Abstract
Positron emission tomography (PET) myocardial perfusion imaging (MPI) has high diagnostic accuracy and prognostic value. PET-MPI can also be used to quantitatively evaluate regional myocardial blood flow (MBF). This technique also allows the calculation of the coronary flow reserve (CFR)/myocardial flow reserve (MFR), which is the ratio of MBF at peak hyperemia to resting MBF. Coronary computed tomography angiography (CTA) is a non-invasive method for accurate detection and exclusion of high-grade coronary stenoses, when compared to an invasive coronary angiography reference standard. However, CTA assessment of coronary stenoses tends toward overestimation, and CTA cannot determine physiologic significance of lesions. Recent advances in computational fluid dynamics and image-based modeling permit calculation of non-invasive fractional flow reserve derived from CT (FFRCT), without the need for additional imaging, modification of acquisition protocols, or administration of medications. In this review, we cover the CFR/MFR assessment by PET and FFR assessment by CT.
Keywords: Coronary flow reserve (CFR), Myocardial flow reserve (MFR), Fractional flow reserve (FFR), Positron emission tomography (PET), Computed tomography angiography (CTA)
Introduction
Positron emission tomography (PET) myocardial perfusion imaging (MPI) allows accurate measurement of myocardial hypoperfusion and function at stress and rest. Absolute myocardial blood flow (MBF) in milliliter/minute/gram can be quantified using PET imaging in the same study. This technique allows the calculation of the coronary flow reserve (CFR) or myocardial flow reserve (MFR), which is the ratio of MBF at peak hyperemia to resting MBF. Fractional flow reserve (FFR) is an invasive approach and functional assessment, which measures the stenosis related decline in distal coronary pressure during maximum hyperemia. FFR at the time of invasive coronary angiography is the gold standard for determining functional consequences of coronary stenosis. Coronary CT angiography (CTA) is a non-invasive method for accurate detection and exclusion of high-grade coronary stenoses, when compared to an invasive coronary angiography reference standard. Recently, computation of FFR from CT (FFRCT) has emerged as a novel non-invasive method that demonstrates high diagnostic performance for identification and exclusion of patients and coronary lesions that cause ischemia. In this review, we discuss CFR/MFR assessment by PET and FFR assessment by CT.
PET Tracers
Amongst several available myocardial perfusion PET tracers, those most widely used in clinical practice include Rubidium-82 (82Rb) and Nitrogen-13-Ammonia (13N-ammonia). In addition to 82Rb and 13N-ammonia, cardiac PET perfusion can also be performed using 15O-water 18F-flurpiridaz. Each of these radiotracers displays different characteristics, which offer both advantages and disadvantages.
Rubidium-82
82Rb is a potassium analog that is a generator product with a physical half-life of 76 seconds [1] and kinetic and biological properties similar to those of Thallium-201 in single-photon emission computed tomography (SPECT) [1]. Because of the distinct advantage of not requiring an on-site cyclotron, 82Rb is the most widely used radionuclide for assessment of myocardial perfusion with PET [1]. Recent data suggest that the total-body effective dose from a stress-rest myocardial perfusion study performed with 82Rb is approximately 4 mSv [2, 3].
Nitrogen-13-Ammonia
13N-ammonia is a cyclotron product and has a physical half-life of 9.96 min [4]. After injection, 13N-ammonia rapidly disappears from the circulation after its cell-trapping conversion to glutamine, permitting the acquisition of images of excellent quality. Although the sequestration of 13N-ammonia in the lungs is usually minimal, it may be increased in patients with depressed left ventricular (LV) systolic function or chronic pulmonary disease and, occasionally, in smokers [4]. This may, in turn, adversely affect the quality of the images. In these cases, it may be necessary to increase the time between injection and image acquisition to optimize the contrast between myocardial and background activity. 13N-ammonia exhibits high resolution and extraction characteristics, but its use is limited to the sites with an on-site cyclotron. The total-body effective dose from a stress-rest myocardial perfusion study performed with 13N-ammonia is less than 3 mSv [5].
Oxygen-15-Water
Theoretically, this radiotracer is ideal for quantitative flow measurements by PET for two reasons: (1) Oxygen-15-Water (15O-water) diffuses freely across myocyte membranes; (2) 15O-water tissue retention of uptake is not affected by metabolic factors [6]. However, unlike 82Rb or 13N-ammonia, 15O-water is neither approved nor reimbursed for clinical imaging in the United States. It is also not suitable for static perfusion imaging [7]. 15O-water images of the myocardium are usually of lower count density, due to its short physical and biological half-life in the myocardium (2.4 min), thereby limiting the visual or semiquantitative assessment of regional myocardial perfusion from the static images [8].
Flurpiridaz F-18
Flurpridaz-F-18 has recently been introduced as a novel radiotracer for myocardial perfusion imaging [9]. Because of its smaller kinetic positron energy and, consequently, short positron range, F-18 tagged agent can take full advantage of PET superior spatial resolution. 18F-flurpiridaz has a relatively long physical half-life of 110 min as compared to currently available tracers and therefore does not require cyclotron on site. Phase 1 and phase 2 clinical studies have been completed with 18F-flurpiridaz, and phase 3 studies are currently ongoing [10]. Results of early clinical trials suggest that both pharmacologically and exercise-induced stress PET imaging protocols can be completed more rapidly and with lower patient radiation exposure than with SPECT tracers [11]. Preliminary data suggested that absolute myocardial blood flow may be determined using 18F-flurpiridaz myocardial perfusion PET imaging [12, 13] (Fig. 1).
Fig. 1.
Comparison of Flurpiridaz F 18 PET MPI and SPECT MPI in (a) diagnostic certainty and (b) sensitivity and specificity. Def. = definitely; Prob. = probably, CAD = coronary artery disease; MPI = myocardial perfusion imaging; PET = positron emission tomography; SPECT = single-photon emission computed tomography (Reproduced with permission from: Berman DS, Maddahi J, Tamarappoo BK, Czernin J, Taillefer R, Udelson JE et al. Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: flurpiridaz F 18 positron emission tomography. J Am Coll Cardiol. 2013;61(4):469–77. doi:10.1016/j. jacc.2012.11.022) [13]
CFR/MFR by PET
With standard list-mode acquisition offered by modern PET/CT scanners, dynamic perfusion images can be obtained with a single injection of the radiopharmaceutical and without additional imaging time or radiation to the patient [1]. Dynamic imaging is essential for evaluating absolute MBF. To obtain MBF values, image-derived time activity curves from the arterial blood and myocardial tissue regions are used as inputs to a tracer kinetic model. The kinetic model describes the exchange of tracer between the blood and the tissue over time. The rate of uptake of the tracer into the myocardial tissue provides an estimate of MBF on an absolute scale of mL/min/g. This technique also allows the calculation of the CFR/MFR, which is the ratio of MBF at peak hyperemia to resting MBF. Automated image analysis tools are required for reliable and robust clinical use of dynamic data for MBF quantification [14]. These clinical tools combine advances in the automated detection of the myocardium with established kinetic modeling techniques and allow rapid and automated analysis of dynamic scans.
Different modeling tools for calculation of MBF and MFR have been developed by several groups [15–19], each of which employ different methods of segmenting and sampling of the myocardial and blood pool activity to obtain input curves. Nevertheless, the agreement in absolute values of MBF and CFR/MFR is relatively high. In a comparative study, three existing clinical implementations of the kinetic modeling packages for the computation of regional stress and rest flow and CFR/MFR for 13N-ammonia with 1 -tissue and 2-tissue compartment models were compared to each other, demonstrating excellent agreement in MBF and MFR for each vascular territory [15]. Similarly, flow and flow reserve obtained with 82Rb tracers showed very good agreement in a comparison among different software programs for the routine kinetic quantification of data from multiple sites, scanners, and protocols [19].
Added Value of MBF, CFR/MFR Measurements by PET
Detecting Coronary Artery Disease
MBF analysis may be able to identify multivessel disease and predict the extent of ischemia more accurately than static perfusion imaging [7, 20]. The ability to quantify absolute MBF with stress PET-MPI permits identification of patients in whom the relative regional distribution of tracer may appear normal because of a balanced reduction of blood flow [21, 22••] (Fig. 2). A preliminary report showed that quantification of MFR in 13N-ammonia PET/CT MPI provides added diagnostic value for detection of coronary artery disease (CAD) over visual analysis of static MPI [23•]. However, further investigation is warranted because other studies by quantitative analysis of static PET images alone have demonstrated very high sensitivity and accuracy [24–26]. Hajjiri et al. reported that absolute quantification of hyperemic MBF is superior to measurements of relative tracer uptake for identification of hemodynamically significant CAD using 13N-ammonia PET [27]. However, numerous caveats apply to this study; in particular, different methods (measure tracer count by placing region of interest over the myocardial segments) from other investigators for relative perfusion assessment were used. Another report using quantitative 82Rb PET demonstrated a nonlinear decrease in hyperemic MBF as the severity of coronary artery stenosis increased [28]. Using 15O-water, a threshold of pharmacologically induced hyperemic MBF of 2.5 ml/g/min was proposed as accurate in the identification of epicardial lesions of >50 % diameter stenosis [29]. Two studies demonstrated better diagnostic performance of quantitative analysis with 82Rb and 15O-water using PET [22••, 30].
Fig. 2.
Clinical example in which flow quantification with PET may improve diagnosis of CAD. a dipyridamole 82Rb PET MPI static images demonstrate normal relative perfusion at rest and during peak stress; b 17-segment model polar maps of rest MBF (lower left; color display scale 0 to 1.5 mL/min/g), stress MBF (upper left; scale: 0–3.0 mL/min/g), MFR (upper right; scale: 0–3.0) and MFD (lower right; scale: 0–2.0) which demonstrate global impairments, absolute values displayed in the table below; c Coronary angiogram reveals significant obstructive three-vessel CAD, relative perfusion underestimated the presence of disease (arrows point out significant stenosis). HLA, horizontal long axis; SA, short axis; VLA, vertical long axis; MBF, myocardial blood flow; MFR, myocardial flow reserve; MFD, myocardial flow difference; LAD, left anterior descending artery; LCX, left circumflex; RCA, right coronary artery; RPLS, right posterolateral branch. (Reproduced with permission from: Ziadi MC, Beanlands RS. The clinical utility of assessing myocardial blood flow using positron emission tomography. J Nucl Cardiol. 2010;17(4):571–81. doi:10.1007/s12350-010-9258-7) [60]
Detecting Microvascular Dysfunction
Hyperemic MBF during pharmacologic vasodilation may be of use to identify microcirculatory dysfunction, as it can be diminished due to coronary microvascular disease in patients with or without focal CAD lesions on coronary angiography but with cardiovascular risk factors [31–33]. For example, in insulin-resistant individuals with normal stress-rest PET-MPI, concurrent MBF quantification has uncovered abnormalities in endothelium-related MBF responses to cold pressor testing, whereas hyperemic flows during pharmacologic vasodilation were preserved [34]. Indeed, coronary circulatory dysfunction in individuals with increasing body weight may progress from an impairment in endothelium-dependent coronary flow response to cold pressor testing, in the early stages of overweight, to an impairment of the predominantly endothelium-independent hyperemic flows during dipyridamole stimulation in the later stages of obesity [31]. An inverse relationship between CFR/MFR and plasma glucose levels has been shown in patients with type 2 diabetes mellitus, providing direct evidence of an adverse effect of raised plasma glucose concentration on diabetes-related coronary microvasculopathy [34]. Assessment of CFR/MFR with PET allows characterization of endothelial dysfunction and may serve as a future platform for early identification of asymptomatic CAD.
Prognostic Value of MBF and CFR
Prognostically, MBF and CFR/MFR demonstrated value as an additional marker for adverse cardiac events [35–37, 38••, 39]. Herzog et al. demonstrated that in the setting of a normal MPI result, a preserved CFR/MFR of more than 2.0 may provide a warranty period of 3 years versus a reduced CFR/MFR [35]. Also, CFR/MFR results were found to stratify patients—both those with normal and those with abnormal stress perfusion— with respect to their risk of adverse cardiovascular events. PET-MPI in combination with quantification of regional MBF or regional CFR/MFR may therefore improve our ability to identify patients who might benefit from revascularization and/or intensified medical therapy, and may provide better prognostic estimates of future cardiac events.
Strengths and Limitations
In addition to absolute quantification of myocardial perfusion, because stress images can be obtained immediately after infusion of a vasodilator, the results of a stress gated PET represent peak stress wall motion (unlike in SPECT where stress images are acquired up to 45 minutes after stress). Although vasodilator-stress gated images are not equivalent to exercise gated images, investigators have found that the failure to show an increased ejection fraction on stress gated PET images compared with rest images is strongly associated with the presence of extensive CAD [40]. This ejection fraction reserve has been shown to be prognostically significant [41]. PET perfusion stress/rest imaging (including absolute quantification of myocardial perfusion) can be performed with much lower radiation doses (< 4 mSv), especially on new 3D PET/CT scanners [42].
Almost all new PET scanners models are currently sold in the hybrid PET/CT configuration, in which the CT-based attenuation correction has replaced the traditional transmission CT-based attenuation correction technique. CT-based attenuation correction for PET studies has proven challenging because of the effects of possible respiratory motion in general [43] and for myocardial perfusion studies more specifically [44, 45]. It has been shown that there are significant differences between traditional transmission CT-based attenuation correction and CT-based attenuation correction applied to cardiac PET/CT studies, which may remain after alignment of CT maps to emission data [46]. This problem is not yet fully resolved; however, careful visual verification of alignment usually ensures that these artifacts are minimized.
Although absolute quantification of myocardial perfusion has a great clinical potential, there are several limitations that need to be recognized. The knowledge of optimal cut-off values for absolute perfusion needs to be studied in large populations and for various tracers. Furthermore, additional information is needed about expected flow and flow reserve values in different subpopulations such as diabetic and obese patients, patients with heart failure and revascularized patients. Further, mean flow rate measurements may be obtainable from PET-based perfusion imaging, potential sources of errors in measurement, including the effect of high driving pressure and high resting flow rates, are still being characterized. Furthermore, still unexplored is how CCR/MFR may differentiate between patients who have epicardial stenoses and patients who have abnormalities with subendocardial microvascular perfusion.
FFR Assessment by CT
Recent advances in computational fluid dynamics (CFD) enable calculation of coronary flow and pressure fields from anatomic image data [47]. Applied to CT, these technologies enable calculation of FFR, defined as the ratio of maximal myocardial blood flow through a diseased artery to the blood flow in the hypothetical case that this artery is normal, without additional imaging or medications (Fig. 3).
Fig. 3.
Simplified schematic of computation fluid dynamic techniques applied to CTA data for simulation of hyperemic coronary artery flow and pressure. (Reproduced with permission from: Min JK, Berman DS, Budoff MJ, Jaffer FA, Leipsic J, Leon MB et al. Rationale and design of the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy) study. J Cardiovasc Comput Tomogr. 2011;5(5):301–9. doi:S1934-5925(11)00317-0 [pii]) [61]
Coronary flow and pressure can be computed by solving the governing equations of fluid dynamics, which have been known in their current form as the Navier-Stokes equations. These equations are solved for the unknown pressure, which varies with position and time, and for the three components of blood velocity, each of which are functions of position and time. The physical properties of blood, the fluid density, and the fluid viscosity, are assumed when solving these equations. For realistic patient-specific models of the human coronary arteries, a numerical method must instead be used to approximate the governing equations and obtain a solution for velocity and pressure at a finite number of points [48•].
Coupled to CFD, the computation of FFR from CT requires methods to extract models from image data and incorporate boundary conditions that model the effect of the microcirculation [48•]. For that, image segmentation algorithms extract the luminal surface of the major vessels and branches, up to the limits imposed by the resolution of CT. Further, simulating maximum hyperemia by modeling mimics the effect of adenosine on reducing the peripheral resistance of the coronary microcirculation downstream of the epicardial arteries extracted from CT. This change in resistance of normal coronary arteries provides an upper bound on the maximal change that can be achieved in patients with microcirculatory dysfunction and represents an identical assumption made with invasive FFR, wherein the hyperemic microcirculatory resistance distal to a stenosis is assumed to be the same as that in the hypothetical case that the coronary arteries have no disease [49]. Upon generation of a discrete model of the ascending aorta and epicardial coronary arteries, and the definition of the boundary conditions for rest and hyperemic conditions, FFRCT can then be determined by solving the equations of blood flow for the velocity and pressure fields. FFRCT is then obtained by normalizing the mean hyperemic pressure field by the average mean hyperemic pressure in the aorta. The end result is a complete spatial distribution of FFRCT (Fig. 4).
Fig. 4.
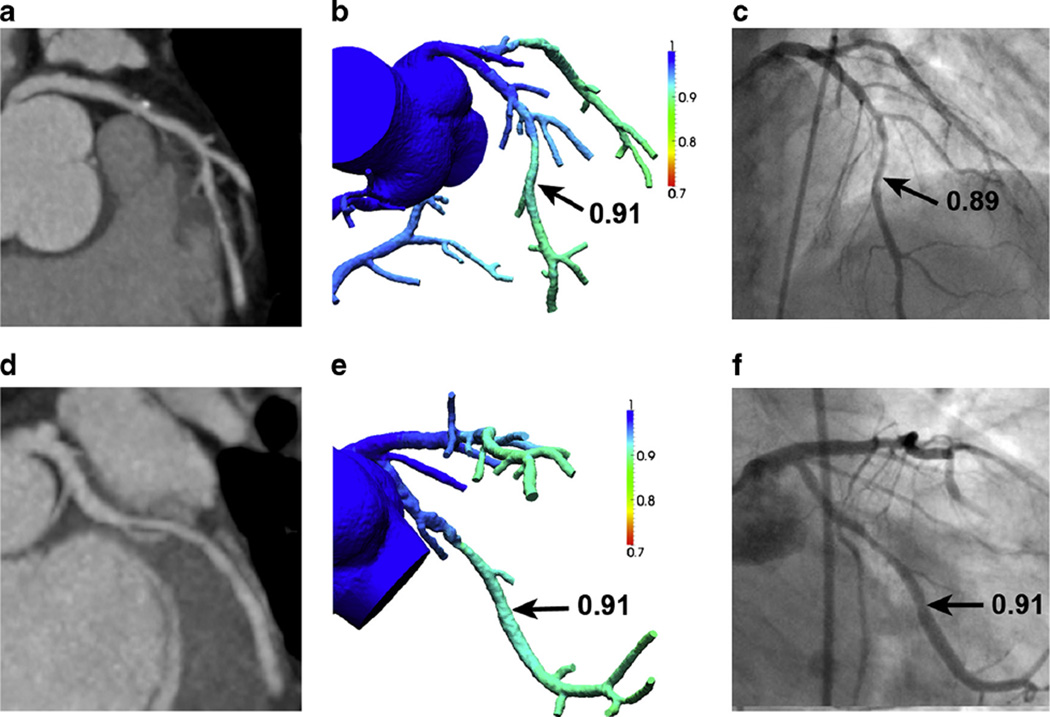
Representative examples of two patients from the DeFACTO study. (1) FFRCTA results for 66-year-old man with multivessel CAD but no lesion-specific ischemia (a) Coronary computed tomography angiography (CTA) demonstrating stenosis in the left anterior descending coronary artery (LAD). (b) Fractional flow reserve (FFR) derived from CTA (FFRCTA) demonstrates no ischemia in the LAD, with a computed value of 0.91. c Invasive coronary angiography (ICA) with FFR also demonstrates no ischemia in the LAD, with a measured value of 0.89. d CTA demonstrating stenosis in the left circumflex coronary (LCx) artery. e FFRCTA demonstrates no ischemia in the LCx, with a computed value of 0.91. f ICA with FFR also demonstrates no ischemia in the LCx, with a measured value of 0.91. (2) FFRCTA results for 66-year-old man with multivessel CAD and lesion-specific ischemia (a) CTA demonstrating stenosis in the LAD. b FFRCTA demonstrates ischemia in the LAD, with a computed value of 0.64. c ICA with FFR also demonstrates ischemia in the LAD, with a measured value of 0.72. d CTA demonstrating stenosis in the LCx. e FFRCTA demonstrates ischemia in the LCx, with a computed value of 0.61. f ICA with FFR also demonstrates ischemia in the LCx, with a measured value of 0.52. Abbreviations as in Fig. 4(1). (Reproduced with permission from: Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61(22):2233–41. doi:10.1016/j.jacc.2012.11.083) [48•]
Diagnostic Performance of FFRCT
Several studies showed incremental value of FFRCT in diagnosis of CAD. In the Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial, compared to invasive FFR, non-invasive FFR derived from CT (FFRCT), demonstrated per-vessel accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for lesions causing ischemia of 84.3 %, 87.9 %, 82.2 %, 73.9 %, 92.2 %, respectively, for FFRCT [50]. The performance of FFRCT was superior to CT stenosis for diagnosing ischemic lesions, the latter of which demonstrated an accuracy, sensitivity, specificity, PPV, and NPV of 58.5%, 91.4%, 39.6%, 46.5%, 88.9 %, respectively. More recently, the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) trial—a pivotal multicenter international study evaluating FFRCT against CT for diagnostic accuracy of ischemia, has been published [51••]. This trial consisted of 252 patients for which 407 vessels were directly interrogated by invasive FFR. On a per-patient basis, FFRCT was superior to CT stenosis for diagnosis of ischemic lesions for accuracy (73 vs. 64 %), sensitivity (90 % vs. 84 %), specificity (54 % vs. 42 %), PPV (67 % vs. 61 %), and NPV (84 % vs. 72 %). Area under the receiver operating characteristic curve of FFRCT also showed improved discrimination over CT alone [0.68 (95 % CI 0.62 to 0.74) vs. 0.81 (0.75–0.86); p <0.001]. For lesions of intermediate stenosis severity (30 % to 69 %), there was a more than two-fold increase in sensitivity, from 34 to 74 %, with no loss of specificity [52••]. FFRCT demonstrated superior discrimination compared with CT stenosis on a per-vessel basis (area under the receiver operating characteristic curve, 0.79 versus 0.53; p<0.0001). FFRCT demonstrated significant reclassification of CT stenosis for lesion-specific ischemia [net reclassification improvement (NRI), 0.45; 95 % confidence interval, 0.25–0.65; p= 0.01].
Strengths and Limitations
CTA is a non-invasive method for accurate detection and exclusion of high-grade coronary stenoses, when compared to an invasive coronary angiography reference standard [53]. Although current generation CTA exhibits high NPV to exclude ≥50 % obstructive coronary artery stenosis, CTA cannot determine physiologic significance of lesions and even among CT-identified obstructive stenoses confirmed by invasive coronary angiography, fewer than half are ischemia-causing [54, 55]. Thus, FFRCT has emerged as a novel non-invasive method that demonstrates high diagnostic performance for identification and exclusion of patients and coronary lesions that cause ischemia [51••]. Further FFRCT possesses high diagnostic performance for diagnosis of ischemic for lesions of intermediate stenosis severity (30 % to 69 %). Notably, the high sensitivity and negative predictive value suggest the ability of FFRCT to effectively rule out intermediate lesions that cause ischemia. In addition to FFRCT, CT also enables assessment of several coronary atherosclerotic plaque characteristics with high accuracy; including positive arterial remodeling; low attenuation plaque (marker for necrotic lipid laden intra-plaque core); and spotty intra-plaque calcification, which have been related to acute coronary syndrome [56–59]. FFRCT offers several operational advantages in that it does not require modification of CT angiography protocols, does not require administration of additional medications beyond what is typically administered for CTA, and does not confer any additional radiation. Yet this technology, while now studied in three prospective multicenter studies, is not without limitations. The calculation of FFRCT is based upon a proprietary algorithm that is at present available only through a single commercial entity. Further, given the computational time and intensity required for calculation of FFRCT, the workflow of obtaining this as-yet not FDA approved software requires sending of images to a 3rd party rather than on-site assessment. Given the importance of coronary artery segmentation, technical artifacts such as misregistration or coronary motion may affect measures.
Conclusions
Stress/rest PET perfusion imaging including absolute myocardial blood flow imaging can be performed in one scanning session. PET-MPI in combination with quantification of regional MBF or regional CFR/MFR may improve the ability to identify patients who might benefit from revascularization and may provide better prognostic estimates of future cardiac events. Recent advances in computational fluid dynamics enable calculation of non-invasive FFR derived from CT (FFRCT). Prospective international multicenter trial demonstrates FFRCT is superior to measures of CTA stenosis severity for determination of lesion-specific ischemia. Further comparative and/or complementary studies are needed to precisely identify the proper clinical role of FFRCT in the context of traditional MPI imaging.
Acknowledgments
This study was funded by grants from the National Institutes of Health (R01HL11515002 and R01HL11801901). This study was also funded by a gift from the Dalio Institute of Cardiovasular Imaging and the Michael Wolk Foundation.
Abbreviations
- CAD
Coronary artery disease
- CFD
Computational fluid dynamics
- CFR
Coronary flow reserve
- CTA
Computed tomographic angiography
- FFR
Fractional flow reserve
- FFRCT
Fractional flow reserve derived from CT
- MBF
Myocardial blood flow
- MFR
Myocardial flow reserve
- NPV
Negative predictive value
- PET
Positron emission tomography
- PPV
Positive predictive value
- SPECT
Single-photon emission computed tomography
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Ryo Nakazato declares that he has no conflict of interest.
Ran Heo declares that he has no conflict of interest.
Jonathon Leipsic has received grant support from and been a consultant for HeartFlow. He has received payment for development of educational presentations including service on speakers’ bureaus from GE Healthcare.
James K. Min has received grant support from HeartFlow. He serves as a consultant to HeartFlow.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Ryo Nakazato, St. Luke’s International Hospital, Tokyo, Japan.
Ran Heo, Weill Cornell Medical College and the New York-Presbyterian Hospital, New York, NY, USA.
Jonathon Leipsic, St. Paul’s Hospital, Vancouver, British Columbia, Canada.
James K. Min, Weill Cornell Medical College and the New York-Presbyterian Hospital, New York, NY, USA Departments of Radiology and Medicine, Weill Cornell Medical College, New York-Presbyterian Hospital, 520 E. 70th Street, New York, NY 10021, USA, jkm2001@med.cornell.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Di Carli MF, Murthy VL. Cardiac PET/CT for the evaluation of known or suspected coronary artery disease. Radiographics. 2011;31(5):1239–1254. doi: 10.1148/rg.315115056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senthamizhchelvan S, Bravo PE, Lodge MA, Merrill J, Bengel FM, Sgouros G. Radiation dosimetry of 82Rb in humans under pharmacologic stress. J Nucl Med Off Publ Soc Nucl Med. 2011;52(3):485–491. doi: 10.2967/jnumed.110.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stabin MG. Proposed revision to the radiation dosimetry of 82Rb. Health Phys. 2010;99(6):811–813. doi: 10.1097/HP.0b013e3181e47b33. [DOI] [PubMed] [Google Scholar]
- 4.Di Carli MF, Dorbala S, Meserve J, El Fakhri G, Sitek A, Moore SC. Clinical myocardial perfusion PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2007;48(5):783–793. doi: 10.2967/jnumed.106.032789. [DOI] [PubMed] [Google Scholar]
- 5.Stabin MG. Radiopharmaceuticals for nuclear cardiology: radiation dosimetry, uncertainties, and risk. J Nucl Med Off Publ Soc Nucl Med. 2008;49(9):1555–1563. doi: 10.2967/jnumed.108.052241. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann SR, Herrero P, Markham J, Weinheimer CJ, Walsh MN. Noninvasive quantitation of myocardial blood flow in human subjects with oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol. 1989;14(3):639–652. doi: 10.1016/0735-1097(89)90105-8. [DOI] [PubMed] [Google Scholar]
- 7.Knuuti J, Kajander S, Mäki M, Ukkonen H. Quantification of myocardial blood flow will reform the detection of CAD. J Nucl Cardiol. 2009;16(4):497–506. doi: 10.1007/s12350-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 8.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3(6):623–640. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Dilsizian V, Taillefer R. Journey in evolution of nuclear cardiology: will there be another quantum leap with the F-18-labeled myocardial perfusion tracers? JACC Cardiovasc Imaging. 2012;5(12):1269–1284. doi: 10.1016/j.jcmg.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Maddahi J. Properties of an ideal PET perfusion tracer: new PET tracer cases and data. J Nucl Cardiol. 2012;19(Suppl 1):S30–7. doi: 10.1007/s12350-011-9491-8. [DOI] [PubMed] [Google Scholar]
- 11.Maddahi J, Czernin J, Lazewatsky J, Huang S-C, Dahlbom M, Schelbert H, et al. Phase I, first-in-human study of BMS747158, a novel F-18-labeled tracer for myocardial perfusion PET: dosimetry, biodistribution, safety, and imaging characteristics after a single injection at rest. J Nucl Med. 2011;52(9) doi: 10.2967/jnumed.111.092528. [DOI] [PubMed] [Google Scholar]
- 12.Maddahi J, Huang S, Truong D, Lazewatsky J, Ehlgen A, Schelbert H, et al. Preliminary results of absolute quantification of rest and stress myocardial blood flow with flurpiridaz F-18 PET in normal and coronary artery disease patients in a single-center study. J Nucl Cardiol. 2010;17:743. abstract. [Google Scholar]
- 13.Berman DS, Maddahi J, Tamarappoo BK, Czernin J, Taillefer R, Udelson JE, et al. Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: flurpiridaz F 18 positron emission tomography. J Am Coll Cardiol. 2013;61(4):469–477. doi: 10.1016/j.jacc.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein R, Beanlands R, deKemp R. Quantification of myocardial blood flow and flow reserve: technical aspects. J Nucl Cardiol. 2010;17(4):555–570. doi: 10.1007/s12350-010-9256-9. [DOI] [PubMed] [Google Scholar]
- 15.Slomka PJ, Alexanderson E, Jácome R, Jiménez M, Romero E, Meave A, et al. Comparison of clinical tools for measurements of regional stress and rest myocardial blood flow assessed with 13N–ammonia PET/CT. J Nucl Med. 2012;53(2):171–181. doi: 10.2967/jnumed.111.095398. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Renaud JM, Ziadi MC, Thorn SL, Adler A, Beanlands RS, et al. Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 pet and a highly automated analysis program. J Nucl Cardiol. 2010;17(4):600–616. doi: 10.1007/s12350-010-9225-3. [DOI] [PubMed] [Google Scholar]
- 17.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med. 2009;50(7):1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kajander S, Joutsiniemi E, Saraste M, Pietilä M, Ukkonen H, Saraste A, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122(6):603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 19.Dekemp RA, Declerck J, Klein R, Pan XB, Nakazato R, Tonge C, et al. Multisoftware reproducibility study of stress and rest myocardial blood flow assessed with 3D dynamic PET/CT and a 1-tissue-compartment model of 82Rb kinetics. J Nucl Med. 2013;54(4):571–577. doi: 10.2967/jnumed.112.112219. [DOI] [PubMed] [Google Scholar]
- 20.Beanlands RS, Ziadi MC, Williams K. Quantification of myocardial flow reserve using positron emission imaging the journey to clinical use. J Am Coll Cardiol. 2009;54(2):157–159. doi: 10.1016/j.jacc.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 21.Parkash R, deKemp RA, Ruddy TD, Kitsikis A, Hart R, Beauchesne L, et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11(4):440–449. doi: 10.1016/j.nuclcard.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 22. Ziadi MC, Dekemp RA, Williams K, Guo A, Renaud JM, Chow BJ, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol. 2012;19(4):670–680. doi: 10.1007/s12350-011-9506-5.This study provides usefulness of myocardial flow reserve (MFR) for 3-vessel coronary artery disease
- 23. Fiechter M, Ghadri JR, Gebhard C, Fuchs TA, Pazhenkottil AP, Nkoulou RN, et al. Diagnostic value of 13N–ammonia myocardial perfusion PET: added value of myocardial flow reserve. J Nucl Med. 2012;53(8):1230–1234. doi: 10.2967/jnumed.111.101840.This study provides substantial added diagnostic value of myocardial flow reserve (MFR) for detection of coronary artery disease
- 24.Santana CA, Folks RD, Garcia EV, Verdes L, Sanyal R, Hainer J, et al. Quantitative (82)Rb PET/CT: development and validation of myocardial perfusion database. J Nucl Med. 2007;48(7):1122–1128. doi: 10.2967/jnumed.107.039750. [DOI] [PubMed] [Google Scholar]
- 25.Nakazato R, Berman DS, Dey D, Le Meunier L, Hayes SW, Fermin JS, et al. Automated quantitative Rb-82 3D PET/CT myocardial perfusion imaging: normal limits and correlation with invasive coronary angiography. J Nucl Cardiol. 2012;19(2):265–76. doi: 10.1007/s12350-011-9496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaster T, Mylonas I, Renaud JM, Wells GA, Beanlands RS, Dekemp RA. Accuracy of low-dose rubidium-82 myocardial perfusion imaging for detection of coronary artery disease using 3D PET and normal database interpretation. J Nucl Cardiol. 2012 doi: 10.1007/s12350-012-9621-y. [DOI] [PubMed] [Google Scholar]
- 27.Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. JACC Cardiovasc Imaging. 2009;2(6):751–758. doi: 10.1016/j.jcmg.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Anagnostopoulos C, Almonacid A, El Fakhri G, Curillova Z, Sitek A, Roughton M, et al. Quantitative relationship between coronary vasodilator reserve assessed by 82Rb PET imaging and coronary artery stenosis severity. Eur J Nucl Med Mol Imaging. 2008;35(9):1593–1601. doi: 10.1007/s00259-008-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesterov SV, Han C, Maki M, Kajander S, Naum AG, Helenius H, et al. Myocardial perfusion quantitation with 15O–labelled water PET: high reproducibility of the new cardiac analysis software (Carimas) Eur J Nucl Med Mol Imaging. 2009;36(10):1594–602. doi: 10.1007/s00259-009-1143-8. [DOI] [PubMed] [Google Scholar]
- 30.Kajander SA, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, et al. Clinical value of absolute quantification of myocardial perfusion with (15)O-water in coronary artery disease. Circ Cardiovasc Imaging. 2011;4(6):678–684. doi: 10.1161/CIRCIMAGING.110.960732. [DOI] [PubMed] [Google Scholar]
- 31.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47(6):1188–1195. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 32.Prior JO, Quinones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, et al. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111(18):2291–2298. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann PA, Gnecchi-Ruscone T, Schäfers KP, Lüscher TF, Camici PG. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J Am Coll Cardiol. 2000;36(1):103–109. doi: 10.1016/s0735-1097(00)00697-5. [DOI] [PubMed] [Google Scholar]
- 34.Quiñones MJ, Hernandez-Pampaloni M, Schelbert H, Bulnes-Enriquez I, Jimenez X, Hernandez G, et al. Coronary vasomotor abnormalities in insulin-resistant individuals. Ann Intern Med. 2004;140(9):700–708. doi: 10.7326/0003-4819-140-9-200405040-00009. [DOI] [PubMed] [Google Scholar]
- 35.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N–ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54(2):150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 36.Fukushima K, Javadi MS, Higuchi T, Lautamäki R, Merrill J, Nekolla SG, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52(5):726–732. doi: 10.2967/jnumed.110.081828. [DOI] [PubMed] [Google Scholar]
- 37.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 38. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427.This study provides incremental prognostic significance of coronary flow reserve (CFR) for coronary artery disease
- 39.Schindler TH, Nitzsche EU, Schelbert HR, Olschewski M, Sayre J, Mix M, et al. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45(9):1505–1512. doi: 10.1016/j.jacc.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 40.Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med. 2007;48(3):349–358. [PubMed] [Google Scholar]
- 41.Dorbala S, Hachamovitch R, Curillova Z, Thomas D, Vangala D, Kwong RY, et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging. 2009;2(7):846–854. doi: 10.1016/j.jcmg.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slomka PJ, Dey D, Duvall WL, Henzlova MJ, Berman DS, Germano G. Advances in nuclear cardiac instrumentation with a view towards reduced radiation exposure. Curr Cardiol Rep. 2012;14(2):208–216. doi: 10.1007/s11886-012-0248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan T, Mawlawi O, Nehmeh SA, Erdi YE, Luo D, Liu HH, et al. Attenuation correction of PET images with respiration-averaged CT images in PET/CT. J Nucl Med. 2005;46(9):1481–1487. [PubMed] [Google Scholar]
- 44.Bacharach SL. PET/CT attenuation correction: breathing lessons. J Nucl Med. 2007;48(5):677–679. doi: 10.2967/jnumed.106.037499. [DOI] [PubMed] [Google Scholar]
- 45.Gould KL, Pan T, Loghin C, Johnson NP, Guha A, Sdringola S. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. J Nucl Med. 2007;48(7):1112–1121. doi: 10.2967/jnumed.107.039792. [DOI] [PubMed] [Google Scholar]
- 46.Slomka PJ, Le Meunier L, Hayes SW, Acampa W, Oba M, Haemer GG, et al. Comparison of myocardial perfusion 82Rb PET performed with CT- and transmission CT-based attenuation correction. J Nucl Med. 2008;49(12):1992–1998. doi: 10.2967/jnumed.108.056580. [DOI] [PubMed] [Google Scholar]
- 47.Kim H, Vignon-Clementel I, Coogan J, Figueroa C, Jansen K, Taylor C. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng. 2010;38(10):3195–3209. doi: 10.1007/s10439-010-0083-6. [DOI] [PubMed] [Google Scholar]
- 48. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61(22):2233–2241. doi: 10.1016/j.jacc.2012.11.083.This review provides a thorough description of how FFRCT can be calculated
- 49.Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354–1367. doi: 10.1161/01.cir.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 50.Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58(19):1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 51. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308(12):1237–245. doi: 10.1001/2012.jama.11274.This study provides substantial diagnostic value of FFRCT for the detection of hemodynamically significant coronary artery disease
- 52. Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis A, et al. Noninvasive Fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ Cardiovasc Imaging. 2013;6(6):881–889. doi: 10.1161/CIRCIMAGING.113.000297.This study provides the ability of FFRCT to effectively rule out intermediate stenosis lesions that cause ischemia
- 53.Min J, Shaw L, Berman D. The present state of coronary computed tomography angiography a process in evolution. J Am Coll Cardiol. 2010;55(10):957–965. doi: 10.1016/j.jacc.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 54.Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52(8):636–643. doi: 10.1016/j.jacc.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 55.Schuijf JD, Bax JJ. CT angiography: an alternative to nuclear perfusion imaging? Heart. 2008;94(3):255–257. doi: 10.1136/hrt.2006.105833. [DOI] [PubMed] [Google Scholar]
- 56.Leber AW, Knez A, White CW, Becker A, von Ziegler F, Muehling O, et al. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am J Cardiol. 2003;91(6):714–718. doi: 10.1016/s0002-9149(02)03411-2. [DOI] [PubMed] [Google Scholar]
- 57.Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47(8):1655–1662. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 58.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50(4):319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 59.Nakazato R, Shalev A, Doh JH, Koo BK, Dey D, Berman DS, et al. Quantification and characterisation of coronary artery plaque volume and adverse plaque features by coronary computed tomographic angiography: a direct comparison to intravascular ultrasound. Eur Radiol. 2013;23(8):2109–2117. doi: 10.1007/s00330-013-2822-1. [DOI] [PubMed] [Google Scholar]
- 60.Ziadi MC, Beanlands RS. The clinical utility of assessing myocardial blood flow using positron emission tomography. J Nucl Cardiol. 2010;17(4):571–581. doi: 10.1007/s12350-010-9258-7. [DOI] [PubMed] [Google Scholar]
- 61.Min JK, Berman DS, Budoff MJ, Jaffer FA, Leipsic J, Leon MB, et al. Rationale and design of the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy) study. J Cardiovasc Comput Tomogr. 2011;5(5):301–309. doi: 10.1016/j.jcct.2011.08.003. [DOI] [PubMed] [Google Scholar]