Abstract
Traumatic brain injury (TBI) is the leading cause of death and disability in children. Diffusion weighted imaging (DWI) methods have been shown to be especially sensitive to white matter abnormalities in TBI. We used our newly developed autoMATE algorithm (automated multi-atlas tract extraction) to map altered WM integrity in TBI. Even so, tractography methods include a free parameter that limits the maximum permissible turning angles for extracted fibers, with little investigation of how this may affect statistical group comparisons. Here, we examined WM integrity calculated over a range of fiber turning angles to determine to what extent this parameter affects our ability to detect group differences. Fiber turning angle threshold has a subtle, but sometimes significant, effect on the differences we were able to detect between TBI and healthy children.
Index Terms: High angular resolution diffusion imaging (HARDI), traumatic brain injury, tractography, fiber turning angle
1. INTRODUCTION
Traumatic brain injury (TBI) is associated with widespread disruptions in white matter integrity (WM), due in part to diffuse axonal injury (DAI). Lesions associated with DAI are found in many children with TBI, and are associated with a wide range of impairments. DAI is frequently detectable in the corpus callosum, brain stem, gray-white matter junctions, and the parasagittal white matter. DAI can only be definitively diagnosed only post mortem, but imaging methods such as DWI can show compelling evidence of DAI.
TBI during development can be especially disruptive as it may delay or alter the maturation of WM tracts. Studies in animals have found that TBI can decrease experience-dependent plasticity, a key neural contributor to brain maturation and development [1]. There are few large, well-controlled, prospective studies on TBI in children, leaving many unknowns about the expected course of recovery, and what factors may help speed the process.
Fiber angle is a key parameter for many tractography methods, limiting the maximum turning angle of extracted fibers. In the literature, it varies between 30°–70°. The intent of including this parameter is to track trajectories of water diffusion along axons and tracts while favoring less erratic paths. Arguably, this may make the fiber reconstruction more robust to image regions with poor signal to noise or uncertain fiber directions (e.g. where fibers cross). Recently it has been proposed that 90° turns may even be biologically plausible [2]. More lenient thresholds allow for the inclusion of tightly turning tracts, such as the uncinate, or U-fibers near the cortex, but they increase computational load and may include a greater proportion of false positive fibers. There has been little investigation into the effect turning angle has on reconstruction and statistical comparisons. Here we examined WM integrity using our newly developed automate algorithm, which yields in tract-based indices of WM integrity, that may be more robustly identifiable than when using methods that employ only one brain template or atlas.
2. METHODS
2.1. Subjects and Image Acquisition
TBI participants were recruited from 4 Pediatric Intensive Care Units (PICUs) at Level 1 Trauma Centers in Los Angeles County. Healthy controls, matched for age, sex, and educational level, were recruited from the community through flyers, magazines, and school postings. Participants were studied in the post-acute phase (1–5 months post-injury) and chronic phase (13–19 months post-injury). In the post-acute phase, we included 29 TBI participants (9 female) and 30 controls (15 female). In the chronic phase, we included 17 TBI participants (4 female) and 22 controls (7 female). Inclusion criteria: non penetrating moderate-severe TBI (intake or post-resuscitation GCS score between 3 and 12), 8–19 years old, right handed, normal vision, English proficiency. Exclusion criteria: history of neurological illness or injury, motor deficits or metal implant preventing safe MRI scanning, history of psychosis, ADHD, Tourette’s, learning disability, mental retardation, autism, or substance abuse.
Participants were scanned using 3T MRI (Siemens Trio) with whole-brain anatomical and 66-gradient diffusion imaging. Diffusion-weighted images (DWI) were acquired with the following acquisition parameters: GRAPPA mode; acceleration factor PE=2; TR/TE=9500/87 ms; FOV=256x256mm; isotropic voxel size=2 mm. 66 images were collected per subject: 2 b0 and 64 diffusion-weighted images (b=1000 s/mm2).
2.2 AutoMATE
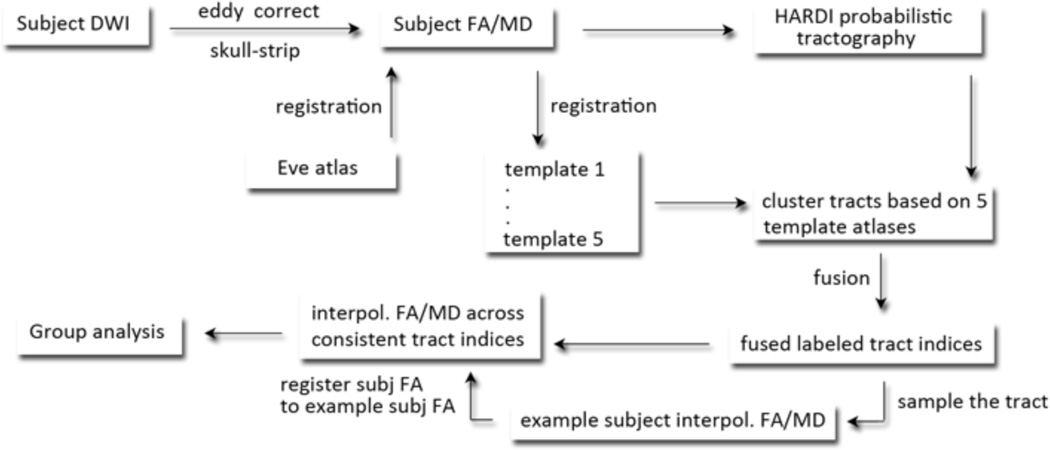
AutoMATE (automated multi-atlas tract extraction), developed by our lab, is described fully in a prior paper [3]. The workflow is shown in Figure 1. Diffusion images were corrected for eddy-current induced distortions using the FSL tool “eddy_correct” (http://fsl.fmrib.ox.ac.uk/fsl/). DWI scans were skull-stripped using “BET”. FA and MD maps were computed using “dtifit”. Whole-brain tractography was performed with Camino (http://cmic.cs.ucl.ac.uk/camino/). We varied the maximum fiber turning angle, running separate experiments at 30°/voxel, 40°/voxel, 50°/voxel and 60°/voxel, consistent with the wide range of thresholds now used in the literature. We were curious if this parameter affected our ability to detect group differences. Tracing stopped when fractional anisotropy (FA) dropped below 0.2, as is standard in the field.
Figure 1. Workflow.
The workflow of autoMATE, described in the methods section and further detailed in [1].
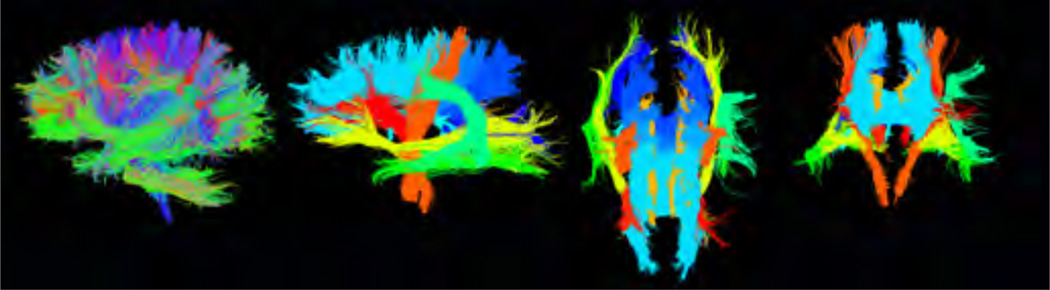
As part of autoMATE, five WM tract atlases were reconstructed from healthy young adults’ (20–30 years old) DWI data, as detailed previously [3]. The atlas, based on the “Eve” brain atlas [4], which is based of a 32-year old female, includes 18 major WM tracts: the anterior thalamic radiation (left and right – atr_l and atr_r), corticospinal tract (left and right – cst_l and cst_r), cingulum (left and right – cgc_l and cgc_r), inferior fronto-occipital fasciculus (left and right – ifo_l and ifo_r), inferior longitudinal fasciculus (left and right – ilf_l and ilf_r), arcuate fasciculus (left only), fornix, and corpus callosal tracts divided into 6 segments – frontal, precentral gyrus, postcentral gyrus, parietal, temporal, and occipital. The Eve atlas was registered, linearly and then non-linearly, to each subject’s FA map using ANTs (Advanced Normalization Tools [5]) and its ROIs were correspondingly warped to extract 18 tracts of interest for each subject based on a look-up table [4]. Each subjects’ FA map was further registered non-linearly to each of the 5 manually constructed atlases. Registrations were visually inspected for quality. We refined each tract’s fiber extractions based on the distance between the corresponding tract of each atlas and the subject’s fiber candidates from the ROI extraction. Individual results from the 5 atlases were fused. We visually inspected the resulting fiber bundles. For each of the 18 WM tracts, we selected one example subject to display results of group analyses. Tracts output by autoMATE are shown in Figure 2 for one example subject, along with the whole brain tractography (leftmost panel).
Figure 2. Output of autoMATE.
Left – whole brain tractography, right – 3 views of the 18 tract ROIs, extracted from one example subject.
2.3 Group Comparison
To limit our search area, as the fiber data results in >100,000 data points, we averaged FA and MD within each of the 18 tracts and ran group analyses on those summary measures. We corrected for multiple statistical comparisons using the FDR method (false discovery rate) (q < 0.05). We then followed up on those tracts for which we found significant differences in average diffusivity measures, examining the point-wise data with a point-wise matching scheme across the entire cohort [6]. We ran a post-hoc element-wise linear regression testing for group differences, including age, sex, and scanner as covariates. This was run separately for the post-acute and chronic data. Results were corrected for multiple comparisons using FDR across all points on all tracts tested (q < 0.05). As SNR (signal to noise ratio) can affect tractography, we verified there were no group differences that would impact our results (p > 0.30 for both post-acute and chronic).
3. RESULTS
3.1. Post-acute
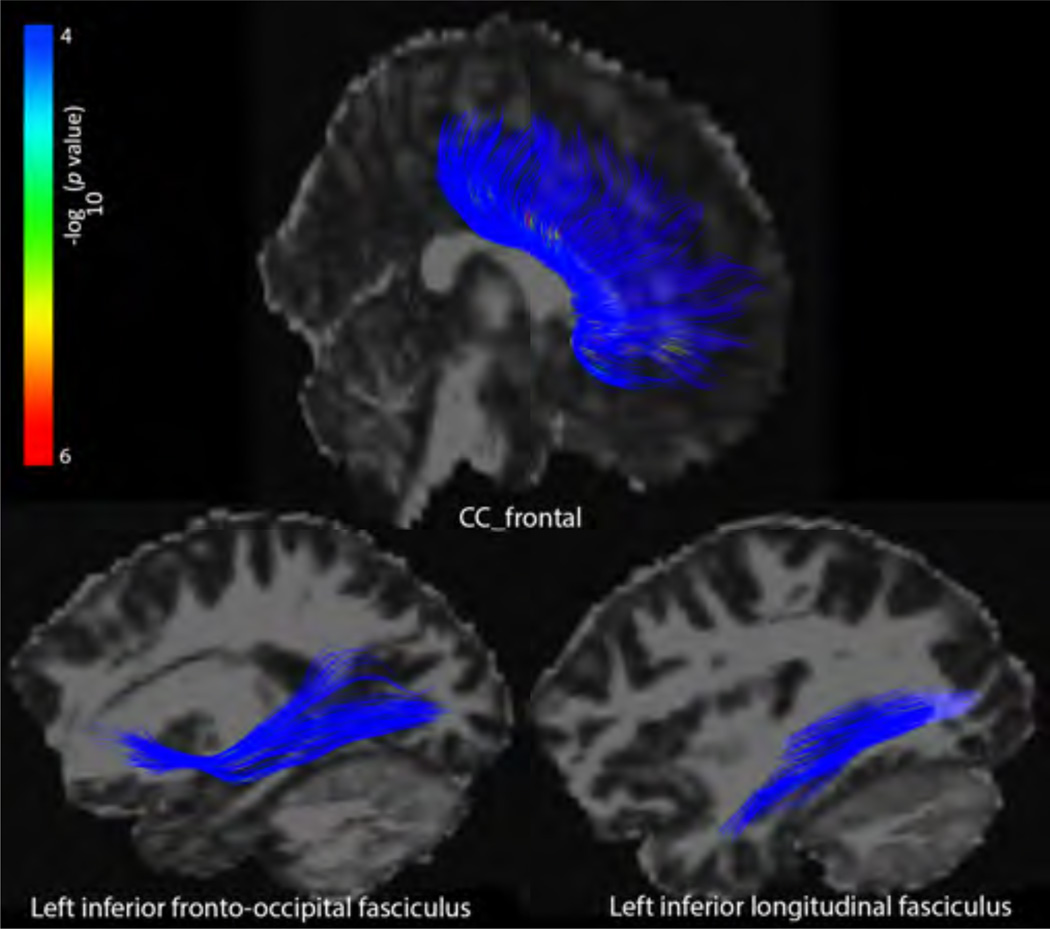
In the post-acute phase, we detected group differences in average FA of the ifo_l and ilf_l across all 4 angle thresholds tested, and in the cc_frontal in the 30° condition (Table 1). When we followed up on these significant results point-wise post hoc, only the 30° results yielded significant differences between TBI and controls (Figure 2).
Table 1. Tract results – post-acute.
Only tracts showing significant group differences in average FA or MD are shown here. The value indicates the percent of the tract that passed the FDR threshold on the point-wise analyses and represents areas of significant differences between TBI and controls. ‘0’ indicates tracts with significant differences in average measures but not point-wise measures.
| 30° | 40° | 50° | 60° | |||||
|---|---|---|---|---|---|---|---|---|
| FA | MD | FA | MD | FA | MD | FA | MD | |
| CC_frontal | 2.2 | - | - | - | - | - | - | - |
| Ifo_l | 1.1 | - | 0 | - | 0 | - | 0 | - |
| Ilf_l | 1.3 | - | 0 | - | 0 | - | 0 | - |
Figure 2. Tract results – post-acute.
Point-wise differences in FA between TBI and controls in the post-acute phase in the 30° condition. The –log10 p-values correspond to the color bar.
FA was significantly lower in the TBI subjects in the cc_frontal, ifo_l, and ilf_l segments in the 30° condition. The same trends existed in the other conditions, but they did not survive FDR correction, indicating that fiber turning angle indeed made a small, but significant, impact on our group comparisons.
3.2. Chronic
In the chronic phase, group differences were much more pronounced, with nearly all tracts showing differences in FA and MD (Table 2). We followed up on all tracts with significant results from the average analyses (FDR q < 0.05). Table 2 shows the results of the post hoc point-wise analysis, with a ‘-‘ for tracts that did not yield significant differences in the averaged analyses.
Table 2. Tract results – chronic.
Only tracts showing significant group differences in average FA or MD are shown here. The value indicates the percent of the tract that passed the FDR threshold on the point-wise analyses and thus represents areas of significant differences between TBI and controls
| 30 | 40 | 50 | 60 | |||||
|---|---|---|---|---|---|---|---|---|
| FA | MD | FA | MD | FA | MD | FA | MD | |
| CC_frontal | 12.6 | 11.2 | 11.5 | 10.2 | 11.4 | 10.9 | 12.1 | 11.0 |
| CC_prcg | 2.3 | 2.7 | 1.4 | 2.7 | 2.1 | 2.8 | 2.6 | 3.4 |
| CC_pocg | 0.6 | 5.2 | 0.1 | 5.1 | 0.03 | 5.1 | 0.08 | 5.4 |
| CC_parietal | 3.0 | 11.9 | 1.6 | 11.9 | 2.2 | 12.4 | 2.2 | 12.1 |
| CC_temporal | 13.1 | 10.2 | 7.1 | 5.0 | 7.7 | 7.5 | 7.8 | 6.2 |
| CC_occipital | 2.6 | 9.3 | 2 | 9.2 | 2.6 | 10.3 | 3.2 | 9.8 |
| Atr_l | - | 10.2 | 1.1 | 11.4 | 1.5 | 10.5 | 1.0 | 11.0 |
| Atr_r | 2.5 | 6.2 | 2.5 | 6.9 | 0.9 | 5.6 | - | 6.2 |
| Cgc_l | 1.6 | 4.5 | 1.2 | 4.6 | 1.6 | 5.2 | 1.2 | 5.2 |
| Cgc_r | 2.5 | 3.7 | 2.4 | 3.6 | 3.8 | 3.7 | 1.7 | 3.4 |
| Cst_l | - | 0.4 | 0.07 | 0.6 | - | 0.4 | 0.1 | 0.4 |
| Cst_r | 0.8 | 0.6 | 2.3 | 0.9 | 3.0 | 0.7 | 2.1 | - |
| Ifo_l | - | - | 0.8 | - | 0.6 | - | 0.9 | - |
| Ifo_r | 2.1 | 11.3 | 2.2 | 9.5 | 3.1 | 10.1 | 1.7 | 9.8 |
| Ilf_l | - | 13.2 | 0.9 | 13.4 | 0.9 | 14.2 | - | 15.1 |
| Ilf_r | 2.3 | 6.0 | 3.2 | 5.0 | 2.4 | 5.9 | 2.3 | 4.6 |
| Fornix | 5.3 | 6.9 | 3.8 | 7.6 | 3.3 | 5.9 | 4.1 | 6.2 |
| Slf_l | - | 3.8 | - | 5.0 | - | 4.2 | - | 4.6 |
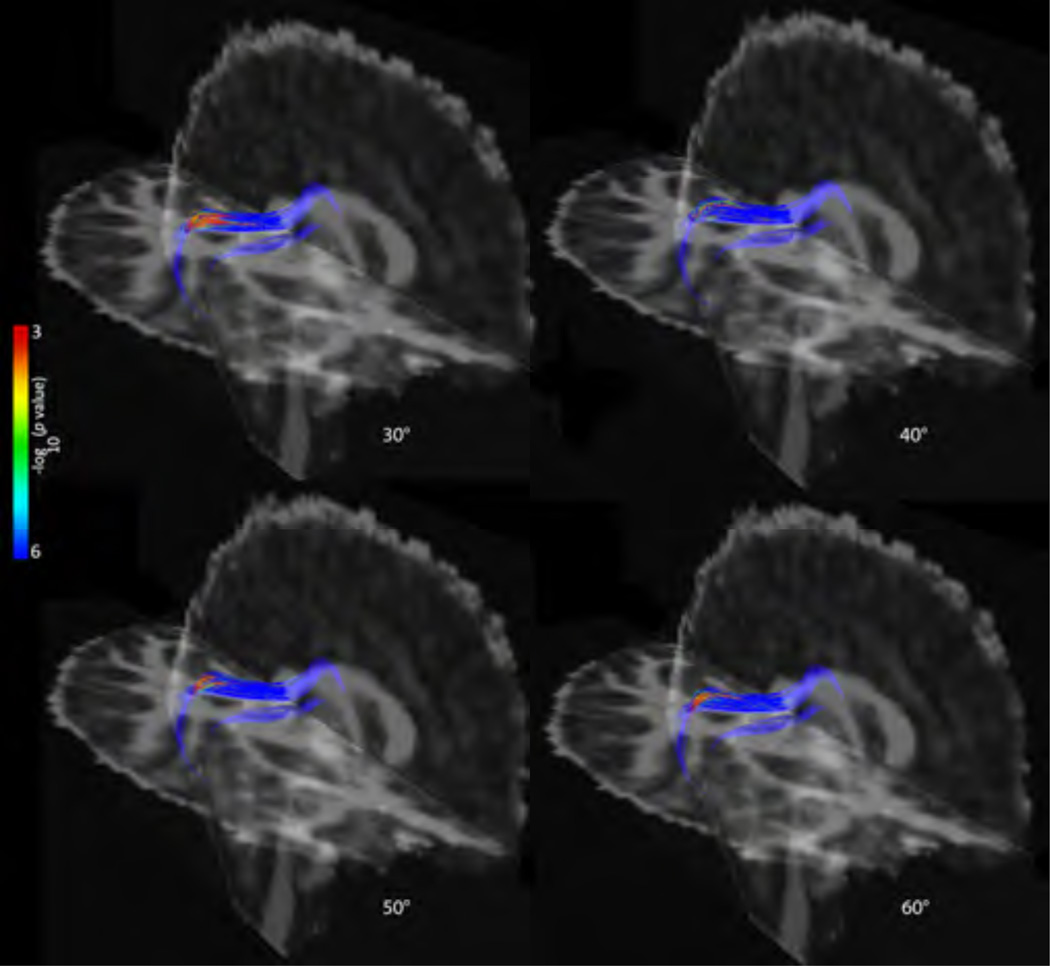
Again fiber turning angle had small but noticeable effects on group comparisons, with some tracts no longer passing FDR in the average analysis. Tracts that showed significant TBI effects across all degree conditions differed slightly in the spatial extent of the significance, but the overarching trend was a larger extent of the areas of significance with the lower fiber angle thresholds. As an example, the point-wise results of FA of the cc_temporal tract are shown in Figure 3.
Figure 3. Tract results – chronic (sample).
Point-wise differences in FA between TBI and controls in the chronic phase in the cc_temporal tract across degree thresholds. The –log10 p-values corresponding to the color bar; we only show cc_temporal here due to space constraints.
4. DISCUSSION
In this paper we applied a recently developed method for assessing white matter integrity along a tract. We found widespread group differences in the chronic phase: TBI patients had lower FA and higher MD, as expected from prior studies [7]. The CC was the most reliably found area of group differences: all 6 CC segments showed differences in FA and MD, across all 4 fiber angle conditions.
The corpus callosum is one of the most frequently reported regions of disruption in TBI, perhaps because midline differences in WM are easiest to detect in a cohort with a heterogeneous distribution of injury locations. Or, it may be that the CC, as the largest fiber bundle connecting the two hemispheres, is especially vulnerable to the acceleration/deceleration and shearing forces during a TBI. We were also able to detect group differences in a number of other tracts. Interestingly, the differences in MD were more extensive than the differences in FA in almost every case. This has also been reported before in chronic TBI [8].
We saw a slight effect of turning angle in the significant group differences we detected across our 4 fiber turning angle thresholds, with slightly more extensive group differences detected at the lower, more stringent fiber turning angle threshold (30°). The relative stability of our results across thresholds is important – a method that detected drastically different results across thresholds would not be reliable. The slight differences that we found across thresholds can be explained by understanding the different numbers of fibers that are recovered across thresholds. At low fiber turning angle thresholds, fewer fibers are recovered, which may miss areas where group differences exist, but may also exclude more false positive fibers. At high fiber turning angle thresholds, more fibers are recovered, which could mean a larger search area for group analyses, but may also include more inaccurate fibers in areas of poor signal to noise. As we found strongest group results at 30°, we can interpret this as indicating that more “non-useful” or “non-discriminative” fibers may be included at the higher fiber angle thresholds.
The low FA frequently seen in TBI can lead to dropouts and premature terminations of fibers in tractography. While autoMATE does use FA to reconstruct tracts, group analyses are not limited to where tracts were reconstructed. In the case of subject data with low FA, where a tract may be dropped, the FA at the location of the warped template point in that subject space can still be used.
Finding more group differences in the chronic phase than the post-acute phase was unexpected, and could indicate a number of things about TBI. This could indicate that our TBI participants are experiencing progressive disruption with time, or it could be that they are indeed improving, but not at the same rate as their age-matched peers who are developing at a normal rate. It could also be that in the post-acute phase local disruption predominates in our statistical comparisons, but over time the disruption spreads through a phenomenon known as diaschisis, where regions connected to the disrupted brain area progressively become disrupted as well, until the disruption becomes more of a global phenomenon. Our planned longitudinal analyses will shed light on these interesting and important questions.
5. CONCLUSION
Diffusion tensor imaging has recently been applied to traumatic brain injury, with great potential for detecting disrupted WM integrity. With the low FA characteristic of TBI, “dropouts” make tractography difficult, but our current method is somewhat robust to these FA dropouts, by virtue of using multiple templates or atlases. We investigated tract-based indices of WM integrity using autoMATE across 4 fiber angle turning thresholds, finding slightly stronger group difference effects at the lowest threshold (30°). Overall, group differences were greater in the chronic phase than in the post-acute phase, which may suggest ongoing and progressive injury. Our planned longitudinal analyses will address these questions.
ACKNOWLEDGMENTS
This study was supported by the NICHDS (R01 HD061504). ELD, YJ, JV, LZ, and PT are also supported by NIH grants to PT: U54 EB020403 (BD2K), R01 EB008432, R01 AG040060, and R01 NS080655. CCG is supported by the UCLA BIRC, NS027544, NS05489, Child Neurology Foundation, and the Jonathan Drown Foundation. Scanning was supported by the Staglin IMHRO Center for Cognitive Neuroscience. We gratefully acknowledge the contributions of Alma Martinez and Alma Ramirez in assisting with participant recruitment and study coordination. We thank the participants and their families for contributing their time to this study.
References
- 1.Giza CC, et al. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J Neurotrauma. 2006;23:950–961. doi: 10.1089/neu.2006.23.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wedeen VJ, et al. The geometric structure of the brain fiber pathways. Science. 2012;335:1628–1634. doi: 10.1126/science.1215280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin Y, et al. Labeling white matter tracts in HARDI by fusing multiple tract atlases with applications to genetics. ISBI. 2013:512–515. doi: 10.1109/ISBI.2013.6556524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. NeuroImage. 2010;52:1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avants BB, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y, et al. Automatic clustering of white matter fibers in brain diffusion MRI with an application to genetics. NeuroImage. 2014;100:75–90. doi: 10.1016/j.neuroimage.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, et al. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J Neurotrauma. 24:753–765. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- 8.Wilde EA, et al. Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: changes within an 18 month post-injury interval. Brain Imaging and Behavior. 6:404–416. doi: 10.1007/s11682-012-9150-y. [DOI] [PubMed] [Google Scholar]