the focus was laid on miR-181b, since it was one of the most clearly altered miRNAs and is known to be deregulated in inflammation, although its function remains unclear (27,28). In addition, miR-181b has recently been identified as a key player in a positive feedback loop linking inflammation to an epigenetic switch that controls cellular transformation in human mammary epithelial MCF-10A cells (20). The results of the current assay showed that the miR-181b expression level in the BEAS-2B cells was <50% of that in the LPS-stimulated BEAS-2B cells (Fig. 2), suggesting that miR-181b expression may be positively correlated with the LPS-induced ALI model.
Table I.
MicroRNA array analysis identified that 41 miRNAs were in response to lipopolysaccharide stimulation in human bronchial epithelial cells.
| No. | Probe_ID | Sample A signal (prestimulation) | Sample B signal (poststimulation) | Log2 (Sample B/Sample A) |
|---|---|---|---|---|
| 1 | hsa-miR-3613-3p | 206.97 | 520.07 | 1.27 |
| 2 | hsa-miR-335 | 338.68 | 853.34 | 1.25 |
| 3 | hsa-miR-26b | 638.32 | 1,490.33 | 1.20 |
| 4 | hsa-miR-23c | 778.64 | 1,834.46 | 1.19 |
| 5 | hsa-miR-181b | 380.03 | 821.69 | 1.15 |
| 6 | hsa-miR-1275 | 1,079.12 | 567.94 | −1.00 |
| 7 | hsa-miR-155 | 1,623.95 | 812.00 | −0.98 |
| 8 | hsa-miR-4324 | 450.65 | 838.80 | 0.91 |
| 9 | hsa-miR-27a | 1,120.89 | 2,037.10 | 0.86 |
| 10 | hsa-miR-15a | 338.01 | 610.37 | 0.86 |
| 11 | hsa-miR-320d | 3,035.04 | 1,678.92 | −0.82 |
| 12 | hsa-miR-224 | 1,684.58 | 2,870.00 | 0.80 |
| 13 | hsa-miR-320e | 2,374.19 | 1,410.80 | −0.75 |
| 14 | hsa-let-7e | 3,773.71 | 6,116.64 | 0.70 |
| 15 | hsa-miR-27b | 2,148.34 | 3,339.14 | 0.68 |
| 16 | hsa-let-7g | 2,219.58 | 3,507.05 | 0.67 |
| 17 | hsa-miR-320a | 4,671.19 | 3,001.94 | −0.64 |
| 18 | hsa-miR-320b | 4,521.04 | 2,934.64 | −0.62 |
| 19 | hsa-let-7b | 11,003.42 | 7,216.83 | −0.62 |
| 20 | hsa-miR-320c | 4,945.14 | 3,247.34 | −0.56 |
| 21 | hsa-miR-107 | 1,142.26 | 1,659.61 | 0.54 |
| 22 | hsa-miR-17 | 998.00 | 1,441.09 | 0.53 |
| 23 | hsa-miR-1246 | 5,445.48 | 3,735.81 | −0.51 |
| 24 | hsa-miR-181a | 626.31 | 889.46 | 0.51 |
| 25 | hsa-miR-92b | 1,925.05 | 1,373.15 | −0.49 |
| 26 | hsa-miR-21 | 15,567.78 | 21,195.97 | 0.46 |
| 27 | hsa-miR-103 | 1,464.21 | 1,991.04 | 0.44 |
| 28 | hsa-miR-222 | 6,340.95 | 4,743.61 | −0.42 |
| 29 | hsa-miR-92a | 4,248.12 | 3,181.43 | −0.41 |
| 30 | hsa-miR-25 | 2,094.70 | 2,733.08 | 0.38 |
| 31 | hsa-miR-15b | 4,844.80 | 5,967.15 | 0.34 |
| 32 | hsa-miR-26a | 5,491.24 | 6,963.52 | 0.34 |
| 33 | hsa-miR-20a | 1,251.63 | 1,569.61 | 0.33 |
| 34 | hsa-let-7a | 18,911.99 | 15,386.43 | −0.30 |
| 35 | hsa-miR-638 | 3,327.89 | 2,721.15 | −0.29 |
| 36 | hsa-let-7i | 3,071.09 | 3,725.29 | 0.28 |
| 37 | hsa-miR-23b | 12,277.94 | 15,069.06 | 0.27 |
| 38 | hsa-miR-3665 | 12,100.79 | 10,305.79 | −0.23 |
| 39 | hsa-miR-23a | 12,915.30 | 14,893.35 | 0.21 |
| 40 | hsa-miR-16 | 9,758.49 | 8,615.40 | −0.18 |
| 41 | hsa-let-7c | 13,839.79 | 12,196.52 | −0.17 |
The microarray contained three probe replicates for each miRNA. Dual-color fluorescence hybridization results of each probe were calculated based on the ratio Cy5/Cy3. For signal homogeneity, the median signal of each probe was defined as the real signal. The log ratio of each probe was calculated and the median log ratio of three replicated probes was defined as the real ratio. miRNA or miR, microRNA; hsa, Homo sapiens.
Figure 2.
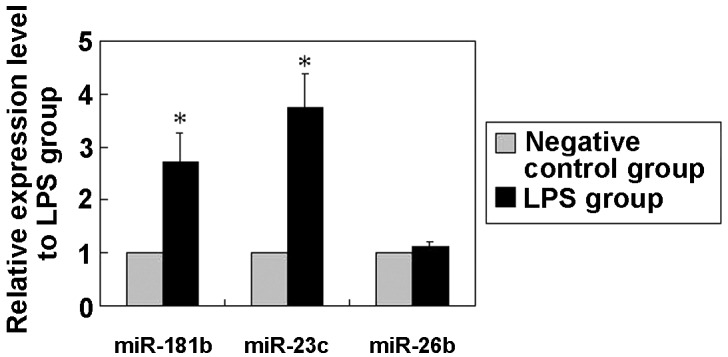
Expression levels of miR-181b, miR-23c and miR-26b in BEAS-2B human bronchial epithelial cells with or without LPS stimulation (LPS and negative control groups, respectively) was assessed using reverse transcription-quantitative polymerase chain reaction. The expression levels of miR-181b and miR-23c appeared significantly increased following LPS stimulation in BEAS-2B cells. The miRNA expression values in the untreated group were set to 1, and the relative amount of the miRNAs in the LPS group was plotted as a logarithmic fold induction. The data are expressed as the mean ± standard error (n=3). *P<0.05, vs. negative control group. LPS, lipopolysaccharide; miR or miRNA, microRNA.
Increased levels of IL-6 in BEAS-2B cells following syn-hsa-miR-181b transfection
Following syn-hsa-miR-181b transfection, the levels of IL-6 in the cultured supernatants of the BEAS-2B cells were determined using ELISA. Fig. 3 shows that the IL-6 levels were clearly elevated in the syn-hsa-miR-181b-transfected cells compared with the negative control levels. The lack of detectable TNF-α was unexpected, since this particular cytokine has been reported to be involved in the regulation of IL-6 and IL-8 (29).
Figure 3.
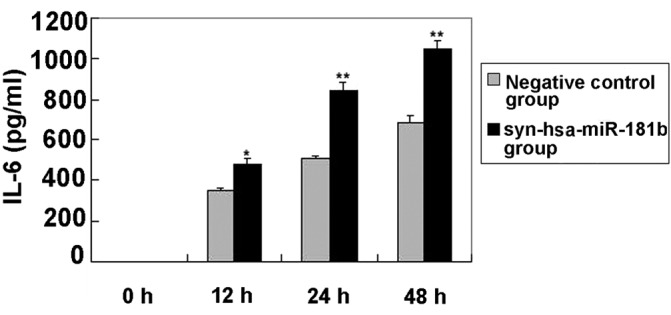
Syn-hsa-miR-181b-induced time-dependent release of IL-6 in BEAS-2B human bronchial epithelial cells. Cells were cultured with the negative control and syn-hsa-miR-181b, for 0, 12 and 48 h. Conditioned media were aspirated at each time interval and assayed for cytokine release by ELISA. The data are expressed as pg/ml of conditioned media and presented as the mean ± standard error (n=3). *P<0.05 and **P<0.01, vs. negative control group. IL, interleukin; miR or miRNA, microRNA.
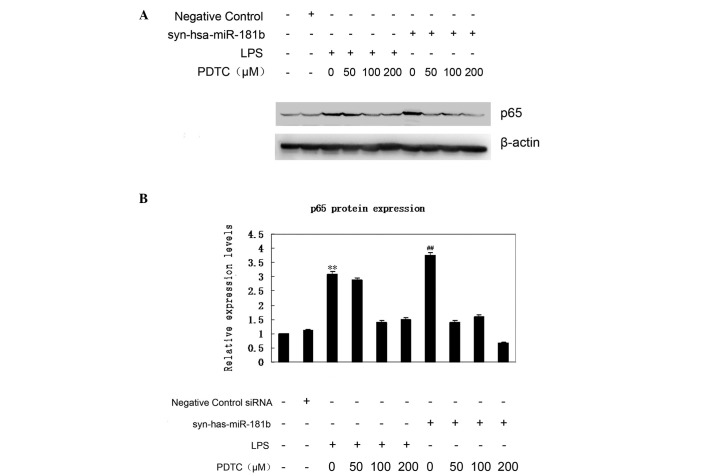
NF-κB inhibitors abrogate upregulation of p65 expression in response to syn-hsa-miR-181b transfection
Increased nuclear p65 protein shows that the NF-κB signaling pathway is activated. The p65 expression was therefore first examined using western blot analysis (Fig. 4A). According to the data, an elevated expression of p65 was observed in the syn-hsa-miR-181b-transfected BEAS-2B cells, which was comparable with that of the LPS treatment group. By contrast, the negative control had little effect on p65 expression (Fig. 4B). To further confirm the effect of miR-181b on the expression of p65, BEAS-2B cells were treated with PDTC 1.5 h prior to syn-hsa-miR-181b-transfection. As shown in Fig. 4A, even a low dose (50 µM) of PDTC treatment markedly abrogated the upregulation of p65 expression. This inhibition of PDTC on p65 expression was also observed in the LPS-treated cells. These findings demonstrated a critical link between miR-181b and the NF-κB signaling pathway in ALI.
Figure 4.
PDTC abrogated upregulation of p65 expression in response to syn-hsa-miR-181b transfection. p65 expression was significantly increased following transfection with syn-has-miR-181b, while the negative control group had little effect on p65 expression. (A) Representative image of western blotting from three repeated experiments, and (B) relative protein expression levels of p65, as determined using ImageJ software. **P<0.01 vs. negative control group. PDTC can abrogate the upregulation of p65 expression. ##P<0.01, vs. 100 µM PDTC group. Representative images were acquired from three different experiments. LPS, lipopolysaccharide; PDTC, ammonium pyrrolidinedithiocarbamate.
Discussion
miRNAs have been demonstrated to play a central role in the regulation of the immune system development, proliferation of monocytes and neutrophils, antibody production, differentiation of B- and T-cells, release of inflammatory mediators (30) and certain inflammatory lung diseases. Thus, miRNAs may also contribute to the pathogenesis of ALI/ARDS. miR-181b has been found to be a key player in a positive feedback loop that links inflammation to an epigenetic switch controlling cellular transformation in MCF-10A human mammary epithelial cells (20). Sun et al (31) revealed that miR-181b regulates NF-κB-mediated endothelial cell activation and vascular inflammation in response to proinflammatory stimuli. In addition, the rescue of miR-181b expression may provide a novel target for the treatment of critical diseases, such as diabetes, arthritis, and other chronic inflammatory diseases, as well as for anti-inflammatory treatment (31). In order to identify potential miRNAs involved in ALI, the miRNAs expression profile was analyzed in BEAS-2B cells with or without LPS treatment. Notably, 41 miRNAs displayed significantly differential expression levels (Table I). The results of qPCR revealed a 2–3-fold increase in miR-181b expression in the LPS-treated cells compared with the non-LPS-treated BEAS-2B cells. Transfection based approaches were further utilized in order to establish the promoting role of miR-181b in BEAS-2B cells.
BEAS-2B cells were selected as representative airway epithelial cell lines (32) for the purpose of studying the LPS-induced effects in the airway epithelium. BEAS-2B cells mimic the primary bronchial epithelial cells considerably well (33) and have been extensively used to investigate LPS-induced activation of pro-inflammatory cytokines as an in vitro model based on the first steps in the development of sepsis-induced ALI/ARDS (34,35). E. coli LPS treatment was selected due to its use in the majority of endotoxin-induced lung injury models (36,37). Furthermore, LPS is a key pathogen recognition molecule for sepsis (38), inducing apoptosis in lung cells (39).
For the examination of the cellular function of miR-181b, an overexpression approach in cultured BEAS-2B cells was used to detect the levels of TNF-α and IL-6. In the present study, it was demonstrated that the upregulation of miR-181b in BEAS-2B cells can increase the IL-6 expression. Iliopoulos et al (20) reported that, in human mammary epithelial MCF-10A cells, the inhibition of miR-181b expression, which is accomplished by treating cells with antisense RNAs against miR-181b, results in a reduced production of IL-6, a direct NF-κB target gene, and reduced NF-κB activity. BEAS-2B cells are also epithelial cells and therefore the use of anti-miR-181b was not required in the present study. IL-8 was not detected, since the release of inflammation factor IL-8 in response to particulate matter (≤2.5µm) and LPS treatment was qualitatively similar to the IL-6 responses, suggesting a common or closely-associated mechanism (40). However, besides the secretion of inflammatory factors, other aspects, such as the anti-apoptosis of lung cells (41) and the promotion of immunocyte transmigration (42), may also be involved in ALI.
NF-κB, a type of multidirectional nuclear transcriptional regulatory factor, regulates the expression of proteins and genes associated with inflammation, immunization, and growth regulation (43). In the present study, it was found that the overexpression of miR-181b leads to the upregulation of p65 expression, which is a member of the NF-κB signaling pathway. The present findings indicated that miR-181b acts as a proinflammatory factor through the targeting of the NF-κB signaling pathway in vitro. This conclusion is supported by the following evidence: First, the present study demonstrated that the upregulation of miR-181b in BEAS-2B cells can increase the expression of IL-6, a direct NF-κB target gene. In addition, western blot analysis identified that p65 was upregulated in the BEAS-2B cells following miR-181b overexpression. It was further demonstrated that PDTC abrogated an miR-181b-mediated p65 increase. Compared with the negative control group, p65 expression exhibited an ~3.7-fold increase following miR-181b overexpression, whereas the inhibition of NF-κB reduced the p65 expression by 50% following miR-181b overexpression; however the exact mechanism remains unclear. It has been reported that PDTC potently inhibits the activation and/or interaction of NF-κB with its upstream regulatory binding sites, thereby preventing NF-κB-mediated transcriptional activation (44,45). Furthermore, PDTC restrains IκB degradation, thus specifically inhibiting NF-κB activation (46).
The present study revealed that a collection of miRNAs was aberrantly expressed in the LPS-treated BEAS-2B cells, and focused on the effects of miR-181b on inflammation in BEAS-2B cells; however, several other miRNAs, such as miR-23c, were also dynamically regulated in LPS-induced ALI. Whether these miRNAs are also associated with LPS-induced lung injury remains to be elucidated. The use of bioinformatics to predict specific targets of miR-181b and the use of luciferase assay to show whether these genes are specific targets of miR-181b should be investigated in future studies.
In conclusion, while >1,000 human mature miRNA sequences are listed in the miRNA registry (47), only a handful have been characterized as functional regulators of leukocyte or endothelial cell inflammatory responses (48,49). The present study demonstrated that miR-181b is involved in LPS-induced lung injury. Specifically, miR-181b was found to serve as a potent regulator to promote inflammation through the NF-κB signaling pathway in the BEAS-2B cells. These findings may have important implications in the regulation of the adaptive immune response in ALI. Thus far, there is promising evidence supporting the potential application of miRNAs as novel therapeutic targets, as well as biomarkers for ALI; however, this requires further investigation prior to application in the daily management of ALI. Further studies on the genetic variation associated with miRNAs in real patient populations may help achieve the ultimate goal of providing personalized medical care for inflammatory lung disease. Considering inflammation as a system disorder (50), it would be interesting to examine whether miR-181b is also involved in the inflammation in vivo.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 31201040), Science Technology Department of Zhejiang Province (no. 2012C24005), Health Bureau of Zhejiang Province (nos. 11-CX01 and 2013ZDA002), Zhejiang Provincial Administration of traditional Chinese Medicine (no. 2012-XK-A04) and Natural Science Foundation of Zhejiang Province (no. Y14H010013). The authors would like to thank all the members of the laboratory for helpful discussions and comments on the manuscript.
References
- 1.Avecillas JF, Freire AX, Arroliga AC. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: Incidence, diagnosis and outcomes. Clin Chest Med. 2006;27:549–557. doi: 10.1016/j.ccm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 5.Hoare GS, Chester AH, Yacoub MH, Marczin N. Regulation of NF-kappaB and ICAM-1 expression in human airway epithelial cells. Int J Mol Med. 2002;9:35–44. [PubMed] [Google Scholar]
- 6.Li Z, Zhang de K, Yi WQ, Ouyang Q, Chen YQ, Gan HT. NF-kappaB p65 antisense oligonucleotides may serve as a novel molecular approach for the treatment of patients with ulcerative colitis. Arch Med Res. 2008;39:729–734. doi: 10.1016/j.arcmed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 11.Port JD, Sucharov C. Role of microRNAs in cardiovascular disease: Therapeutic challenges and potentials. J Cardiovasc Pharmacol. 2010;56:444–453. doi: 10.1097/FJC.0b013e3181f605b6. [DOI] [PubMed] [Google Scholar]
- 12.Buechner J, Henriksen JR, Haug BH, Tømte E, Flaegstad T, Einvik C. Inhibition of mir-21, which is upregulated during MYCN knockdown-mediated differentiation, does not prevent differentiation of neuroblastoma cells. Differentiation. 2011;81:25–34. doi: 10.1016/j.diff.2010.09.184. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Zhang Y, Shi Y, Dong G, Liang J, Han Y, Wang X, Zhao Q, Ding J, Wu K, et al. MicroRNA-107, an oncogene microRNA that regulates tumour invasion and metastasis by targeting DICER1 in gastric cancer. J Cell Mol Med. 2011;15:1887–1895. doi: 10.1111/j.1582-4934.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 15.Oglesby IK, McElvaney NG, Greene CM. MicroRNAs in inflammatory lung disease-master regulators or target practice? Respir Res. 2010;11:148. doi: 10.1186/1465-9921-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roggli E, Britan A, Gattesco S, Lin-Marq N, Abderrahmani A, Meda P, Regazzi R. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes. 2010;59:978–986. doi: 10.2337/db09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angulo M, Lecuona E, Sznajder JI. Role of MicroRNAs in lung disease. Arch Bronconeumol. 2012;48:325–330. doi: 10.1016/j.arbr.2012.06.015. (In English, Spanish) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai ZG, Zhang SM, Zhang Y, Zhou YY, Wu HB, Xu XP. MicroRNAs are dynamically regulated and play an important role in LPS-induced lung injury. Can J Physiol Pharmacol. 2012;90:37–43. doi: 10.1139/y11-095. [DOI] [PubMed] [Google Scholar]
- 19.Xie T, Liang J, Liu N, Wang Q, Li Y, Noble PW, Jiang D. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcγ receptor I. J Immunol. 2012;188:2437–2444. doi: 10.4049/jimmunol.1101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YZ, Mao GX, Lv YD, Huang QD, Wang GF. MicroRNA-181b stimulates inflammation via the NF-kappa B signaling pathway in vitro. J Am Geriatr Soc. 2014;62:S394–S394. [Google Scholar]
- 22.Schulz C, Farkas L, Wolf K, Kratzel K, Eissner G, Pfeifer M. Differences in LPS-induced activation of bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes (A-549) Scand J Immunol. 2002;56:294–302. doi: 10.1046/j.1365-3083.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 23.Pencheva N, Tran H, Buss C, Huh D, Dorbnjak M, Busam K, Tavazoie SF. Concergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell. 2012;151:1068–1082. doi: 10.1016/j.cell.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Zhang P, Wang F, Zhang H, Yang J, Peng J, Liu W, Qin H. miR-150 as a potential biomarker associated with prognosis and therapeutic outcome in colorectal cancer. Gut. 2012;61:1447–1453. doi: 10.1136/gutjnl-2011-301122. [DOI] [PubMed] [Google Scholar]
- 25.Elder ACP, Gelein R, Finkelstein JN, Cox C, Oberdorster G. Endotoxin priming affects the lung response to ultrafine particles and ozone in young and old rats. Inhalation Toxicology. 2000;12:85–98. doi: 10.1080/089583700196419. (Suppl 1) [DOI] [Google Scholar]
- 26.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 27.Dave RS, Khalili K. Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: Impact on inflammation and oxidative stress in the central nervous system. J Cell Biochem. 2010;110:834–845. doi: 10.1002/jcb.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 2011;3:159–166. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson S, Martin TR. Cytokines in Pulmonary Disease: Infection and Inflammation (Lung Biology in Health and Disease) In: Martin T, editor. 1st. Vol. 141. Marcel Dekker; New York, NY: 2000. [Google Scholar]
- 30.Pedersen I, David M. MicroRNAs in the immune response. Cytokine. 2008;43:391–394. doi: 10.1016/j.cyto.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, MICU Registry, Blackwell TS, et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyama S, Sato E, Nomura H, Kubo K, Miura M, Yamashita T, Nagai S, Izumi T. The potential of various lipopolysaccharides to release monocyte chemotactic activity from lung epithelial cells and fibroblasts. Eur Respir J. 1999;14:545–552. doi: 10.1034/j.1399-3003.1999.14c11.x. [DOI] [PubMed] [Google Scholar]
- 33.Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–1909. [PubMed] [Google Scholar]
- 34.Boots AW, Gerloff K, Bartholomé R, van Berlo D, Ledermann K, Haenen GR, Bast A, van Schooten FJ, Albrecht C, Schins RP. Neutrophils augment LPS-mediated pro-inflammatory signaling in human lung epithelial cells. Biochim Biophys Acta. 2012;1823:1151–1162. doi: 10.1016/j.bbamcr.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Yeh CH, Cho W, So EC, Chu CC, Lin MC, Wang JJ, Hsing CH. Propofol inhibits lipopolysaccharide-induced lung epithelial cell injury by reducing hypoxia-inducible factor-1alpha expression. Br J Anaesth. 2011;106:590–599. doi: 10.1093/bja/aer005. [DOI] [PubMed] [Google Scholar]
- 36.Fortis S, Spieth PM, Lu WY, Parotto M, Haitsma JJ, Slutsky AS, Zhong N, Mazer CD, Zhang H. Effects of anesthetic regimes on inflammatory responses in a rat model of acute lung injury. Intensive Care Med. 2012;38:1548–1555. doi: 10.1007/s00134-012-2610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal N, Sanyal SN. In vivo effect of surfactant on inflammatory cytokines during endotoxin-induced lung injury in rodents. J Immunotoxicol. 2011;8:274–283. doi: 10.3109/1547691X.2011.591294. [DOI] [PubMed] [Google Scholar]
- 38.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 39.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: How many ways can cells die? Am J Physiol Lung Cell Mol Physiol. 2008;294:L632–L641. doi: 10.1152/ajplung.00262.2007. [DOI] [PubMed] [Google Scholar]
- 40.Veranth JM, Reilly CA, Veranth MM, Moss TA, Langelier CR, Lanza DL, Yost GS. Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide and surface-modified particles. Toxicol Sci. 2004;82:88–96. doi: 10.1093/toxsci/kfh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern JB, Jaffré S, Dehoux M, Crestani B. Keratinocyte growth factor and Hepatocyte growth factor: Their roles in alveolar epithelial repair. Rev Mal Respir. 2003;20:896–903. [PubMed] [Google Scholar]
- 42.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 43.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 44.Kawai M, Nishikomori R, Jung EY, Tai G, Yamanaka C, Mayumi M, Heike T. Pyrrolidine dithiocarbamate inhibits intercellular adhesion molecule-1 biosynthesis induced by cytokines in human fibroblasts. J Immunol. 1995;154:2333–2341. [PubMed] [Google Scholar]
- 45.Schreck R, Meier B, Männel DN, Dröge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Zhou SH, Li XP, Shen XQ, Fang ZF, Liu QM, Qiu SF, Zhao SP. Atorvastatin downregulates BMP-2 expression induced by oxidized low-density lipoprotein in human umbilical vein endothelial cells. Circ J. 2008;72:807–812. doi: 10.1253/circj.72.807. [DOI] [PubMed] [Google Scholar]
- 47.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suárez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: Feedback control of inflammation. J Immunol. 2010;184:21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996;153:1850–1856. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]