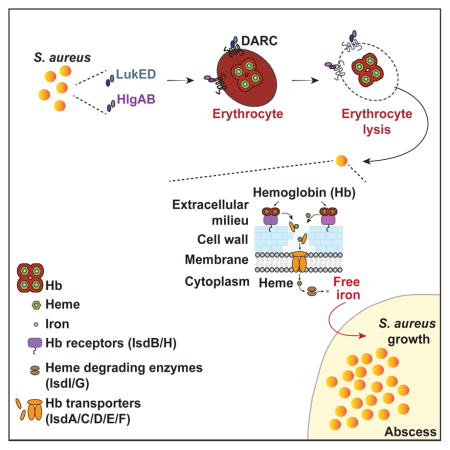
SUMMARY
In order for Staphylococcus aureus to thrive inside the mammalian host, the bacterium has to overcome iron scarcity. S. aureus is thought to produce toxins that lyse erythrocytes, releasing hemoglobin, the most abundant iron source in mammals. Here we identify the Duffy antigen receptor for chemokines (DARC) as the receptor for the S. aureus hemolytic leukocidins LukED and HlgAB. By assessing human erythrocytes with DARC polymorphisms, we determined that HlgAB and LukED-mediated lysis directly relates to DARC expression. DARC is required for S. aureus-mediated lysis of human erythrocytes and DARC overexpression is sufficient to render cells susceptible to toxin-mediated lysis. HlgA and LukE bind directly to DARC through different regions, and by targeting DARC, HlgAB and LukED support S. aureus growth in a hemoglobin acquisition-dependent manner. These findings elucidate how S. aureus targets and lyses erythrocytes to release one of the scarcest nutrients within the mammalian host.
Graphical Abstract
INTRODUCTION
Staphylococcus aureus (S. aureus) is an important human pathogen that plagues patients in hospitals worldwide, especially since the emergence of antibiotic resistance. S. aureus infections range from minor skin and soft tissue infections, to more invasive and life threating infections like sepsis, endocarditis, osteomyelitis, and pneumonia (Chambers and Deleo, 2009). The pathogenesis of this bacterium is multifactorial, and is exacerbated by the combination of resistance to many antibiotics and the production of an arsenal of virulence (DeLeo et al., 2009).
To prevail in the harsh environments encountered in the bloodstream and deeper body tissues of the human host, pathogenic organisms must cope with the onslaught carried by the host innate immune system (Spaan et al., 2013b). S. aureus addresses this by producing a large number of immune-modulatory factors, including potent pore-forming toxins, that tamper with innate immunity (Spaan et al., 2013b; Vandenesch et al., 2012). Among these toxins, a single S. aureus clone associated with human infections can produce up to five bi-component leukocidins: LukSF-PV (or PVL), HlgAB and HlgCB (also known as gamma-toxins), LukED, and LukAB (also known as LukGH) (Alonzo and Torres, 2014) that target and eliminate specific innate and adaptive immune cell populations, contributing to S. aureus pathobiology.
Invading pathogens also have to overcome the barrier posed by nutrient limitation (Waldron and Robinson, 2009). As with many pathogens (Schaible and Kaufmann, 2004), iron is essential for proliferation and virulence of S. aureus (Cassat and Skaar, 2013). In the host, however, free iron is scarce, an innate immune defense strategy of the host known as “nutritional immunity” (Andrews et al., 2003; Schaible and Kaufmann, 2004; Weinberg, 1975). The main reservoir of iron in the host lies within erythrocytes, where iron is sequestered and bound to heme within hemoglobin. Heme iron is S. aureus’s preferred iron source during infection (Skaar et al., 2004). Although hemoglobin usage by S. aureus has been extensively investigated, little is known about the actual release of hemoglobin from erythrocytes. While it is presumed that S. aureus releases hemoglobin from erythrocytes by the action of hemolytic toxins, experimental data supporting this notion are limited (Bernheimer et al., 1968; Skaar and Schneewind, 2004).
Staphylococcal toxins with hemolytic activity include α-toxin, β-hemolysin, and phenol soluble modulins (PSMs). Although a potent hemolysin on mouse, rabbit and sheep erythrocytes, α-toxin has limited hemolytic activity towards human erythrocytes (Hildebrand et al., 1991) due to the lack of its receptor, ADAM10 (Wilke and Bubeck Wardenburg, 2010). β-hemolysin (encoded by the hlb gene) is an enzyme with sphingomyelinase activity. Erythrocyte lysis by β-hemolysin is observed only after heat-cold shock incubation (Bernheimer et al., 1974) and in 90% of S. aureus isolates the hlb gene is inactivated by integration of β-hemolysin-converting bacteriophages (van Wamel et al., 2006). Phenol soluble modulins (PSMs), including δ-toxin, are small amphipathic peptides, which perforate lipid bilayer membranes in a receptor-independent manner (Wang et al., 2007). Cytotoxic activity of PSMs is neutralized by serum lipoproteins (Surewaard et al., 2012). As such, the contribution of α-toxin, β-hemolysin, and PSMs to the release of iron from human erythrocytes seems unlikely.
The bi-component pore-forming leukocidins are considered a pathogenic strategy to evade the cellular host immune response (Alonzo and Torres, 2014; Spaan et al., 2013b). The respective immune cell receptor counterparts of these leukocidins have been identified (Alonzo et al., 2013; DuMont et al., 2013; Reyes-Robles et al., 2013; Spaan et al., 2013a; Spaan et al., 2014). Interestingly, several of the leukocidins also exhibit hemolytic activity (Vandenesch et al., 2012). Despite this, the hemolytic activity of the toxins remains unexplained by the reported leukocyte receptors.
Here, we identify the Duffy antigen receptor for chemokines (DARC) as the erythroid receptor for both HlgAB and LukED, the main hemolytic leukocidins in S. aureus. Using DARC positive and negative erythrocytes, we show that hemolysis induced by S. aureus is strictly mediated by DARC and these leukocidins and that leukocidin-dependent hemolysis favors S. aureus growth.
RESULTS
Hemolytic activity of HlgAB and LukED depends on erythroid expression of DARC
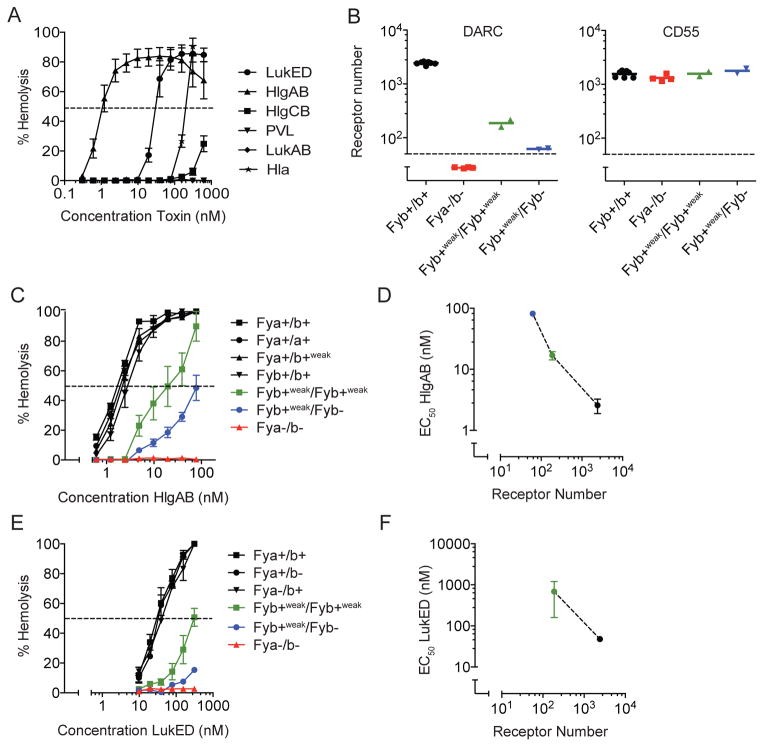
Of the β-barrel pore-forming toxins produced by S. aureus, HlgAB and LukED are the most potent hemolytic leukocidins against human erythrocytes (Figure 1A). In contrast, HlgCB and α-toxin induce only limited hemolysis, while PVL and LukAB are inactive towards human erythrocytes (Figure 1A). The divergent activity of HlgAB and HlgCB, which share HlgB, indicates that HlgA drives erythroid specificity. The restricted hemolytic activity of the different but closely related bi-component toxins suggests targeting of specific cellular receptors.
Figure 1. Hemolytic activity of HlgAB and LukED depends on DARC.
(A) Susceptibility of human erythrocytes to S. aureus β-barrel pore forming toxins. The dashed line indicates 50% hemolysis. n = 6 ± SEM.
(B) Levels of DARC and CD55 on erythrocytes of donors with different Fy phenotypes. The dashed line indicates the detection threshold. n = 2–7 ± SEM.
(C) Susceptibility of human erythrocytes with different Fy phenotypes to HlgAB. The dashed line indicates 50% hemolysis. n = 2–7 ± SEM.
(D) Correlation of half-maximal effective concentrations (EC50) of HlgAB with the total number of receptors expressed on the erythrocyte surface. n = 2–7 ± SEM.
(E) Susceptibility of human erythrocytes with different Fy phenotypes to LukED. The dashed line indicates 50% hemolysis. n = 2–7 ± SEM.
(F) Correlation of half-maximal effective concentrations (EC50) of LukED with the total number of receptors expressed on the erythrocyte surface. For Fyb+weak/Fyb− donors, EC50 could not be calculated.
n = 2–7 ± SEM.
While attempting to elucidate the molecular mechanisms of hemolytic activity induced by these toxins, we observed differential susceptibility of human donors to the toxins, which was reminiscent of the susceptibility of erythrocytes to the malarial parasites Plasmodium vivax and Plasmodium knowlesi, which target the Duffy antigen receptor for chemokines (DARC) for entry into erythrocytes (Miller et al., 1976; Miller et al., 1975). P. vivax uses its surface molecule P. vivax Duffy Binding Protein (PvDBP) to dock to the receptor (Choe et al., 2005; Tournamille et al., 2005). DARC is an atypical chemokine receptor and the antigen of the Duffy (Fy) blood group system (Tournamille et al., 1995; Tournamille et al., 1998; Wasniowska and Hadley, 1994). This system discriminates two antigens (Fya and Fyb), based on a codon 42 polymorphism encoding a glycine in approximately half of human alleles (FY*A) and an aspartic acid in the other half (FY*B) (Iwamoto et al., 1995). A substantial part of the human population carries a homozygous mutation (FY*AES or FY*BES alleles for erythrocyte silent) in the erythroid-specific promoter of the FY gene, resulting in a lack of expression of the receptor on erythrocytes (Fya−/b− phenotype) but not in non-erythroid tissues (Tournamille et al., 1995; Zimmerman et al., 1999). This mutation is highly prevalent in individuals of African descent (Tournamille et al., 1995) and lack of erythroid DARC has been suggested to be an evolutionary adaptation to resist malaria in endemic areas (Miller et al., 1976; Miller et al., 1975). Other, yet rare, mutations result in low-level expression of DARC (allele FY*X resulting in Fyb+weak phenotype) (Tournamille et al., 1998).
To directly investigate if hemolysis by HlgAB and LukED is mediated by DARC, we took advantage of erythrocyte samples from genotyped individuals from the French National Blood Transfusion Institute (INTS). In addition to genotyping, we evaluated DARC expression on erythrocyte surfaces (Figure 1B). Indeed, DARC-negative erythrocytes (phenotype Fya−/b− with genotype FY*BES/FY*BES) were fully resistant to HlgAB and LukED (Figure 1C and 1E). Compared to individuals expressing normal levels of DARC (phenotypes Fya+/b+, Fya+/b+weak, Fya+/a+, and Fyb+/b+ with respective genotypes FY*A/FY*B, FY*A/FY*X, FY*A/FY*A, and FY*B/FY*B), individuals expressing intermediate (phenotype Fyb+weak/b+weak with genotype FY*X/*X) or very low levels of DARC (phenotype Fyb−/b+weak with genotype FY*BES/FY*X) (Figure 1B) showed intermediate susceptibility to both HlgAB and LukED (Figure 1C and 1E). Half-maximal effective concentration (EC50) of the toxins directly correlated with the total number of receptors on the erythrocyte surface (Figure 1D and 1F). The difference in susceptibility to HlgAB and LukED between donors was specifically related to erythroid expression levels of DARC, as levels of CD55 (also known as DAF) were equal in all donors (Figure 1B). Taken together, these data demonstrate that the hemolytic activity of HlgAB and LukED is dependent on DARC.
DARC is the receptor for HlgAB and LukED in erythrocytes
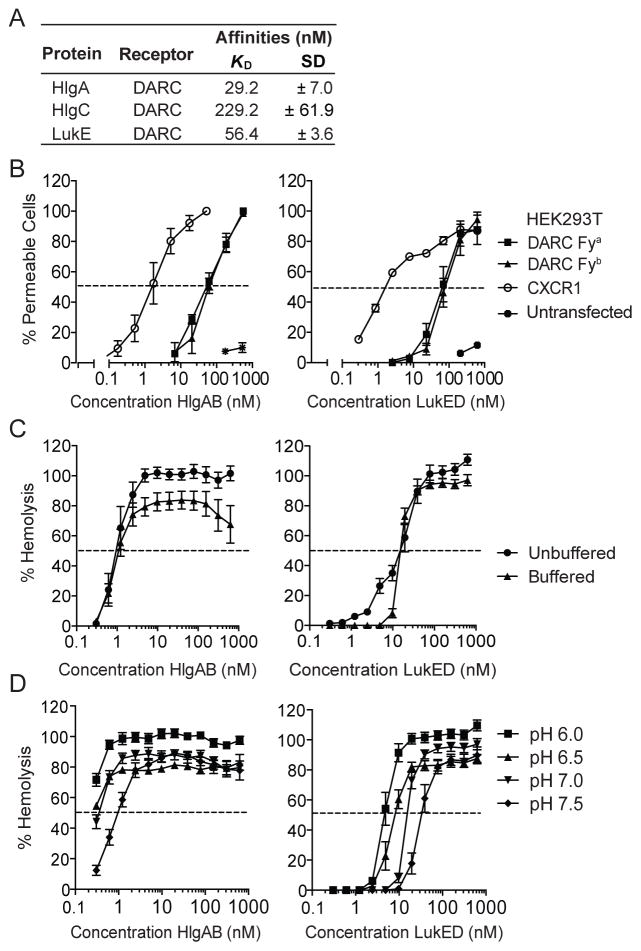
To determine if HlgAB and LukED directly bind DARC, surface plasmon resonance (SPR) was performed using purified toxins and receptor. HlgA and LukE bound DARC with a KD of 29.2 nM and 56.4 nM, respectively (Figure 2A). Consistent with the limited hemolytic activity of HlgCB, the KD of HlgC for DARC was 229.0 nM (Figure 2A). The KD-value of HlgA for DARC is approximately 6-fold higher compared to CXCR1, which serves as a neutrophil receptor for HlgA (Spaan et al., 2014). To investigate if DARC was sufficient to render host cells susceptible to HlgAB and LukED, human embryonic kidney (HEK293T) cells, which are resistant to both HlgAB and LukED (Figure 2B), were transfected with DARC-Fya or DARC-Fyb-encoding plasmids (Figure S1). Consistent with the obtained affinities, at least a 10-fold higher EC50 was observed for both HlgAB and LukED for DARC when compared to CXCR1 (Figure 2B). DARC-Fya and -Fyb conferred equal susceptibility to these toxins (Figure 2B).
Figure 2. HlgAB and LukED directly target DARC.
(A) Apparent dissociation constants (KD) of HlgA, HlgC, and LukE for purified DARC as determined by surface plasmon resonance.
(B) Pore formation following HlgAB or LukED treatment in HEK293T cells transfected with plasmids encoding DARC Fya, DARC Fyb, or CXCR1. The dashed line indicates 50% pore formation. n = 3–5 ± SEM.
(C) Hemolytic activity of HlgAB and LukED on human erythrocytes incubated in unbuffered or buffered media. The dashed line indicates 50% hemolysis. Bars indicate SEM, with n = 3.
(D) Hemolytic activity of HlgAB and LukED in human erythrocytes under different pH conditions. The dashed line indicates 50% hemolysis. Bars indicate SEM, with n = 6.
See also Figures S1 and S2.
Several of the S. aureus leukocidins exhibit species specificity, which render them highly active against human cells, but not murine cells (DuMont and Torres, 2014). Thus, we also evaluated if HlgAB and LukED can target murine DARC, which shares amino acid sequence homology of approximately 63% with human DARC (Luo et al., 1997). HEK293T cells were transfected with plasmids encoding either murine or human DARC, and the susceptibility to the toxins was evaluated. In contrast to human DARC, we observed that cells transfected with murine DARC were more susceptible to LukED than to HlgAB (Figure S2A and S2B). Consistent with the transfection data, primary murine erythrocytes were susceptible to both HlgAB and LukED (Figure S2B).
It has been hypothesized that during skin infections and abscess formation, ischemia and necrosis cause a local acidification of the infected tissue, which leads to the conversion of ferric ions into ferrous iron, the preferred iron source for S. aureus (Skaar et al., 2004; Skaar and Schneewind, 2004). We observed that the hemolytic activity of both HlgAB and LukED was enhanced in saline (unbuffered) compared to buffer (Figure 2C). Similar results were observed with murine erythrocytes (Figure S2C). Additional studies showed increased hemolytic activity in low pH conditions (Figure 2D). Collectively, these data indicate that DARC is sufficient to render mammalian cells susceptible to HlgAB and LukED, and that the hemolytic activity of these toxins is likely to be influenced by the local environment of infected tissues.
HlgAB and LukED target different DARC regions for cell killing
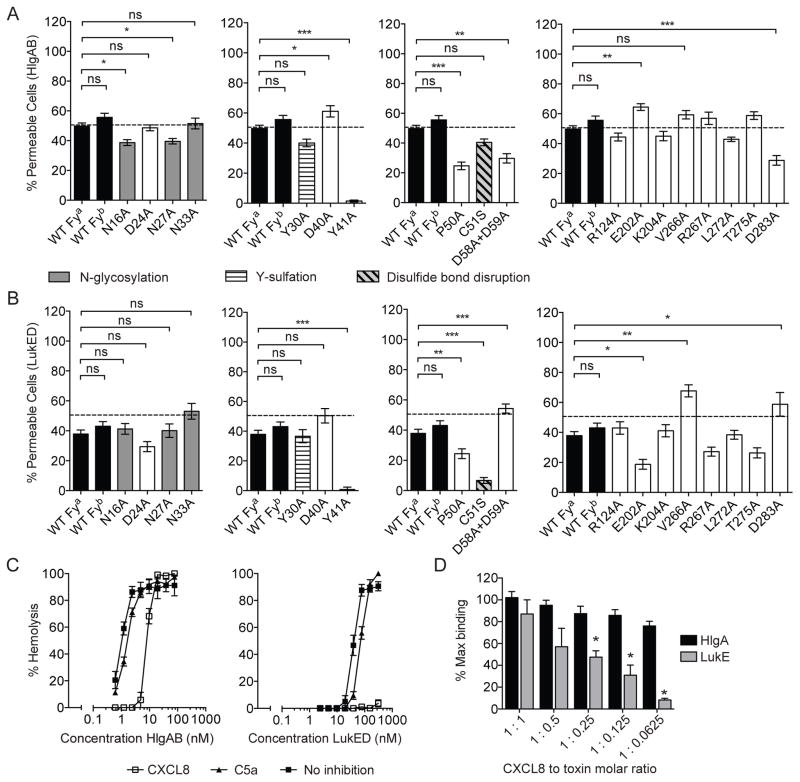
To tease out the interaction of HlgAB and LukED with DARC, we screened HEK293T cells transfected with a collection of plasmids encoding DARC mutants (Tournamille et al., 2003). All receptors were expressed at similar levels on the cell surface of transfected cells (Figure S1). Mutation of N-terminal glycosylation sites (N16A, N27A, or N33A) resulted in moderately reduced susceptibility of cells towards HlgAB or LukED (Figures 3A and 3B). Mutation of the second N-terminal tyrosine (Y41A) but not the first (Y30A) resulted in resistance to both HlgAB and LukED (Figures 3A and 3B), which is similar to what has been observed for PvDBP (Choe et al., 2005; Tournamille et al., 2005). Mutation of a proline in close proximity to the first transmembrane region of the receptor (P50A) reduced susceptibility to both toxins (Figures 3A and 3B). While mutation of two adjacent aspartic acid residues (D58A+D59A) resulted in a partial resistance of cells to HlgAB, mutation of the same residues enhanced susceptibility to LukED (Figures 3A and 3B). Surprisingly, a disulfide bond-disrupting mutation (C51S) strongly affected susceptibility to LukED but not to HlgAB (Figures 3A and 3B). Lastly, mutations in the three extracellular loops (ECLs) differentially affected susceptibility to HlgAB and LukED. Mutation of a glutamic acid in ECL2 (E202A) slightly enhanced susceptibility of cells to HlgAB, but reduced cytotoxicity of LukED. The inverse effect was observed with aspartic acid in ECL3 (D283A) (Figures 3A and 3B).
Figure 3. HlgAB and LukED target different DARC regions for cell killing.
(A) Pore formation following HlgAB treatment (70 nM) in HEK293T cells transfected with plasmids encoding DARC Fya, DARC Fyb, or DARC alanine substitution mutants. The dashed line indicates 50% pore formation. n ≥ 18 ± SEM. Statistical significance is displayed as ns (not significant), *p<0.05, **p<0.01, ***p<0.005 using One-Way ANOVA with Bonferroni post test correction for multiple comparisons.
(B) Pore formation following LukED treatment (70 nM) in HEK293T cells transfected with plasmids encoding DARC Fya, DARC Fyb, or DARC alanine substitution mutants. The dashed line indicates 50% pore formation. n ≥ 18 ± SEM. Statistical significance is displayed as ns (not significant), *p<0.05, **p<0.01, ***p<0.005 using One-Way ANOVA with Bonferroni post test correction for multiple comparisons.
(C) Effect of pretreatment with CXCL8 (10 μg.mL−1) during hemolysis induced by HlgAB and LukED. n = 3 ± SEM.
(D) Competition between CXCL8 and toxin subunits at varying molar ratios using surface plasmon resonance. n = 3 ± SEM *Indicates significant difference to non-CXCL8 treated response units (p<0.01) using t-Test.
See also Figure S3.
Next, we tested if DARC competition with monoclonal antibodies, chemokines, or PvDBP could interfere with hemolytic activity. Pre-treatment of erythrocytes with the DARC ligand CXCL8 (or IL-8) modestly shifted the EC50 for HlgAB (1.14 to 8.58 nM), while it completely antagonized hemolysis induced by LukED irrespective of buffer conditions (Figure 3C and S3A). In contrast to CXCL8, a commercially available monoclonal against DARC (Fya) did not confer protection to HlgAB or LukED (Figure S3B), nor did high concentrations of a nanobody targeting the DARC Fy6-epitope. Similarly, PvDBP did not antagonize cytotoxicity of HlgAB, while it minimally protected against LukED (Figure S3B).
To directly evaluate if CXCL8 inhibits LukED hemolytic activity by blocking toxin-receptor interaction, we determined the effect of CXCL8 on the formation of LukE-DARC and HlgA-DARC complexes using SPR. CXCL8 did not affect HlgA binding to DARC (Figure 3D). In contrast, CXCL8 significantly inhibited LukE binding to DARC when the chemokine was present at four fold (1:0.25) or greater (1:0.125 and 1:0.0625) concentration than LukE (Figure 3D). Together, these data demonstrate that DARC targeting by these toxins is dictated by different domains of the receptor.
S. aureus lyses erythrocytes in a leukocidin and DARC dependent manner
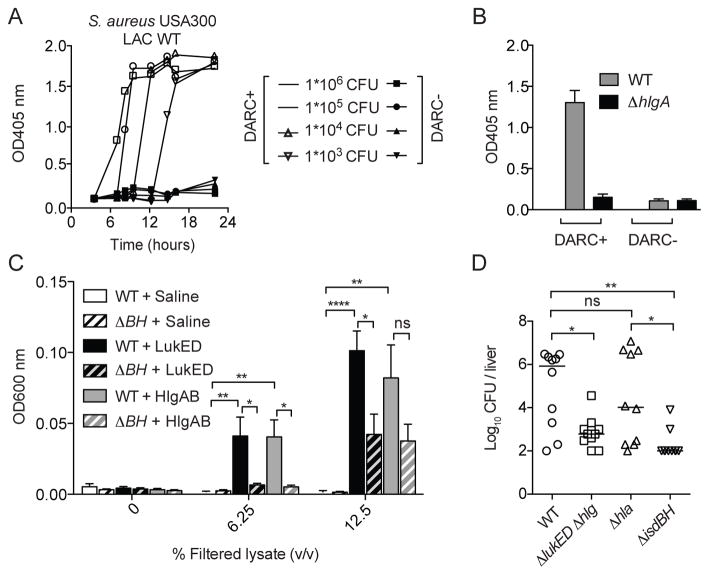
S. aureus presumably releases iron from erythrocytes by secreting various hemolytic toxins, promoting the bacterium’s survival in the host (Bernheimer et al., 1968; Skaar and Schneewind, 2004; Torres et al., 2010). To address whether S. aureus lyses erythrocytes in a DARC-dependent manner, the CA-MRSA USA300 clone LAC was grown in the presence of DARC positive or negative erythrocytes and hemolysis measured over time. We observed that S. aureus induced hemolysis of erythrocytes in a time, bacterial density, and DARC-dependent manner (Figure 4A and Figures S4).
Figure 4. S. aureus lyses erythrocytes in a HlgAB, LukED and DARC dependent manner to release iron and promote growth.
(A) S. aureus USA300 LAC grown in the presence of erythrocytes from donors with or without erythroid expression of DARC and hemolysis measured. Curves depict a representative sample.
(B) Hemolysis induced during overnight growth of S. aureus strain USA300 LAC and its hlgA mutant (hlgA::bursa) strain (infectious dose set at 1 × 106 CFU per sample). n = 3 ± SEM.
(C) Growth after 20 hours of S. aureus strains Newman WT or isogenic ΔisdBH as a result of erythrocyte lysis by LukED and HlgAB in iron-restricted medium. n = 9 ± SEM. Statistical significance is displayed as ns (not significant), *p<0.05, **p<0.01, ****p<0.001 using One-Way ANOVA with Tukey’s post test correction for multiple comparisons. Bacterial growth was measured at OD600 nm.
(D) Swiss-Webster female mice (n = 10 mice per group) infected systemically with S. aureus Newman isogenic strains: WT, Δhlg ΔlukED, Δhla, and ΔisdBH (~1 × 107 colony forming units, CFU). 96-hours post infection, mice were sacrificed and bacterial burden in the liver determined. Lines represent median log CFU. Statistical significance is displayed as ns (not significant), *p<0.05, **p<0.01, ****p<0.001 using One-way ANOVA with Tukey’s post test correction for multiple comparisons.
See also Figure S4.
We next defined the contribution of HlgAB and LukED to S. aureus mediated lysis of DARC positive erythrocytes. Growth of isogenic strains revealed that HlgAB is sufficient for the observed erythrocyte lysis in this ex vivo experiment (Figure 4B) and this was recapitulated with different S. aureus strains (Figure S4B). Notably, the lack of a LukED contribution is not surprising due to low production of this toxin in vitro (Alonzo et al., 2012; Gravet et al., 1998).
To directly evaluate if LukED and HlgAB promote bacterial replication as a result of erythrocyte lysis, S. aureus was grown in iron-starved medium supplemented with cell-free extracts of erythrocytes treated with saline, LukED or HlgAB. We observed that HlgAB and LukED were each capable of promoting S. aureus growth (Figure 4C). To determine if this growth was dependent of hemoglobin acquisition, we used an isogenic strain lacking the S. aureus hemoglobin receptors IsdB and IsdH (ΔisdBH) (Pishchany et al., 2014; Torres et al., 2006). In contrast to wild type (WT) S. aureus, the ΔisdBH strain was significantly growth impaired in medium supplemented with cell-free extracts from toxin treated erythrocytes (Figure 4C).
To elucidate the potential role of toxin-mediated erythrocyte targeting in vivo, bacterial burden of mice infected systemically with S. aureus WT or isogenic mutants lacking hlgACB and lukED (Δhlg ΔlukED), the gene encoding for α-toxin (hla; Δhla), or isdBH (ΔisdBH) (Figure S4C) were evaluated. Inactivation of lukED and hlgACB phenocopied the deletion of the hemoglobin receptors, resulting in a 3-log and 4-log reductionin bacterial burden, respectively (Figure 4D). The bacterial burden reduction observed with the Δhlg ΔlukED strain was specific, with no significant difference between the bacterial burden of WT and Δhla-infected mice, even though α-toxin exhibits hemolytic activity in vitro towards murine erythrocytes (Figure S2C). Altogether, these data suggest that S. aureus targets DARC to lyse erythrocytes in a HlgAB and LukED-dependent manner to release hemoglobin, promoting bacterial replication.
DISCUSSION
It is presumed that S. aureus releases hemoglobin from erythrocytes by secreting hemolytic toxins. Strikingly however, the contribution of toxins to hemolysis-mediated bacterial growth has never been proven empirically. By identifying DARC as the erythroid receptor for HlgAB and LukED, we show that these staphylococcal bi-component toxins play a central role in human erythrocyte lysis.
Our data shows that the hemolytic activity of HlgAB and LukED is in the nanomolar range and is exclusively driven by DARC expression. The affinity of the binding components HlgA and LukE for DARC is lower than their myeloid receptors, CXCR1 and CXCR2 (Reyes-Robles et al., 2013; Spaan et al., 2014). High expression levels of DARC and possibly lack of cellular membrane repair mechanisms make erythrocytes highly susceptible to the toxins. Consistent with this, our data suggests that the actual number of receptors on the cell surface dictates susceptibility to these hemolytic toxins. Using alanine substitution mutants, we identified a tyrosine in the N-terminus of DARC that is essential for the interaction with both HlgAB and LukED and has been described as sulfated (Choe et al., 2005). For PVL, sulfation of tyrosines in the N-terminal C5aR is critical for initial binding of LukS-PV (Spaan et al., 2013a). Possibly, sulfated N-terminal tyrosines define a conserved host interaction site for the staphylococcal leukocidins. Otherwise, our data show that HlgAB and LukED interact differentially with DARC. An N-terminal cysteine (C51) identified as involved in the interaction of DARC with LukED is also involved in binding CXCL8 (Tournamille et al., 2003), supporting the notion that this chemokine directly blocks receptor binding by LukE.
The genes encoding HlgAB are present in over 99.5% of human S. aureus isolates (Prevost et al., 1995). Strictly following clonal lineage, approximately 80% of S. aureus strains carry the genes encoding LukED (McCarthy and Lindsay, 2013). The S. aureus strains investigated in this study all contain the genes encoding HlgAB and LukED, thus demonstrating that S. aureus-mediated hemolysis requires DARC and these leukocidins.
S. aureus is remarkably well adapted to the human host, thus multiple virulence factors of this bacterium are not compatible with non-human species frequently used during in vivo studies. One such factor is the staphylococcal hemoglobin receptor IsdB, which exhibits low affinity for murine hemoglobin as compared to human hemoglobin (Pishchany et al., 2010). Nevertheless, our in vivo studies revealed a remarkable similarity in the phenotypes of isogenic mutants lacking either the hemoglobin receptors or the hemolytic leukocidins, suggesting that these toxins contribute to nutrient acquisition during infection. However, to unequivocally demonstrate that the attenuated phenotype exhibited by the Δhlg ΔlukED strain is due to impaired erythrocyte lysis, additional studies uncoupling the leukocidal and hemolytic activities of HlgAB and LukED are required.
The current epidemic of CA-MRSA in the United States and elsewhere disproportionally affects individuals of African descent with severe and invasive infections (Fridkin et al., 2005). Socio-economic factors and other underlying diseases likely contribute to this predisposition, precluding epidemiological assessment of the contribution of erythroid DARC expression to S. aureus infection. However, the resistance of DARC negative erythrocytes to the parasites P. vivax and P. knowlesi together with our findings, further support the notion that this gene could undergo positive selection in response to different diseases caused by important human pathogens.
EXPERIMENTAL PROCEDURES
Ethics statement
DARC blood samples were provided by the Centre National de Référence sur les Groupes Sanguins (CNRGS, Paris). Additional blood samples of consenting, healthy volunteers were obtained in accordance with the Declaration of Helsinki. Approval was obtained from the medical ethics committee of the UMC Utrecht, The Netherlands. Blood was also obtained from de-identified, consenting donors from the New York Blood Center.
All experiments involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of New York University and were performed according to NIH guidelines, the Animal Welfare Act, and US Federal law.
Hemolysis assays with recombinant toxins
Erythrocytes were washed thrice in 0.9% saline, adjusted to 5 × 107 cells.ml−1 then intoxicated at a final of 2.5 × 107 cells.ml−1 per reaction with purified recombinant toxins for 30 minutes at 37°C + 5% CO2 in a final volume of 160 μl. Equimolar concentrations of 6xHis-tagged proteins were used. Samples were centrifuged for 10 min at 1,780 g, 4°C, and 100 μl of cell-free lysates were used to measure absorbance (OD405 nm). Hemolysis is expressed as the OD405 nm of cell-free lysates using an EnVision Plate Reader. The hemolysis experiments with recombinant proteins were performed using buffer containing 30 mM Tris with 100 mM NaCl (pH 7.0) (buffered) or 0.9% saline (unbuffered) as indicated.
S. aureus burden in vivo
To evaluate bacterial burden in vivo, 4-week old Swiss-Webster mice (Harlan) were anesthetized intraperitoneally with Avertin, and infected retro-orbitally with 100 μl of Newman isogenic strains WT, Δhlg ΔlukED, Δhla, and ΔisdBH (1 × 107 CFU). 96-hours post infection, mice were sacrificed, the livers harvested and homogenized, and serially diluted.
Graphical and statistical analyses
Flow cytometric analyses were performed with FlowJo (Tree Star Software). Statistical analyses were performed with Prism (GraphPad Software). Statistical significance was calculated using ANOVA and Student’s t-tests where appropriate.
Supplementary Material
Acknowledgments
We thank Manoj Duraising (Harvard School of Public Health) for the DARC expressing plasmid, Olivier Bertrand and Stéphane Grangnard (Inserm U1134) for the nanobody targeting the DARC F6 epitope and PvDBP, Gérard Lina (Inserm U1111) for stimulating discussions, Raïhane Massulaha-Ahmed and Pauline Abrial (CIRI, Lyon) for technical assistance, Thierry Peyrard (CNRGS, Paris) for Fy-genotyped frozen blood samples, Eric Skaar (Vanderbilt University) for the Newman ΔisdBH strain, and Kayan Tam (NYU) for purified recombinant α-toxin.
This work was supported in part by grants from: the French Agence Nationale de la Recherche (ANR-12-BSV3-0003 to F.V. and T.H.), the Finovi foundation to T.H., the NIH (T32 AI007180 and F31 AI112290 to T.R-R., T32 GM007308 to K.B., and R01 AI099394 and R01 AI105129 to V.J.T.); the NHMRC (565526 to M.P.J.), and the Smart Futures Fund Research Partnerships Program (M.P.J.). This work was performed within the framework of the LABEX ECOFECT (ANR-11-LABX-0048) of Université de Lyon, of the program “Investissements d’Avenir” (ANR-11-IDEX-0007) operated by the ANR. V.J.T. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
Footnotes
Supplemental Information includes detailed supplemental Experimental Procedures, one table and four figures.
AUTHOR CONTRIBUTIONS
Conceptualization, A.S., T.R.-R., T.H., and V.J.T.; Methodology, A.S., T.R.-R., T.H. and V.J.T.; Investigation, A.S., T.R.-R., C.B., S.C., K.M.B., P.Y., C.J.C.d.H., K.v.K; Resources, F.V., M.P.J., C.l.V.K., Y.C.; Funding Acquisition, M.P.J., J.A.S., F.V., T.H. and V.J.T; Writing, A.S., T.R.-R., T.H. and V.J.T; Resources, M.P.J., J.A.S., T.H. and V.J.T; Supervision, J.A.S., T.H. and V.J.T.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonzo F, 3rd, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol. 2012;83:423–435. doi: 10.1111/j.1365-2958.2011.07942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo F, 3rd, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493:51–55. doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo F, 3rd, Torres VJ. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiology and molecular biology reviews : MMBR. 2014;78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS microbiology reviews. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- Bernheimer AW, Avigad LS, Grushoff P. Lytic effects of staphylococcal alpha-toxin and delta-hemolysin. J Bacteriol. 1968;96:487–491. doi: 10.1128/jb.96.2.487-491.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer AW, Avigad LS, Kim KS. Staphylococcal sphingomyelinase (beta-hemolysin) Annals of the New York Academy of Sciences. 1974;236:292–306. doi: 10.1111/j.1749-6632.1974.tb41499.x. [DOI] [PubMed] [Google Scholar]
- Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Moore MJ, Owens CM, Wright PL, Vasilieva N, Li W, Singh AP, Shakri R, Chitnis CE, Farzan M. Sulphated tyrosines mediate association of chemokines and Plasmodium vivax Duffy binding protein with the Duffy antigen/receptor for chemokines (DARC) Mol Microbiol. 2005;55:1413–1422. doi: 10.1111/j.1365-2958.2004.04478.x. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am. 2009;23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuMont AL, Torres VJ. Cell targeting by the Staphylococcus aureus pore-forming toxins: it’s not just about lipids. Trends in microbiology. 2014;22:21–27. doi: 10.1016/j.tim.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuMont AL, Yoong P, Day CJ, Alonzo F, 3rd, McDonald WH, Jennings MP, Torres VJ. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A. 2013;110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. Methicillin-resistant Staphylococcus aureus disease in three communities. The New England journal of medicine. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- Gravet A, Colin DA, Keller D, Girardot R, Monteil H, Prevost G. Characterization of a novel structural member, LukE-LukD, of the bi-component staphylococcal leucotoxins family. FEBS Lett. 1998;436:202–208. doi: 10.1016/s0014-5793(98)01130-2. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Pohl M, Bhakdi S. Staphylococcus aureus alpha-toxin. Dual mechanism of binding to target cells. J Biol Chem. 1991;266:17195–17200. [PubMed] [Google Scholar]
- Iwamoto S, Omi T, Kajii E, Ikemoto S. Genomic organization of the glycoprotein D gene: Duffy blood group Fya/Fyb alloantigen system is associated with a polymorphism at the 44-amino acid residue. Blood. 1995;85:622–626. [PubMed] [Google Scholar]
- Luo H, Chaudhuri A, Johnson KR, Neote K, Zbrzezna V, He Y, Pogo AO. Cloning, characterization, and mapping of a murine promiscuous chemokine receptor gene: homolog of the human Duffy gene. Genome research. 1997;7:932–941. doi: 10.1101/gr.7.9.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AJ, Lindsay JA. Staphylococcus aureus innate immune evasion is lineage-specific: A bioinfomatics study. Infect Genet Evol. 2013;19C:7–14. doi: 10.1016/j.meegid.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. The New England journal of medicine. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189:561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- Pishchany G, McCoy AL, Torres VJ, Krause JC, Crowe JE, Jr, Fabry ME, Skaar EP. Specificity for human hemoglobin enhances Staphylococcus aureus infection. Cell Host Microbe. 2010;8:544–550. doi: 10.1016/j.chom.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishchany G, Sheldon JR, Dickson CF, Alam MT, Read TD, Gell DA, Heinrichs DE, Skaar EP. IsdB-dependent hemoglobin binding is required for acquisition of heme by Staphylococcus aureus. J Infect Dis. 2014;209:1764–1772. doi: 10.1093/infdis/jit817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost G, Couppie P, Prevost P, Gayet S, Petiau P, Cribier B, Monteil H, Piemont Y. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J Med Microbiol. 1995;42:237–245. doi: 10.1099/00222615-42-4-237. [DOI] [PubMed] [Google Scholar]
- Reyes-Robles T, Alonzo F, 3rd, Kozhaya L, Lacy DB, Unutmaz D, Torres VJ. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell host & microbe. 2013;14:453–459. doi: 10.1016/j.chom.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- Skaar EP, Schneewind O. Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect. 2004;6:390–397. doi: 10.1016/j.micinf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJ, van Kessel KP, Vandenesch F, et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell host & microbe. 2013a;13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils Versus Staphylococcus aureus: A Biological Tug of War. Annu Rev Microbiol. 2013b doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Vrieling M, Wallet P, Badiou C, Reyes-Robles T, Ohneck EA, Benito Y, de Haas CJ, Day CJ, Jennings MP, et al. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nature communications. 2014;5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewaard BG, Nijland R, Spaan AN, Kruijtzer JA, de Haas CJ, van Strijp JA. Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog. 2012;8:e1002606. doi: 10.1371/journal.ppat.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, Anderson KL, Stauff DL, McDonald WH, Zimmerman LJ, et al. Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun. 2010;78:1618–1628. doi: 10.1128/IAI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188:8421–8429. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournamille C, Colin Y, Cartron JP, Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- Tournamille C, Filipe A, Badaut C, Riottot MM, Longacre S, Cartron JP, Le Van Kim C, Colin Y. Fine mapping of the Duffy antigen binding site for the Plasmodium vivax Duffy-binding protein. Molecular and biochemical parasitology. 2005;144:100–103. doi: 10.1016/j.molbiopara.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Tournamille C, Filipe A, Wasniowska K, Gane P, Lisowska E, Cartron JP, Colin Y, Le Van Kim C. Structure-function analysis of the extracellular domains of the Duffy antigen/receptor for chemokines: characterization of antibody and chemokine binding sites. British journal of haematology. 2003;122:1014–1023. doi: 10.1046/j.1365-2141.2003.04533.x. [DOI] [PubMed] [Google Scholar]
- Tournamille C, Le Van Kim C, Gane P, Le Pennec PY, Roubinet F, Babinet J, Cartron JP, Colin Y. Arg89Cys substitution results in very low membrane expression of the Duffy antigen/receptor for chemokines in Fy(x) individuals. Blood. 1998;92:2147–2156. [PubMed] [Google Scholar]
- van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol. 2006;188:1310–1315. doi: 10.1128/JB.188.4.1310-1315.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F, Lina G, Henry T. Staphylococcus aureus Hemolysins, bi-component Leukocidins, and Cytolytic Peptides: A Redundant Arsenal of Membrane-Damaging Virulence Factors? Front Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Wasniowska K, Hadley TJ. The Duffy blood group antigen: an update. Transfusion medicine reviews. 1994;8:281–288. doi: 10.1016/s0887-7963(94)70119-x. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A. 2010;107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman PA, Woolley I, Masinde GL, Miller SM, McNamara DT, Hazlett F, Mgone CS, Alpers MP, Genton B, Boatin BA, et al. Emergence of FY*A(null) in a Plasmodium vivax-endemic region of Papua New Guinea. Proc Natl Acad Sci U S A. 1999;96:13973–13977. doi: 10.1073/pnas.96.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.