Abstract
Reference intervals (RIs) for common clinical laboratory tests are usually not developed separately for different subpopulations. The aim of this study was to investigate racial/ethnic differences in RIs of common biochemical and hematological laboratory tests using the National Health and Nutrition Examination Survey (NHANES) 2011–2012 data. This current study included 3,077 participants aged 18–65 years who reported their health status as “Excellent,” “Very good,” or “Good,” with known race/ethnicity as white, black, Hispanic, or Asian. Quantile regression analyses adjusted for sex were conducted to evaluate racial/ethnic differences in the normal ranges of 38 laboratory tests. Significant racial/ethnic differences were found in almost all laboratory tests. Compared to whites, the normal range for Asians significantly shifted to higher values in globulin and total protein and to lower values in creatinine, hematocrit, hemoglobin, mean cell hemoglobin, mean cell hemoglobin concentration, and mean platelet volume. These results indicate that racial/ethnic subpopulations have unique distributions in the labortoary tests and race/ethnicity may need to be incorporated in the development of their RIs. Establishment of racial/ethnic-specific RIs may have significant clinical and public health implication for more accurate disease diagnosis and appropriate treatment to improve quality of patient care, especially for a state with diverse racial/ethnic subpopuations such as Hawai‘i.
Keywords: Race/ethnicity, reference interval, laboratory test, sex, NHANES
Introduction
Reference intervals (RIs) of clinical laboratory tests are frequently established using distribution-based (eg, normal or log normal) 95% confidence intervals or nonparametric 2.5th and 97.5th percentiles of healthy subjects' laboratory test results. The RIs have an important role in clinical practice in screening for diseases, assessing disease progression and treatment response. The use of accurate RIs can reduce disease misdiagnosis and improve patient care.
The guidelines by International Federation of Clinical Chemistry (IFCC) recommend that every country must establish RIs for health.1 For example, there were movements to develop locally relevant RIs in Ghana and India.1,2 In most other non-industrialized nations, however, RIs have not been adequately addressed. Instead, clinicians in those countries adopt the textbook RIs that were mainly developed in Western countries predominantly with Caucasian populations, without consideration of potential racial/ethnic differences.
Several studies have recognized racial/ethnic differences in RIs of various laboratory tests, mainly between blacks and whites.3–15 Compared with whites, blacks show significantly lower thyrotropin,12 total white blood cell (WBC), neutrophil counts,13 platelet counts,5 hematocrit, mean cell hemoglobin centration (MCHC), mean cell hemoglobin,13 and hemoglobin13,14 and significantly higher mononuclear and lymphocyte percent.13 For example, the hematological (hemoglobin, mean cell volume, platelets, WBC) reference values for the Gambian population encompasses lower limits compared with Western standards and shifted to the lower values.16
A few studies have evaluated other racial/ethnic differences in RIs for some laboratory tests. Hispanics were found to have similar RIs as whites in WBC, absolute neutrophil counts17 and albumin.18 Similarly, Cheng, et al, (2004) concluded no significant trend differences between whites and Mexican Americans for blood chemistries such as hemoglobin.13 In a multicenter study from four regions (Milan Italy, Bursa Turkey, Beijing China and Nordic Countries), Ceriotti, et al, (2010) concluded that common RIs for aminotransferase (ALT) and aspartate aminotransferase (AST) are reasonable but that for gamma-glutamyl transferase (GGT) may not be applicable due to differences among regions.15 Such findings have led many researchers to advocate for usage of racial/ethnic-specific RIs for laboratory tests. This has direct and significant clinical and public health implications, especially for a state like Hawai‘i with its diverse racial/ethnic population (Hawai‘i, white 24.7%, Asian 38.6%, and Native Hawaiian and other Pacific Islander 10.0% versus the United States, 72.4%, 4.8%, and 0.2%, respectively).19
To our knowledge, there are no studies comparing RIs of Asians to other racial/ethnic groups across common laboratory tests in the United States. In studies comparing different racial/ethnic groups, Asians are often ignored due to small sample size. For example, the National Health and Nutrition Examination Survey (NHANES), one of the largest nationwide surveys, combined Asians (until recently) into the “other race” category. Given this important and fast growing racial/ethnic subpopulation, the NHANES 2011–2012, for the first time, included Asians as a separate racial/ethnic group. This study aimed to address the question on whether the RIs of common laboratory tests are different between major racial/ethnic groups including Asians from a representative sample of US healthy adults using NHANES 2011–2012 data.
Methods
Data Source and Study Population
The latest NHANES 2011–2012 data were utilized for this study. NHANES uses a multistage, stratified, cluster sampling design to generate a representative sample of the civilian US population. The data were collected from surveys, examinations, and laboratory tests. The detailed description of survey methods and laboratory and examination data collection procedures is available at the NHANES website (www.cdc.gov/nchs/nhanes.htm). Unlike the previous years in which Asians were combined into the “other” racial/ethnic group, the 2011–2012 data oversampled Asians and categorized them as a separate racial/ethnic group. As a result, race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, non-Hispanic Asian and other race/ethnicity categories.
To compare RIs of laboratory tests in healthy adults by race/ethnicity, only adults aged between 18 and 65 years (inclusive) who rated their overall health status as either “Excellent,” “Very Good,” or “Good” were included. Mexican American and other Hispanic groups were combined into one group for our analysis. Participants who did not specify their race/ethnicity or identified themselves as other mixed race were not included because their sample sizes were too small to produce reliable estimates.
Laboratory Tests
The following 38 biochemical and hematological laboratory tests were examined: albumin, ALT, ALP, basophils percent, bicarbonate, blood urea nitrogen (BUN), calcium, chloride, creatine phosphokinase (CPK), creatinine, eosinophils percent, GGT, globulin, glucose, hematocrit, hemoglobin, iron, lactate dehydrogenase (LDH), lymphocytes percent, mean cell hemoglobin, MCHC, mean cell volume, mean platelet volume, monocytes percent, osmolality, phosphorus, platelet count, potassium, red blood cell count (RBC), red blood cell distribution width (RCDW), segmented neutrophils percent (SNP), sodium, total bilirubin, total cholesterol, total protein, triglycerides, uric acid, and white blood cell count (WBC). Missing laboratory test rates were relatively small, ranging from 3.44% to 6.11%.
Statistical Methods
Descriptive statsitics were reported on subject charcteristics for the healthy adult population sampled, both unweighted and weighted for complex sampling design. Unadjusted/unweighted upper and lower limits of normal ranges were calculated for the laboratory tests stratified by sex and race/ethnicity. Lower and upper limits of normal range were defined as 2.5th and 97.5th values in percent, respectively. Adjusting for sex, quantile regression models were conducted for the lower and upper limit of normal range for each laboratory test comparing across racial/ethnic groups. Quantile regression is a robust statistical method that models the shape and location of a distribution since it avoids parametric assumptions about the error distribution. Standard error for each parameter was estimated based on a bootstrapping method with 1,000 bootstrap samples and was reported at one more decimal point than its parameter estimate. Sensitivity analyses were performed using the participants who reported “Excellent” or “Very Good” health status to investigate whether different health status provided similar patterns. Finally, weighted quantile regressions were also implemented with consideration of the NHANES complex sampling design. P-value < .05 was considered statistically significant. All analyses were conducted in SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Sample Characteristics
Among the 4,711 participants in NHANES 2011–2012 data, 3,077 subjects met the inclusion criteria. The average age was 39.9 years (standard error=0.3), with about half being male (52.1%) (Table 1). Of the participants, 37.7% were white, 27.4% black, 19.2% Hispanic (about half were Mexican Americans), and 15.6% Asian. About forty-five percent were married, 19.3% had annual household income less than $20,000, and 13.2% self-reported “Excellent” health status.
Table 1.
Subject Characteristics
| Variable | n | Unweighted % | Weighted % |
| Sex | |||
| Male | 1,603 | 52.1 | 51.1 |
| Female | 1,474 | 47.9 | 48.9 |
| Race/Ethnicity | |||
| White | 1,160 | 37.7 | 70.8 |
| Black | 844 | 27.4 | 11.0 |
| Hispanic | 592 | 19.2 | 13.1 |
| Asian | 481 | 15.6 | 5.1 |
| Education | |||
| Less than High School | 432 | 14.0 | 10.4 |
| High School Gradaute/GED or Equivalent | 643 | 20.9 | 19.7 |
| Some College | 939 | 30.5 | 32.0 |
| College Graduate or Above | 895 | 29.1 | 34.8 |
| Refused/Don't Know/Missing | 168 | 5.5 | 3.1 |
| Marital Status | |||
| Married | 1,389 | 45.1 | 51.2 |
| Widowed/Divorced/Separated | 428 | 13.9 | 13.5 |
| Never Married | 769 | 25.0 | 22.5 |
| Living with Partner | 253 | 8.2 | 8.3 |
| Refused/Missing | 238 | 7.7 | 4.5 |
| Annual Household Income* | |||
| <$20,000 | 595 | 19.3 | 13.5 |
| $20,000–$55,000* | 1,103 | 35.9 | 32.4 |
| $55,000–$100,000 | 622 | 20.2 | 24.1 |
| ≥$100,000 | 628 | 20.4 | 27.5 |
| Refused/Don't Know/Missing | 129 | 4.2 | 2.5 |
| Self-Reported Health Status | |||
| Excellent | 407 | 13.2 | 14.8 |
| Very Good | 1,113 | 36.2 | 40.4 |
| Good | 1,557 | 50.6 | 44.8 |
| Age, mean ± SE | 3,077 | 39.9 ± 0.3 | 41.1 ± 0.4 |
| BMI, mean ± SE | 3,056 | 28.1 ± 0.1 | 28.2 ± 0.2 |
N=3,077. SE = Standard error. BMI = Body mass index.
‘$20,000 and Over’ (n=115, unweighted percent=3.8%, weighted percent=2.7%) in income variable of NHANES data was combined to the category of $20,000–$55,000.
Normal Ranges of Laboratory Tests by Sex and Race/Ethnicity
Table 2 summarizes unweighted lower and upper limits of normal ranges for the 38 laboratory tests stratified by sex and race/ethnicity (Asian, black, Hispanic, and white). For comparison, the RIs from the NHANES laboratory manual are also included. Although most normal ranges appeared to be close to the relevant RIs, some normal ranges deviated significantly from the corresponding RIs. For example, the NHANES RIs for ALT are 11–47 U/L for male and 7–30 U/L for female but the normal ranges are 12–80 U/L for male and 10–56 U/L for female. The RIs of GGT are 10–65 IU/L for male and 8–36 IU/L for female but the normal ranges are 9–103 IU/L for male and 6–76 IU/L for female. More importantly, shifts in normal ranges among different races/ethnicities were observed in multiple laboratory tests. For example, the normal range of ALP for white males was 35–107 IU/L but for Hispanic males was 43–126 IU/L. The normal range of creatinine for white females was 0.50–1.10 mg/dL but for Asian females was 0.43–0.88 mg/dL.
Table 2.
Unweighted Normal Ranges of Clinical Laboratory Tests by Sex and Race/Ethnicity
| Laboratory Test | % Missing | Male | Female | ||||||||||
| NHANES Reference | All (n=1,603) | White (n=608) | Black (n =425) | Hispanic (n=316) | Asian (n=254) | NHANES Reference | All (n=1,474) | White (n=552) | Black (n=419) | Hispanic (n=276) | Asian (n=227) | ||
| Albumin, g/dL | 5.98 | 3.7–4.7 | 3.6–5.0 | 3.9–5.1 | 3.8–4.9 | 3.9–5.1 | 4.0–5.1 | 3.7–4.7 | 3.6–5.0 | 3.5–4.8 | 3.5–4.7 | 3.5–4.8 | 3.7–4.9 |
| ALT*, U/L | 6.01 | 11–47 | 12–80 | 12–87 | 11–64 | 12–102 | 12–76 | 7–30 | 10–56 | 11–58 | 9–41 | 10–62 | 10–47 |
| ALP, IU/L | 6.01 | 36–113 | 34–115 | 35–107 | 38–114 | 43–126 | 38–105 | 36–113 | 34–115 | 31–115 | 33–121 | 40–123 | 29–94 |
| Basophils Percent*, % | 3.61 | 0.1–1.6 | 0.0–2.7 | 0.0–2.7 | 0.0–3.2 | 0.1–2.2 | 0.0–2.0 | 0.1–1.7 | 0.0–2.5 | 0.0–1.9 | 0.0–3.0 | 0.0–1.7 | 0.1–1.8 |
| Bicarbonate, mmol/L | 6.01 | 22–29 | 21–29 | 21–29 | 22–30 | 22–29 | 22–29 | 22–29 | 21–29 | 21–28 | 20–29 | 20–28 | 21–28 |
| BUN, mg/dL | 5.98 | 6–23 | 6–21 | 6–21 | 6–20 | 7–22 | 6–21 | 6–23 | 6–21 | 5–22 | 4–20 | 6–22 | 6–18 |
| Calcium, mg/dL | 6.01 | 8.5–10.5 | 8.8–10.1 | 8.8–10.2 | 8.8–10.1 | 8.8–10.1 | 8.8–10.1 | 8.5–10.5 | 8.8–10.1 | 8.7–10.1 | 8.7–10.2 | 8.7–10.0 | 8.6–10.0 |
| Chloride, mEq/L | 6.01 | 102–110 | 99–109 | 98–109 | 99–109 | 99–108 | 98–108 | 102–110 | 99–109 | 98–109 | 99–110 | 100–110 | 98–108 |
| CPK*, IU/L | 6.14 | 22–334 | 56–805 | 50–534 | 82–997 | 62–805 | 56–1008 | 22–100 | 35–372 | 31–247 | 45–487 | 38–317 | 31–227 |
| Creatinine*, mg/dL | 5.98 | 0.7–1.3 | 0.69–1.37 | 0.70–1.27 | 0.73–1.45 | 0.65–1.34 | 0.68–1.24 | 0.6–1.1 | 0.47–1.10 | 0.50–1.10 | 0.52–1.15 | 0.46–0.99 | 0.43–0.88 |
| Eosinophils Percent*, % | 3.61 | 0.7–8.5 | 0.6–8.4 | 0.6–7.6 | 0.6–9.6 | 0.7–7.7 | 0.7–8.9 | 0.6–7.3 | 0.6–7.6 | 0.6–7.4 | 0.6–6.9 | 0.5–7.4 | 0.6–8.3 |
| GGT*, IU/L | 6.01 | 10–65 | 9–103 | 9–93 | 10–119 | 9–96 | 10–96 | 8–36 | 6–76 | 6–86 | 7–78 | 6–64 | 6–49 |
| Globulin, g/dLa | 6.11 | 2.3–3.5 | 2.1–3.8 | 1.9–3.5 | 2.3–4.4 | 2.1–3.8 | 2.1–3.8 | 2.3–3.5 | 2.1–3.8 | 2.0–3.6 | 2.5–4.1 | 2.3–3.8 | 2.4–3.8 |
| Glucose, mg/dL | 5.98 | 60–110 | 69–178 | 66–161 | 69–220 | 72–211 | 67–193 | 60–110 | 69–178 | 70–155 | 70–178 | 69–140 | 66–142 |
| Hematocrit*, % | 3.44 | 38.7–51.4 | 37.0–49.6 | 38.7–50.0 | 36.1–49.6 | 38.8–49.5 | 36.7–49.4 | 32.0–45.9 | 31.3–44.3 | 33.6–44.9 | 29.5–43.6 | 31.0–44.1 | 32.2–43.8 |
| Hemoglobin, g/dL* | 3.44 | 13.1–17.5 | 12.5–17.1 | 13.4–17.3 | 12.0–16.4 | 13.5–17.0 | 12.2–16.9 | 10.6–15.6 | 10.4–15.1 | 11.4–15.6 | 9.6–14.6 | 10.2–14.8 | 10.5–14.9 |
| Iron*, µg/dL | 6.08 | 50––160 | 41–177 | 46–177 | 34–175 | 40–192 | 43–173 | 40–150 | 20–156 | 28–159 | 17–141 | 17–144 | 31–167 |
| LDH, U/L | 6.08 | 93–198 | 86–182 | 87–178 | 87–206 | 83–170 | 87–183 | 93–198 | 86–182 | 86–172 | 89–188 | 85–174 | 83–171 |
| Lymphocyte Percent*, % | 3.61 | 16.1–47.9 | 16.0–51.3 | 16.0–43.5 | 16.8–54.2 | 15.6–47.8 | 16.5–48.8 | 14.1–47.6 | 16.3–48.5 | 16.2–45.3 | 17.1–51.3 | 15.2–46.1 | 16.7–49.6 |
| Mean Cell Hemoglobin*, pg | 3.44 | 26.3–34.0 | 25.6–34.3 | 28.5–34.8 | 24.2–34.2 | 27.3–34.2 | 22.3–34.0 | 24.3–33.8 | 23.2–34.2 | 26.3–34.6 | 21.0–33.7 | 23.2–33.7 | 22.1–33.8 |
| MCHC*, g/dL | 3.44 | 32.3–35.3 | 31.7–36.2 | 32.7–36.3 | 31.4–35.8 | 32.4–35.8 | 31.8–36.0 | 32.1–35.3 | 31.8–36.0 | 32.6–36.3 | 31.1–35.6 | 32.3–35.7 | 32.3–35.8 |
| Mean Cell Volume*, fL | 3.44 | 79.8–99.1 | 77.6–98.9 | 82.6–99.1 | 74.1–99.1 | 82.3–98.4 | 69.9–99.8 | 74.6–98.2 | 72.0–98.6 | 78.7–99.4 | 66.8–97.8 | 72.1–96.4 | 67.8–97.8 |
| Mean Platelet Volume*, fL | 3.48 | 6.8–10.1 | 6.8–10.5 | 6.8–10.4 | 6.9–10.8 | 6.9–10.5 | 6.6–10.0 | 6.8–10.2 | 6.9–10.4 | 6.9–10.4 | 7.1–10.6 | 7.0–10.4 | 6.8–10.0 |
| Monocyte Percent*, % | 3.61 | 4.4–13.5 | 3.8–12.9 | 3.8–12.6 | 3.4–12.0 | 4.4–12.6 | 3.8–11.1 | 3.8–11.6 | 3.3–11.9 | 3.5–12.0 | 3.3–12.5 | 3.3–11.0 | 3.3–10.6 |
| Osmolality, mOsm/kga | 6.01 | 275–295 | 268–286 | 269–285 | 271–286 | 271–286 | 269–285 | 275–295 | 268–286 | 266–285 | 268–287 | 268–286 | 267–286 |
| Phosphorus, mg/dL | 5.98 | 2.6–4.4 | 2.7–4.9 | 2.6–4.8 | 2.6–4.9 | 2.7–4.9 | 2.8–4.8 | 2.6–4.4 | 2.7–4.9 | 2.7–4.8 | 2.7–4.9 | 2.6–4.9 | 2.7–5.0 |
| Platelet Count*, % | 3.48 | 152–386 | 139–339 | 136–336 | 134–349 | 138–343 | 152–325 | 168–441 | 148–385 | 132–337 | 153–402 | 160–386 | 139–370 |
| Potassium, mEq/L | 6.01 | 3.5–5.0 | 3.3–4.5 | 3.4–4.6 | 3.3–4.6 | 3.4–4.6 | 3.4–4.7 | 3.5–5.0 | 3.3–4.5 | 3.2–4.4 | 3.2–4.5 | 3.4–4.4 | 3.3–4.6 |
| RBC*, SI | 3.44 | 4.18–5.86 | 4.07–5.70 | 4.18–5.62 | 3.99–5.79 | 4.14–5.68 | 4.06–5.97 | 3.64–5.2 | 3.66–5.13 | 3.70–5.14 | 3.55–5.16 | 3.71–5.06 | 3.66–5.05 |
| RCDW*, % | 3.44 | 11.4–14.5 | 11.5–14.7 | 11.5–14.1 | 11.4–15.5 | 11.6–14.3 | 11.4–14.6 | 11.4–16.3 | 11.4–17.5 | 11.4–16.2 | 11.6–18.8 | 11.6–18.8 | 11.3–15.7 |
| SNP*, % | 3.61 | 37.8–74.6 | 36.2–75.3 | 43.2–75.3 | 32.3–75.3 | 37.5–75.0 | 40.2–75.4 | 39.8–78.1 | 40.3–75.4 | 42.3–75.4 | 36.1–74.3 | 42.4–76.5 | 39.8–75.0 |
| Sodium, mEq/L | 6.01 | 136–144 | 135–143 | 134–142 | 135–143 | 135–143 | 135–143 | 136–144 | 135–143 | 134–143 | 135–143 | 135–142 | 134–143 |
| Total Bilirubin, mg/dL | 6.08 | 0.2–1.3 | 0.3–1.4 | 0.4–1.7 | 0.4–1.7 | 0.4–1.5 | 0.4–1.6 | 0.2–1.3 | 0.3–1.4 | 0.3–1.3 | 0.3–1.2 | 0.3–1.2 | 0.3–1.2 |
| Total Cholesterol, mg/dL | 6.01 | <200 | 121–276 | 124–270 | 111–247 | 115–278 | 118–259 | <200 | 121–276 | 130–297 | 114–286 | 127–274 | 127–278 |
| Total Protein, g/dL | 6.11 | 6.4–7.7 | 6.3–8.2 | 6.2–8.1 | 6.5–8.6 | 6.5–8.3 | 6.5–8.2 | 6.4–7.7 | 6.3–8.2 | 6.1–7.9 | 6.4–8.2 | 6.3–8.0 | 6.4–8.2 |
| Triglycerides, mg/dL | 6.04 | 0–1000 | 37–455 | 40–512 | 37–370 | 46–586 | 40–520 | 0–1000 | 37–455 | 42–448 | 30–257 | 32–349 | 35–466 |
| Uric Acid*, mg/dL | 6.01 | 3.6–8.4 | 3.8–8.8 | 3.9–8.7 | 3.7–9.0 | 3.7–8.4 | 3.9–9.1 | 2.9–7.5 | 2.7–7.1 | 3.0–7.2 | 2.8–7.5 | 2.7–6.7 | 2.7–6.8 |
| WBC*, SI | 3.44 | 3.9–11.8 | 3.7–11.7 | 4.0–12.2 | 3.4–10.6 | 3.8–12.3 | 3.8–11.7 | 4.1–12.9 | 3.7–11.9 | 4.1–11.9 | 3.4–11.4 | 3.9–12.0 | 3.9–10.8 |
N = 3,077. % Missing = percent of missing data. Hispanic = Mexican American or Other Hispanic. ALT = Alanine aminotransferase. ALP = Alkaline phosphotase. BUN = Blood urea nitrogen. CPK = Creatine phosphokinase. GGT = Gamma-glutamyl transferase. LDH = lactate dehydrogenase. MCHC = Mean cell hemoglobin concentration. RBC = Red blood cell count. RCDW = Red cell distribution width. WBC = White blood cell count. SNP = Segmented neutrophils percent.
All the laboratory tests in “Standard Biochemistry Profile” and “Complete Blood Count with 5-Part Differential in Whole Blood” data were utilized from the NHANES 2011–2012 Laboratory Data. Lower and upper limits of normal range were defined as 2.5th and 97.5th values in percent, respectively.
Different reference interval by sex by the NHANES manual. If there is no distinction between sex, same reference intervals are given for male and female.
aReference interval is not availabe in the NHANES manual. The common reference interval is given, exerpt from the following website, http://musom.marshall.edu/usmle/usmlelabvalues.htm. Note. According to the NHANES manual, reference intervals for most biochemistry laboratory tests were established from Tietz' textbook and reference intervals for blood chemistry laboratory tests were calculated from the NHANES data set (1999–2004) using 95% reference interval(s) determined non-parametrically, through ranking the observations and determining the lower (2.5th percentile) and the upper (97.5th percentile) reference limits. Reference intervals for blood chemistry laboratory tests are those corresponding to the age group of 19–65.
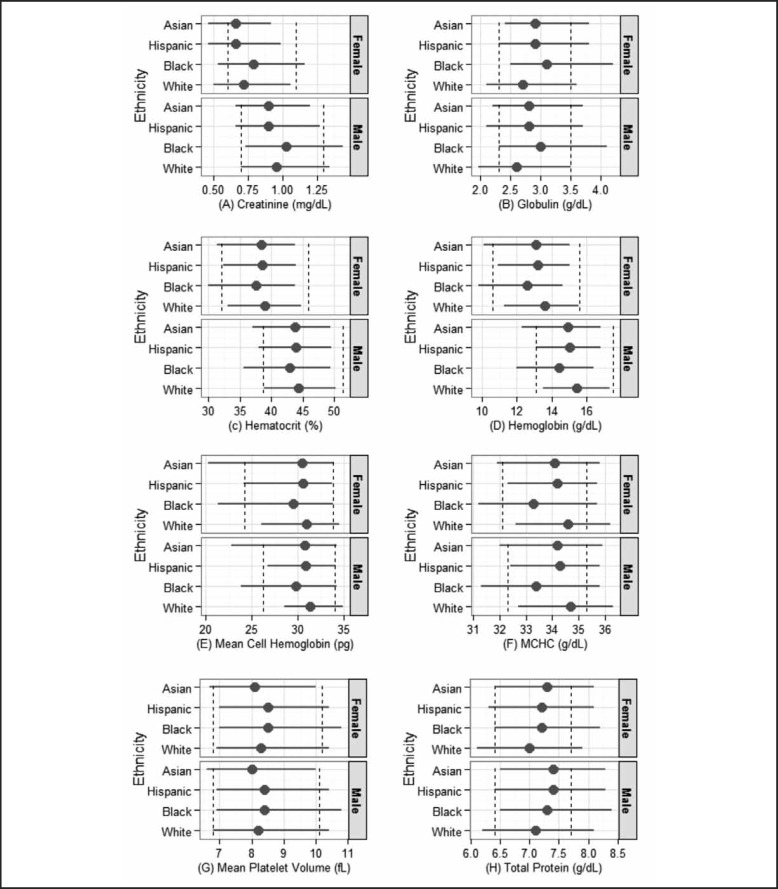
To address whether these shifts in normal range were statistically significant, quantile regressions were conducted using race/ethnicity and sex as independent variables (Table 3). The parameter estimate of each race/ethnicity allowed us to assess whether its normal range is different from whites after adjusting for sex. All except for five laboratory tests (ie, glucose, phosphorus, potassium, total bilirubin, and uric acid) showed significant racial/ethnic difference in either lower or upper percentile. Racial/ethnic differences varied across laboratory tests. Compared to whites, Asians are more likely to have higher lower limits for bicarbonate, globulin, and total protein and reduced lower limits for most hematological laboratory tests (ie, hematocrit, hemoglobin, mean cell hemoglobin, mean cell volume, MCHC, and mean platelet volume) and creatinine. Asians also had lower upper limit estimates for calcium, creatinine, hematocrit, hemoglobin, mean cell hemoglobin, MCHC, mean platelet volume, and monocyte percent. Asians were also more likely to have higher estimates for albumin, eosinophils percent, globulin, lymphocyte percent, RCDW, and total protein. Blacks had significantly higher normal ranges in CPK, globulin, and total protein and lower normal ranges in hematocrit, hemoglobin, mean cell hemoglobin, MCHC, total cholesterol, triglycerides, and WBC than the referent whites. Hispanics had higher normal ranges in total protein and lower normal ranges in mean cell hemoglobin and MCHC. Figure 1 depicts the variation in the estimated normal ranges by sex and race/ethnicity for the eight laboratory tests that showed significant difference bewteen Asians and whites in both percentiles.
Table 3.
Summary of Parameter Estimates Based on Quantile Regression Analysis
| Laboratory Test | Parameter Estimate (Standard Error) for Lower Limit | Parameter Estimate (Standard Error) for Upper Limit | ||||||||
| Reference | Male | Black | Hispanic | Asian | Reference | Male | Black | Hispanic | Asian | |
| Albumin, g/dL | 3.6*** (0.05) | 0.3*** (0.05) | −0.1+ (0.06) | −0.1 (0.07) | 0.1 (0.06) | 4.8*** (0.04) | 0.3*** (0.04) | −0.2 (0.03) | 0.0 (0.04) | 0.1* (0.05) |
| ALT, U/L | 10*** (0.4) | 2** (0.4) | −1* (0.5) | 0 (0.6) | 0 (0.5) | 58*** (7.0) | 29*** (6.7) | −18+ (9.3) | 5 (16.0) | −11 (7.8) |
| ALP, IU/L | 31*** (1.0) | 5*** (1.2) | 2 (1.7) | 9*** (1.2) | −1 (1.6) | 112*** (4.9) | −3 (4.6) | 9 (5.7) | 12+ (6.7) | −11+ (6.5) |
| Basophils Percent, % | 0.0 (0.01) | 0.0 (0.00) | 0.0 (0.01) | 0.1 (0.04) | 0.0 (0.03) | 2.1*** (0.22) | 0.3 (0.21) | 0.8* (0.32) | −0.4 (0.40) | −0.4+ (0.24) |
| Bicarbonate, mmol/L | 20*** (0.2) | 1*** (0.2) | 0 (0.5) | 0 (0.4) | 1*** (0.4) | 28*** (0.0) | 1*** (0.0) | 1*** (0.1) | 0 (0.0) | 0 (0.0) |
| BUN, mg/dL | 5*** (0.2) | 1*** (0.2) | 0 (0.5) | 1* (0.4) | 1+ (0.5) | 22*** (0.8) | 0 (0.8) | −2+ (1.1) | 0 (1.0) | −2.0 (1.4) |
| Calcium, mg/dL | 8.7*** (0.04) | 0.1** (0.04) | 0.0 (0.04) | 0.0 (0.04) | 0.0 (0.06) | 10.1*** (0.04) | 0.1* (0.04) | 0.0 (0.07) | −0.1* (0.05) | −0.1* (0.05) |
| Chloride, mEq/L | 98*** (0.5) | 0 (0.4) | 1+ (0.6) | 1** (0.4) | 0 (0.6) | 109*** (0.2) | −1** (0.3) | 1* (0.5) | 0 (0.5) | 0 (0.4) |
| CPK, IU/L | 28*** (2.4) | 24*** (2.7) | 20*** (4.6) | 10** (3.6) | 4 (3.2) | 211*** (30.9) | 408*** (52.9) | 293*** (60.6) | 106 (91.2) | 25 (103.0) |
| Creatinine, mg/dL | 0.5*** (0.01) | 0.2*** (0.01) | 0.0+ (0.02) | −0.0** (0.01) | −0.0* (0.02) | 1.1*** (0.03) | 0.3*** (0.03) | 0.1* (0.04) | −0.1 (0.07) | −0.1*** (0.04) |
| Eosinophils Percent, % | 0.6*** (0.03) | 0.0 (0.04) | 0.0 (0.04) | 0.0 (0.04) | 0.0 (0.04) | 6.8*** (0.44) | 0.9+ (0.47) | 0.8 (0.70) | 0.0 (0.81) | 1.4* (0.58) |
| GGT, IU/L | 6*** (0.2) | 3*** (0.2) | 1** (0.3) | 0 (0.5) | 0 (0.4) | 75*** (9.4) | 30** (10.7) | 10 (15.0) | −10 (13.7) | −13 (16.8) |
| Globulin, g/dL | 2.1*** (0.04) | −0.2*** (0.04) | 0.4*** (0.05) | 0.2** (0.07) | 0.3*** (0.06) | 3.6*** (0.07) | −0.1 (0.09) | 0.6*** (0.16) | 0.2+ (0.11) | 0.2* (0.10) |
| Glucose, mg/dL | 69*** (1.5) | −1 (1.5) | 1 (2.3) | 1 (1.6) | −3 (3.0) | 142*** (13.1) | 33 (16.9) | 40 (21.2) | 21 (25.8) | 10 (25.6) |
| Hematocrit, % | 33.1*** (0.46) | 5.7*** (0.44) | −3.2*** (0.60) | −0.8 (0.81) | −1.8* (0.80) | 44.7** *(0.25) | 5.6*** (0.34) | −0.9* (0.42) | −0.8+ (0.47) | −0.9** (0.43) |
| Hemoglobin, g/dL | 11.3*** (0.19) | 2.2*** (0.18) | −1.5*** (0.22) | −0.4 (0.28) | −1.2** (0.37) | 15.5*** (0.09) | 1.8*** (0.13) | −0.9***(0.16) | −0.5***(0.15) | −0.5** (0.17) |
| Iron, μg/dL | 28*** (1.9) | 18*** (2.0) | −11*** (2.5) | −10*** (2.2) | 1 (3.7) | 157*** (5.0) | 23*** (5.7) | −13+ (6.8) | 0 (9.3) | −1 (13.5) |
| LDH, U/L | 86*** (1.5) | 1 (1.7) | 2 (2.1) | −1 (2.4) | −1 (2.3) | 172*** (4.2) | 7*** (4.3) | 23*** (5.5) | 0 (5.9) | −1 (8.8) |
| Lymphocyte Percent, % | 16.2*** (0.58) | −0.1 (0.69) | 0.7 (1.06) | −0.7 (0.78) | 0.4 (1.11) | 43.6*** (0.86) | 0.5 (0.86) | 8.8*** (1.10) | 3.5* (1.38) | 5.6*** (1.08) |
| Mean Cell Hemoglobin, pg | 26.1*** (0.46) | 2.5*** (0.41) | −4.7*** (0.52) | −1.9** (0.59) | −5.8*** (0.89) | 34.5***(0.24) | 0.4+ (0.23) | −0.7* (0.32) | −0.8* (0.34) | −0.7* (0.32) |
| MCHC, g/dL | 32.6** *(0.12) | 0.1 (0.11) | −1.4*** (0.13) | −0.3* (0.15) | −0.7** (0.25) | 36.2***(0.08) | 0.1 (0.10) | −0.5*** (0.14) | −0.5***(0.15) | −0.4** (0.13) |
| Mean Cell Volume, fL | 77.8*** (1.26) | 5.1*** (1.17) | −9.0*** (1.68) | −2.5 (1.56) | −12.6*** (2.09) | 99.2***(0.56) | 1.4* (0.65) | −1.4 (0.92) | −2.5*** (0.72) | −0.8 (1.20) |
| Mean Platelet Volume, fL | 6.9*** (0.06) | −0.1+ (0.06) | 0.1 (0.07) | 0.1 (0.13) | −0.2* (0.08) | 10.4*** (0.12) | 0.0 (0.14) | 0.4+ (0.21) | 0.0 (0.19) | −0.4* (0.16) |
| Monocyte Percent, % | 3.4*** (0.18) | 0.5** (0.18) | −0.3 (0.21) | 0.2 (0.25) | −0.1 (0.24) | 11.8*** (0.29) | 1.1*** (0.30) | 1.0+ (0.56) | −0.6 (0.43) | −1.4***(0.38) |
| Osmolality, mOsm/kg | 266*** (0.6) | 3*** (0.6) | 2** (0.7) | 2** (0.8) | 0 (0.9) | 286*** (0.7) | −1 (0.6) | 1 (0.7) | 0 (1.9) | 0 (1.4) |
| Phosphorus, mg/dL | 2.7*** (0.06) | −0.1 (0.06) | 0.0 (0.09) | 0.0 (0.10) | 0.1 (0.10) | 4.5*** (0.05) | 0.1+ (0.05) | 0.0 (0.06) | −0.1 (0.07) | 0.1 (0.07) |
| Platelet Count, % | 142*** (5.6) | −11* (4.8) | 6 (6.2) | 16* (7.6) | 15 (9.5) | 378*** (8.5) | −44*** (7.8) | 18 (14.8) | 8 (9.1) | −8 (10.5) |
| Potassium, mEq/L | 3.2*** (0.04) | 0.1** (0.04) | 0.0 (0.06) | 0.1 (0.08) | 0.1+ (0.10) | 4.8*** (0.07) | 0 (0.07) | 0.1 (0.09) | 0.1 (0.12) | 0.1 (0.10) |
| RBC, SI | 3.7*** (0.03) | 0.4*** (0.04) | −0.2** (0.06) | 0.0 (0.05) | −0.1 (0.06) | 5.1*** (0.05) | 0.6*** (0.05) | 0.1 (0.07) | −0.0 (0.06) | 0.4 (0.12) |
| RCDW, % | 11.4*** (0.05) | 0.0 (0.05) | 0.1 (0.09) | 0.2*** (0.06) | 0.0 (0.06) | 16.8*** (0.27) | −2.8*** (0.24) | 1.9*** (0.36) | 0.4 (0.27) | 0.6* (0.30) |
| SNP, % | 42.9*** (0.78) | −1.9+ (1.00) | −8.2***(1.16) | −2.7+ (1.59) | −2.3+ (1.31) | 75.4*** (0.72) | −0.1 (0.86) | −0.7 (1.24) | 0.0 (0.95) | 0.1 (1.36) |
| Sodium, mEq/L | 134*** (0.3) | 0 (0.4) | 1** (0.4) | 1+ (0.6) | 1 (0.7) | 143*** (0.3) | 0 (0.2) | 0 (0.4) | 0 (0.6) | 0 (0.4) |
| Total Bilirubin, mg/dL | 0.3*** (0.00) | 0.1*** (0.00) | 0.0 (0.00) | 0.0 (0.01) | 0.0 (0.04) | 1.3*** (0.07) | 0.4*** (0.08) | −0.1 (0.10) | −0.2 (0.11) | −0.1 (0.10) |
| Total Cholesterol, mg/dL | 130*** (2.4) | −6* (2.8) | −13*** (3.7) | −8+ (4.1) | −4 (5.0) | 293*** (7.0) | −23*** (6.0) | −18*** (8.8) | −6 (8.3) | −14+ (7.4) |
| Total Protein, g/dL | 6.1*** (0.06) | 0.1* (0.05) | 0.3*** (0.06) | 0.2* (0.08) | 0.3*** (0.06) | 7.9*** (0.05) | 0.2*** (0.06) | 0.3** (0.12) | 0.2** (0.07) | 0.2** (0.07) |
| Triglycerides, g/dL | 38*** (1.8) | 6** (2.0) | −7** (2.3) | −2 (2.9) | −2 (2.8) | 423*** (32.8) | 94** (29.2) | −165*** (31.4) | −7 (55.1) | 20 (62.1) |
| Uric Acid, mg/dL | 2.9*** (0.11) | 1.0*** (0.12) | −0.1 (0.15) | −0.2 (0.23) | −0.1 (0.16) | 7.2*** (0.15) | 1.6*** (0.16) | 0.2 (0.30) | −0.4 (0.26) | −0.3 (0.23) |
| WBC, SI | 4.1*** (0.11) | −0.1 (0.10) | −0.7*** (0.11) | −0.2 (0.21) | −0.2 (0.13) | 12.1*** (0.42) | −0.0 (0.45) | −1.0* (0.51) | −0.1 (0.61) | −0.9 (0.69) |
Hispanic = Mexican American or Other Hispanic. Reference = White female. Lower and upper limits of normal range were defined as 2.5th and 97.5th values in percent, respectively.
ALT = Alanine aminotransferase. ALP = Alkaline phosphotase. BUN = Blood urea nitrogen. CPK = Creatine phosphokinase. GGT = Gamma-glutamyl transferase. MCHC = Mean cell hemoglobin concentration. RBC = Red blood cell count. RCDW = Red cell distribution width. WBC = White blood cell count. SNP = Segmented neutrophils percent.
+P < .10. *P < .05. **P < .01. ***P < .001.
Note. Unweighted quantile regression was fitted for each analyte adjusting for sex and race/ethnicity. A bootstrap resampling method with 1,000 bootstrap samples was applied to compute the standard errors of parameter estimates. Female white was the reference group. Weighted quantile regressions accounting for the NHANES complex sampling design provided similar results (not shown).
Figure 1.
Normal Ranges of Selected Laboratory Tests Adjusted for Sex and Race/Ethnicity.
Dashed lines are the reference intervals for each laboratory test based on the NHANES laboratory manual. The horizontal line represents the lower and upper limits of normal range for the subpopulation and the dot on each line represents the estimated median value based on a median analysis. Lower and upper limits of each normal range are the estimated 2.5th and 97.5th values in percent by sex and race/ethnicity, respectively.
Significant sex differences were also found in both percentiles in the following laboratory tests: albumin, ALT, bicarbonate, calcium, CPK, creatinine, GGT, hematocrit, hemoglobin, iron, mean cell volume, monocyte percent, platelet count, RBC, total bilirubin, total cholesterol, total protein, triglycerides, and uric acid (Table 3). Overall, males had higher estimates except for platelet count and total cholesterol whose direction was opposite.
As a sensitivity analysis, the same models were applied to the participants who reported “Excellent” or “Very Good” health status. The results were very similar in direction and magnitude in parameter estimates for most of all laboratory tests. Weighted quantile regression using the NHANES complex sampling weight also showed comparable patterns (results not shown).
Discussion
Comparing major racial/ethnic subpopulations in the United States, our study aimed to explore whether the use of racial/ethnic-specific RIs is reasonable for common laboratory tests. For this purpose, we used the NHANES 2011–2012 data, a representative nationwide sample, which includes Non-Hispanic Asian as a separate racial/ethnic category. According to the 2010 US Census. Asians alone grew by 43.3 percent from 2000 to 2010.20 As a result, the NHANES oversampled Asians in its 2011–2012 data in order to compare Asians with other racial/ethnic groups.
Even though researchers have acknowledged racial/ethnic differences in RIs for some laboratory tests since the early 1970's,6,8 no racial/ethnic-specific RIs have been developed for clinical settings in the United States. Hence, it is important to evaluate whether a single RI for everyone is appropriate, especially in a multiethnic country like the United States. Laboratory tests play a critical role in physicians' clinical decision-making. According to one study, about 60–70% of all clinical decisions regarding a patient's diagnosis and treatment, hospital admission and discharge are made based on laboratory test results.21 Ignoring the natural variations in the distributions of laboratory test results among racial/ethnic groups could contribute to, among other things, disease misdiagnosis. For example, our study indicated that Asians had lower normal ranges for creatinine than the textbook RI. If our estimated normal ranges are close to true RI for this racial/ethnic group, many healthy Asians with lower creatinine would be considered as having muscle or nerve problems (eg, myasthenia gravis, muscular dystrophy)22 and clinicians may order unnecessary MRI or biopsy to make a clinical diagnosis. Similarly, our study found that blacks have significantly lower values than whites in hematocrit, hemoglobin, mean cell hemoglobin, and MCHC.17 According to the study on Tanzanian children by Buchanan, et al, (2010), about 20% of healthy Tanzanian children would be misclassified as having an adverse event related to hemoglobin if the US National Institute of Health Division of AIDS adverse event grading criteria were applied.23 The development of racial/ethnic-specific RIs for common laboratory tests, therefore, may be important for reducing inaccuracies and misdiagnosis so that treatment can be conducted in a timely manner and patients' health status can be better monitored.
The significant difference between American Asians and whites warrants further discussion. Compared to whites, Asians have lower RIs in creatinine, hematocrit, hemoglobin, mean cell hemoglobin, MCHC, and mean platelet volume and higher normal ranges in globulin and total protein. Asians are the fastest growing population in America, hence, the development of Asian-specific RIs for these laboratory tests may be valuable. This finding is also important to a state like Hawai‘i where a significant Asian population exists. Hawai‘i's Asian population is unique and diverse, with 57.4% of the state population self-identifying as Asian alone or in combination.20 More specified diverse Asian groups may need to be considered when developing RIs. According to the 2009 Asian multicenter study for derivation of reference intervals, Ichihara, et al, found significant regional differences in Asian countries among 11 of 40 laboratory tests.24,25 To our knowledge, there are no published studies comparing the RIs between Asian subpopulations in Hawai‘i or on the mainland. Studies showed that RIs of common laboratory tests tend to vary among people who are usually assigned into the same ethnic or racial group.2,25,26 Therefore, it is anticipated that different Asian populations in Hawai‘i may have different distributions of laboratory tests. Our future work is to develop racial/ethnic-specific RIs for Hawai‘i residents and compare those with the RIs reported in the literature.
Our study revealed some findings that are inconsistent with previous studies. For example, a shift in platelet count among US blacks was not detected, as observed in a study among blacks in Gambia.16 This inconsistent result may be attributed to dissimilarities in nutritional status (eg, Western diet style) or regional factors (eg, no malaria infection that may increase platelet count), among other things. Also, utilizing 33 laboratory tests in the NHANES III, Horn and Pesce (2002) suggested combining Hispanics and whites.27 Our current study, however, showed significant differences in some laboratory tests (ie, mean cell hemoglobin, MCHC, total protein) between Hispanics and whites.
Interestingly, for some laboratory tests (eg, albumin, bicarbonate, calcium, total bilirubin, total cholesterol, and total protein), our analysis results indicate that sex-specific RIs may be more appropriate even though the NHANES provides a single RI for both male and female. Recent studies also reported significant sex differences in albumin,28 total bilirubin,28,29 and cholesterol28,30 among healthy adults in Africa and East Asia. Further study may need to be conducted to address whether sex-specific RIs are relevant for these laboratory tests.
This study has several limitations. First, self-reported health status was used to define healthy adults instead of using other more objective criteria (eg, medical history, medication). Based on the evaluation of laboratory tests, a simple exclusion criterion that could be used to define healthy adults for all 38 laboratory tests was not found. Thus, for simplicity, we selected participants who reported they were healthy. According to Cheng, et al, (2004), however, derivation of RIs in clinical chemistry can be straightforward.13 A simple set of interview questions (eg, body mass index, smoking, drinking, etc) complemented with glucose and creatinine testing can usually exclude most patients with chronic or acute disease. In addition, one well-known problem of self-reporting is response bias which can impact the validity of our results.31 We found that more whites and Asians reported their health status as “Excellent” or “Very Good” than blacks or Hispanics did. Although self-reported current health status was shown to have good reliability32 and predictive validity,33–36 future investigations will be needed to evaluate the validity of NHANES self-reporting health status to ensure the generalizability of our study results. Second, there are missing values in the laboratory tests. For instance, we found blacks and Asians have more missing laboratory tests (P < .001). Although the missing rates were relatively small (< 7%), these unbalanced missing rates could affect our findings. Along with the response bias due to self-reporting, this can also impact the generalizability of our results.
Our findings highlight the complexity of developing RIs. Potentially, racial/ethnic-specific RIs will reduce misdiagnosis, over- and under-estimation of disease prevalence rates, the failure or delay in the required reporting of critical laboratory values;12 however, further work is needed to validate these benefits. Physicians and other healthcare providers use the laboratory test results to track clinical outcomes and make clinical decisions,37,38 to screen asymptomatic people and to identify those at risk and for early detection of diseases.39,40 Therefore, accurate RIs for for laboratory tests are important for patients and their caregivers to monitor their health and disease progress. Further work will be necessary to evaluate the impact of using racial/ethnic-specific RIs to improve health outcomes.
Conclusion
Inter-racial/ethnic differences are usually not reflected in the widely adopted RIs, which would potentially result in lower quality healthcare and unnecessary high healthcare costs. Racial/ethnic-specific RIs for clinical laboratory tests may help improve disease diagnosis, allow for better tracking and monitoring of one's health status, facilitate clinical decision making and improve healthcare in general.
Acknowledgement
We thank Rosa Castro for editing this manuscript.
Conflict of Interest
None of the authors identify any conflict of interest.
Funding Sources
This research was supported in part by grants U54MD007584, G12MD007601, P20GM103466, and U54GM104944 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Malati T. Whether western normative laboratory values used for clinical diagnosis are applicable to Indian population? An overview on reference interval. Indian Journal of Clinical Biochemistry. 2009 Jul 9;24(2):111–122. doi: 10.1007/s12291-009-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dosoo DK, Kayan K, Adu-Gyasi D, et al. Haematological and biochemical reference values for healthy adults in the middle belt of Ghana. PLoS ONE. 2012;7(4):e36308. doi: 10.1371/journal.pone.0036308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groesbeck D, Kottgen A, Parekh R, et al. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol. 2008 Nov;3(6):1777–1785. doi: 10.2215/CJN.00840208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ash KO, Clark SJ, Sandberg LB, Hunter E, Woodward SC. The influences of sample distribution and age on reference intervals for adult males. Am J Clin Pathol. 1983 May;79(5):574–581. doi: 10.1093/ajcp/79.5.574. [DOI] [PubMed] [Google Scholar]
- 5.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996 Aug;49(8):664–666. doi: 10.1136/jcp.49.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg DM, Handyside AJ, Winfield DA. Influence of demographic factors on serum concentrations of seven chemical constituents in healthy human subjects. Clin Chem. 1973 Apr;19(4):395–402. [PubMed] [Google Scholar]
- 7.Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem. 1990 Feb;36(2):265–270. [PubMed] [Google Scholar]
- 8.McPherson K, Healy MJ, Flynn FV, Piper KA, Garcia-Webb P. The effect of age, sex and other factors on blood chemistry in health. Clin Chim Acta. 1978 Mar 15;84(3):373–397. doi: 10.1016/0009-8981(78)90254-1. [DOI] [PubMed] [Google Scholar]
- 9.Nilssen O, Forde OH, Brenn T. The Tromso Study. Distribution and population determinants of gamma-glutamyltransferase. Am J Epidemiol. 1990 Aug;132(2):318–326. doi: 10.1093/oxfordjournals.aje.a115661. [DOI] [PubMed] [Google Scholar]
- 10.PetitClerc C, Solberg HE. Approved recommendation (1987) on the theory of reference values. Part 2. Selection of individuals for the production of reference values. J Clin Chem Clin Biochem. 1987;25:639–644. [Google Scholar]
- 11.Sinton TJ, Cowley DM, Bryant SJ. Reference intervals for calcium, phosphate, and alkaline phosphatase as derived on the basis of multichannel-analyzer profiles. Clin Chem. 1986 Jan;32(1 Pt 1):76–79. [PubMed] [Google Scholar]
- 12.Boucai L, Hollowell JG, Surks MI. An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid : official journal of the American Thyroid Association. 2011 Jan;21(1):5–11. doi: 10.1089/thy.2010.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng CK-W, Chan J, Cembrowski GS, Assendelft OWV. Complete blood count reference interval diagrams derived from NHANES III: stratification by age, sex, and race. Laboratory Hematology. 2004;10(1):42–53. doi: 10.1532/lh96.04010. [DOI] [PubMed] [Google Scholar]
- 14.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? 2006;107(5):1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceriotti F, Henny J, Queralto J, et al. Common reference intervals for aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) in serum: results from an IFCC multicenter study. Clin Chem Lab Med. 2010 Nov;48(11):1593–1601. doi: 10.1515/CCLM.2010.315. [DOI] [PubMed] [Google Scholar]
- 16.Adetifa IMO, Hill PC, Jeffries DJ, et al. Haematological values from a Gambian cohort - possible reference range for a West African population. International Journal of Laboratory Hematology. 2009;31(6):615–622. doi: 10.1111/j.1751-553X.2008.01087.x. [DOI] [PubMed] [Google Scholar]
- 17.Lim EM, Cembrowski G, Cembrowski M, Clarke G. Race-specific WBC and neutrophil count reference intervals. International Journal of Laboratory Hematology. 2010;32(6p2):590–597. doi: 10.1111/j.1751-553X.2010.01223.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Liu Y, Tsilimingras D, Campbell KM. Racial disparity in the associations of microalbuminuria and macroalbuminuria with odds of hypertension: results from the NHANES study in the United States. ISRN Hypertension. 2013;2013:8. [Google Scholar]
- 19.Bureau USC. QuickFacts Beta United States. 2010. [July 27, 2015]. http://www.census.gov/quickfacts/table/PST045214/00,15.
- 20.Hoeffel EM, Rastogi S, Kim MO, Shahid H. The Asian Population: 2010. U.S. Census Bureau; 2012. [Google Scholar]
- 21.MayoClinic, author. Medical Laboratory Sciences. 2015. [June 15, 2015]. http://www.mayo.edu/mshs/careers/laboratory-sciences.
- 22.Dugdale DC. Creatinine blood test. 2013. [June 16, 2015]. http://www.nlm.nih.gov/medlineplus/ency/article/003475.htm.
- 23.Buchanan AM, Muro FJ, Gratz J, et al. Establishment of haematological and immunological reference values for healthy Tanzanian children in Kilimanjaro Region. Trop Med Int Health. 2010 Sep;15(9):1011–1021. doi: 10.1111/j.1365-3156.2010.02585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichihara K, Ceriotti F, Tam TH, et al. The Asian project for collaborative derivation of reference intervals: (1) strategy and major results of standardized analytes. Clin Chem Lab Med. 2013 Jul;51(7):1429–1442. doi: 10.1515/cclm-2012-0421. [DOI] [PubMed] [Google Scholar]
- 25.Ichihara K, Ceriotti F, Kazuo M, et al. The Asian project for collaborative derivation of reference intervals: (2) results of non-standardized analytes and transference of reference intervals to the participating laboratories on the basis of cross-comparison of test results. Clin Chem Lab Med. 2013 Jul;51(7):1443–1457. doi: 10.1515/cclm-2012-0422. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan AM, Muro FJ, Gratz J, et al. Establishment of haematological and immunological reference values for healthy Tanzanian children in Kilimanjaro Region. Tropical medicine & international health : TM & IH. 2010 Jul 15;15(9):1011–1021. doi: 10.1111/j.1365-3156.2010.02585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn PS, Pesce AJ. Effect of ethnicity on reference intervals. Clinical Chemistry. 2002;48(10):1802–1804. October 1, 2002. [PubMed] [Google Scholar]
- 28.Tembe N, Joaquim O, Alfai E, et al. Reference Values for Clinical Laboratory Parameters in Young Adults in Maputo, Mozambique. PLoS ONE. 2014;9(5):e97391. doi: 10.1371/journal.pone.0097391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segolodi TM, Henderson FL, Rose CE, et al. Normal Laboratory Reference Intervals among Healthy Adults Screened for a HIV Pre-Exposure Prophylaxis Clinical Trial in Botswana. PLoS ONE. 2014;9(4):e93034. doi: 10.1371/journal.pone.0093034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kibaya RS, Bautista CT, Sawe FK, et al. Reference Ranges for the Clinical Laboratory Derived from a Rural Population in Kericho, Kenya. PLoS ONE. 2008;3(10):e3327. doi: 10.1371/journal.pone.0003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donaldson S, Grant-Vallone E. Understanding Self-Report Bias in Organizational Behavior Research. Journal of Business and Psychology. 2002;17(2):245–260. 2002/12/01. [Google Scholar]
- 32.Lundberg O, Manderbacka K. Assessing reliability of a measure of self-rated health. Scandinavian Journal of Public Health. 1996;24(3):218–224. doi: 10.1177/140349489602400314. September 1, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Gold M, Franks P, Erickson P. Assessing the health of the nation. The predictive validity of a preference-based measure and self-rated health. Medical care. 1996 Feb;34(2):163–177. doi: 10.1097/00005650-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn R, Rahman O, Menken J, editors. Survey measures of health: how well do self-reported and observed indicators measure health and predict mortality? In: National Research Council (US) Committee on Population. Washington (DC): The National Academies Press (US); 2006. J CBM, ed. Aging in sub-Saharan Africa: recommendation for furthering research. [Google Scholar]
- 35.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. American Journal of Public Health. 1982;72(8):800–808. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38(1):21–37. [PubMed] [Google Scholar]
- 37.Shine B. Use of routine clinical laboratory data to define reference intervals. Ann Clin Biochem. 2008 Sep;45(Pt 5):467–475. doi: 10.1258/acb.2008.008028. [DOI] [PubMed] [Google Scholar]
- 38.Hickner J, Thompson PJ, Wilkinson T, et al. Primary care physicians' challenges in ordering clinical laboratory tests and interpreting results. J Am Board Fam Med. 2014 Mar-Apr;27(2):268–274. doi: 10.3122/jabfm.2014.02.130104. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Huang H, Zelen M. Early detection of disease and scheduling of screening examinations. Statistical methods in medical research. 2004 Dec;13(6):443–456. doi: 10.1191/0962280204sm377ra. [DOI] [PubMed] [Google Scholar]
- 40.Coller JM, Campbell DJ, Krum H, Prior DL. Early identification of asymptomatic subjects at increased risk of heart failure and cardiovascular events: progress and future directions. Heart, lung & circulation. 2013 Mar;22(3):171–178. doi: 10.1016/j.hlc.2012.09.009. [DOI] [PubMed] [Google Scholar]