Abstract
Aims:
To assess the deproteinizing effect of bromelain enzyme and compare it with 5% sodium hypochlorite (NaOCl) on shear bond strength before application of the adhesive system.
Materials and Methods:
A total of 30 extracted human premolars were divided into three groups, each one consisted of 10 teeth. The occlusal surface was wet ground to expose superficial dentin. In Group 1, teeth were etched; in Group 2, teeth were etched and deproteinized with bromelain enzyme; in Group 3, teeth were etched and deproteinized with 5% NaOCl. Upon completion of the adhesive procedures, resin composite was inserted into the plastic tube and light-polymerized. All specimens were stored at 37°C in water for 24 h, and the specimens were transferred to the universal testing machine, and then subjected to shear bond strength analysis at a crosshead speed of 1.0 mm/min.
Statistical Analysis Used:
Data were statistically analyzed using one-way analysis of variance and unpaired t-test at a significance level of 0.05. The statistical analysis was performed using SPSS version 12.0.1 for Windows (SPSS Inc., Chicago, IL, USA).
Results:
The bond strength results were significantly influenced by the application of bromelain enzyme. Statistically significant differences were not demonstrated in control group and NaOCl-treated group. The highest bond strength was seen in bromelain enzyme-treated group.
Conclusions:
Within the limitations of the present study, it was concluded that removal of unsupported collagen fiber with bromelain enzyme after acid etching results in improved bond strength.
Keywords: Bromelain enzyme, shear bond strength, sodium hypochlorite
INTRODUCTION
The creation of a hybrid layer or resin-infiltrated dentin-layers (RIDLs) is considered as the most efficient mechanism of adhesion in recent dentin bonding agents (DBAs).[1,2]
However, controversy exists regarding the role of collagen fibrils on resin adhesion and sealing efficiency of DBAs. Many restorations bonded with different DBAs have shown microleakage.[3] Nanoleakage, a microscopic leakage inside the thickness of RIDL, was first described by Sano et al., collagen matrix degradation occurs either by the breakdown of the polymer phase or collagen fibrils in the hybrid layer. Also, exposure of collagen matrix of dentin by acid etching may activate matrix metalloproteinase (MMP).[4] In order to prevent this biodegradation, various techniques have been employed such as the demineralized collagen removal[5,6] and the use of MMPs inhibitors.[7]
The use of sodium hypochlorite (NaOCl) to deproteinize acid-etched dentin has several disadvantages. It forms a fragility zone and is cytotoxic with a bad taste and odor. As the dentinal depth increases, the negative impact of NaOCl increases. These disadvantages have led to the search for better means to deproteinized dentin.[8]
Newer techniques for removing collagen network include deproteinizing enzymes such as collagenase or bromelain enzyme.[8]
Bromelain is a proteolytic enzyme (proteases) which belongs to a group of protein-digesting enzymes obtained commercially from the fruit or stem of pineapple. The function of proteases is to catalyze the hydrolysis of proteins to give amino acids.[9]
Bromelain enzyme can reduce nanoleakage after collagen removal as compared to NaOCl[8] but there has not been a study to see its effectiveness in improving the bond strength.
Therefore, the aim of this study was to assess the deproteinizing effect of bromelain enzyme and compare it with 5% NaOCl on shear bond strength before application of the adhesive system.
MATERIALS AND METHODS
Thirty extracted human premolar teeth were taken and stored in 0.1% thymol solution until they were subjected to use. Roots were embedded in self-cure acrylic resin. Samples were wet ground on the occlusal surface using a series of silicon carbide discs to prepare flat superficial dentin. Teeth were divided into three groups based on the method of dentin deproteinization.
Group 1: Teeth were etched with 37% phosphoric acid (Scotchbond Multi-purpose Etchant, 3M) for 15 s and then rinsed with water and blot dried.
Group 2: Teeth were etched with 37% phosphoric acid for 15 s, rinsed with water, blot dried and deproteinized with bromelain enzyme (Bangalore Sales Corporation) for 1 min. The bromelain enzyme was washed off with distilled water.
Group 3: Teeth were etched with 37% phosphoric acid for 15 s, rinsed with water, blot dried and deproteinized with 5% NaOCl (Nice Chemicals Pvt. Ltd.) for 1 min. NaOCl was washed off with distilled water.
Fifth generation DBA, Adper single bond 2 (3M ESPE), was applied according to manufacturer's instructions. Upon completion of the adhesive procedures, standardized plastic tubes were placed onto the dentin surface. The resin composite Filtek Z-250 (3M ESPE) was inserted into the plastic tube and light-polymerized. The plastic tube was removed to expose the resin cylinder. All specimens were stored at 37°C in water for 24 h before testing, to simulate the oral environment. After storage, the specimens were transferred to the universal testing machine individually and then subjected to shear bond strength analysis at a crosshead speed of 1.0 mm/min.
Statistical analysis
Data were statistically analyzed using one-way analysis of variance for mean comparison among groups and unpaired t-test for mean comparison of shear bond strength between groups at a significance level of 0.05. The statistical analysis was performed using SPSS version 12.0.1 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
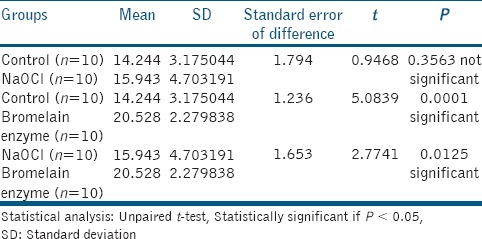
The bond strength results were significantly influenced by the application of bromelain enzyme (P < 0.05) as shown in Table 1. Statistically significant differences were not demonstrated in control group and NaOCl treated group as shown in Table 2. The highest bond strength was seen in bromelain enzyme-treated group.
Table 1.
Mean value of shear bond strength in MPa of all three tested groups (n = 30)
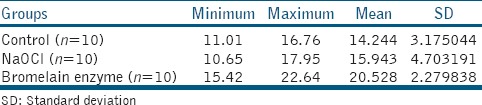
Table 2.
Mean comparison of shear bond strength (MPa) between groups
DISCUSSION
Adhesion to tooth structure depends on various factors including the type of the adhesive system, tooth structure, load cycling, and surface contaminations.[10] Inadequate adhesion of composite resin restorations to dentin results in reduced retention, microleakage, and finally recurrent caries.[11] Progressive loss of bond strength of etch-and-rinse adhesives has been demonstrated in some studies.[12,13] One of the factors that is responsible for this degradation is incomplete infiltration of resin monomers into unsupported collagen networks after acid etching with strong acids which produces a zone of collagen without any support of either minerals or resins in the base of the hybrid layer.[12]
Questions have been raised whether bond strength tests can predict the clinical behavior of adhesively bonded composite resin restorations since they do not represent the complex clinical failure mechanism. In micro tensile tests, the fracture starts at the weakest part of the bond. A disadvantage of these tests is the high technique sensitivity.[14] In shear test, the fracture does not start at the weakest part of the bond, but always at the insertion point of the load.[15] The preference for the conventional shear test instead of micro shear test is justified because they are easy to perform, requiring minimal equipment, and specimen preparation.[14] Also, a comparative bond strength study involving three adhesive systems has shown similarities between “macro” and “micro” counterparts regarding material ranking.[14]
Numerous studies have evaluated the effects of NaOCl on adhesion process, and different results have been achieved.[16,17,18] Some studies, however, have shown lower bond strength using NaOCl.[17] This decrease in bond strength can be attributed to the generation of oxygen after the disintegration of NaOCl into NaCl and O2. The released oxygen in this chemical reaction prevents the polymerization of adhesive agents. These reactive residual free radicals in NaOCl-treated dentin compete with the propagation of vinyl-free radicals generated during light activation of the adhesive system, resulting in premature chain termination and incomplete polymerization.[19]
However, NaOCl may exert different effects on bond strength depending on the chemical structure of the adhesive system and the type of the initiator in the adhesive system used.[10]
In this present study, bromelain enzyme performed better which could be because of reduced nanoleakage as shown by the previous study. It has better effectiveness in removing unsupported collagen matrix as compared to NaOCl, and lower nanoleakage is seen.[8]
This could be because of the depletion of collagen from the surface of acid-etched dentin resulting in increased permeability of dentin substrate due to the enlargement of dentinal tubules near the outer dentin surface. This enhances the spreading and diffusing of adhesive monomers through dentin.[20,21] The surface energy of the dentin is improved, because the hydroxyapatite has a high surface energy substrate while collagen has a low energy surface, and this leads to enhanced diffusion of adhesive monomers through dentin.[22,23] Also, the dentin is very porous and rough with many lateral branches of tubules which are detectable in main tubules, which may contribute to the increase in the spreading of adhesive monomers through dentin.[24,25]
CONCLUSION
Within the limitations of the present study, it was concluded that removal of unsupported collagen fibers with bromelain enzyme after acid etching results in improved bond strength.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 2.Barkmeier WW, Los SA, Triolo PT., Jr Bond strengths and SEM evaluation of Clearfil Liner Bond 2. Am J Dent. 1995;8:289–93. [PubMed] [Google Scholar]
- 3.Tay FR, Gwinnett AJ, Pang KM, Wei SH. Variability in microleakage observed in a total-etch wet-bonding technique under different handling conditions. J Dent Res. 1995;74:1168–78. doi: 10.1177/00220345950740050501. [DOI] [PubMed] [Google Scholar]
- 4.Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: Leakage within the hybrid layer. Oper Dent. 1995;20:18–25. [PubMed] [Google Scholar]
- 5.Perdigão J, Thompson JY, Toledano M, Osorio R. An ultra-morphological characterization of collagen-depleted etched dentin. Am J Dent. 1999;12:250–5. Erratum in: Am J Dent 1999;12:308. [PubMed] [Google Scholar]
- 6.Uceda-Gómez N, Loguercio AD, Moura SK, Grande RH, Oda M, Reis A. Long-term bond strength of adhesive systems applied to etched and deproteinized dentin. J Appl Oral Sci. 2007;15:475–9. doi: 10.1590/S1678-77572007000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley D, Tay F, et al. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. Eur J Oral Sci. 2011;119:79–85. doi: 10.1111/j.1600-0722.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayem RN, Tameesh MA. A new concept in hybridization: Bromelain enzyme for deproteinizing dentin before application of adhesive system. Contemp Clin Dent. 2013;4:421–6. doi: 10.4103/0976-237X.123015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavan R, Jain S, Shraddha, Kumar A. Properties and therapeutic application of bromelain: A review. Biotechnol Res Int 2012. 2012:976203. doi: 10.1155/2012/976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasraei S, Azarsina M, Khamverdi Z. Effect of Ethylene diamine tetra acetic acid and sodium hypochlorite solution conditioning on microtensile bond strength of one-step self-etch adhesives. J Conserv Dent. 2013;16:243–6. doi: 10.4103/0972-0707.111324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue G, Nikaido T, Foxton RM, Tagami J. The acid-base resistant zone in three dentin bonding systems. Dent Mater J. 2009;28:717–21. doi: 10.4012/dmj.28.717. [DOI] [PubMed] [Google Scholar]
- 12.Jacques P, Hebling J. Effect of dentin conditioners on the microtensile bond strength of a conventional and a self-etching primer adhesive system. Dent Mater. 2005;21:103–9. doi: 10.1016/j.dental.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent Mater. 2005;21:864–81. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Braga RR, Meira JB, Boaro LC, Xavier TA. Adhesion to tooth structure: A critical review of “macro” test methods. Dent Mater. 2010;26:e38–49. doi: 10.1016/j.dental.2009.11.150. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe I, Nakabayashi N. Measurement methods for adhesion to dentine: The current status in Japan. J Dent. 1994;22:67–72. doi: 10.1016/0300-5712(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 16.Saboia VP, Rodrigues AL, Pimenta LA. Effect of collagen removal on shear bond strength of two single-bottle adhesive systems. Oper Dent. 2000;25:395–400. [PubMed] [Google Scholar]
- 17.Fuentes V, Ceballos L, Osorio R, Toledano M, Carvalho RM, Pashley DH. Tensile strength and microhardness of treated human dentin. Dent Mater. 2004;20:522–9. doi: 10.1016/j.dental.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Cecchin D, Farina AP, Galafassi D, Barbizam JV, Corona SA, Carlini-Júnior B. Influence of sodium hypochlorite and edta on the microtensile bond strength of a self-etching adhesive system. J Appl Oral Sci. 2010;18:385–9. doi: 10.1590/S1678-77572010000400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai SC, Mak YF, Cheung GS, Osorio R, Toledano M, Carvalho RM, et al. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001;80:1919–24. doi: 10.1177/00220345010800101101. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa SV, Safavi KE, Spångberg SW. Influence of sodium hypochlorite on the permeability and structure of cervical human dentine. Int Endod J. 1994;27:309–12. doi: 10.1111/j.1365-2591.1994.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 21.Inaba D, Iijima Y, Takagi O, Ruben J, Arends J. The influence of air-drying on hyper-remineralization of demineralized dentine: A study on bulk as well as on thin wet section of bovine dentine. Caries Res. 1995;29:231–6. doi: 10.1159/000262074. [DOI] [PubMed] [Google Scholar]
- 22.De Castro AK, Hara AT, Pimenta LA. Influence of collagen removal on shear bond strength of one-bottle adhesive systems in dentin. J Adhes Dent. 2000;2:271–7. [PubMed] [Google Scholar]
- 23.Dayem RN. Assessment of the penetration depth of dental adhesives through deproteinized acid-etched dentin using neodymium: Yttrium-aluminum-garnet laser and sodium hypochlorite. Lasers Med Sci. 2010;25:17–24. doi: 10.1007/s10103-008-0589-4. [DOI] [PubMed] [Google Scholar]
- 24.Inai N, Kanemura N, Tagami J, Watanabe LG, Marshall SJ, Marshall GW. Adhesion between collagen depleted dentin and dentin adhesives. Am J Dent. 1998;11:123–7. [PubMed] [Google Scholar]
- 25.Ferrari M, Mason PN, Vichi A, Davidson CL. Role of hybridization on marginal leakage and bond strength. Am J Dent. 2000;13:329–36. [PubMed] [Google Scholar]


