Abstract
Context:
Where nonsurgical endodontic intervention is not possible, or it will not solve the problem, surgical endodontic treatment must be considered. A major cause of surgical endodontic failures is an inadequate apical seal, so the use of the suitable substance as root-end filling material that prevents egress of potential contaminants into periapical tissue is very critical.
Aims:
The aim of the present ex-vivo study was to compare and evaluate the three root-end filling materials of mineral trioxide aggregate (MTA) family (white MTA [WMTA], grey MTA [GMTA] and Portland cement [PC]) for their marginal adaptation at the root-end dentinal wall using scanning electron microscopy (SEM).
Materials and Methods:
Sixty human single-rooted teeth were decoronated, instrumented, and obturated with Gutta-percha. After the root-end resection and apical cavity preparation, the teeth were randomly divided into three-experimental groups (each containing 20 teeth) and each group was filled with their respective experimental materials. After longitudinal sectioning of root, SEM examination was done to determine the overall gap between retrograde materials and cavity walls in terms of length and width of the gap (maximum) at the interface. Descriptive statistical analysis was performed to calculate the means with corresponding standard errors, median and ranges along with an analysis of variance and Tukey's test.
Results:
The least overall gap was observed in GMTA followed by PC and WMTA. While after statistically analyzing the various data obtained from different groups, there was no significant difference among these three groups in terms of marginal adaptation.
Conclusion:
GMTA showed the best overall adaptation to root dentinal wall compared to PC and WMTA. Being biocompatible and cheaper, the PC may be an alternative but not a substitute for MTA.
Keywords: Grey mineral trioxide aggregate; marginal adaptation, Portland cement, root-end filling materials, white mineral trioxide aggregate
INTRODUCTION
Where nonsurgical intervention is not possible, or it will not solve the problem, surgical endodontic treatment must be considered. This can be defined as an endodontic surgical procedure which involves root-end resection, apical curettage, and root-end filling.[1] Because most endodontic failures occurs as a result of leakage of irritants from pathologically involved root canals, the root-end filling materials should provide an apical seal to an otherwise unobturated root canal or improve the seal of existing root canal filling materials and be biocompatible with the periradicular tissues.[2]
Once the root-end preparation has been completed, a suitable root-end filling material must be chosen. According to Gartner and Dorn,[3] an ideal material to seal the root-end cavities should prevent the leakage of microorganisms and their by-products into the periradicular tissues. Numerous materials have been suggested as root-end filling materials[2,4] like Gutta-percha (GP), amalgam, polycarboxylate cement, IRM cement, EBA cement, Cavit, glass-ionomer cement, composite resins, mineral trioxide aggregate (MTA) and Portland cement (PC).
Many investigations have compared MTA as a root-end filling material with other currently used materials, and numerous investigations have proved the biocompatibility and superior apical sealing capacity of MTA when compared with other root-end filling materials.[4]
MTA was given approval for endodontic use by the USA Food and Drug Administration in 1998.[5] Two commercial forms of MTA have been available in either the grey or white forms. Recently, MTA-Angelus (Angelus Solucoes Odontologicas Londrina, Brazil) has become available. The use of MTA as a root-end filling material was identified because the material is the hydraulic cement that sets in the presence of water.[6]
MTA materials are a mixture of refined PC and bismuth oxide and are reported to contain trace amounts of SiO2, CaO, MgO, K2SO4 and Na2 SO4. The major component, PC, is a mixture of dicalcium silicate, tricalcium silicate, tricalcium aluminate, gypsum, and tetracalcium alumino-ferrite, although to a lesser extent. Although, the physical properties and biocompatibility of MTA are well documented, there have been fewer studies evaluating the white MTA (WMTA). Also, studies comparing the properties of grey MTA (GMTA) and WMTA with PC had conflicting results.[7]
In view of above goal of searching an ideal root-end filling material, the present ex-vivo study was undertaken to analyze the marginal adaptation of three different root-end filling materials of MTA family, that is, GMTA, WMTA, and PC (Birla white, UltraTech Cement Ltd., India) in terms of length and width of the gap between the material and root-end dentinal wall using scanning electron microscopy (SEM).
MATERIALS AND METHODS
Sixty human single-rooted teeth, freshly extracted for periodontal cause at the Department of Oral and Maxillofacial Surgery, Dr. R. Ahmed Dental College and Hospital, Kolkata, were selected. Teeth were stored in a solution of 2% formaldehyde after cleaning in distill water. Teeth were excluded having an immature apex, root fracture, root resorption, multiple canals on radiograph and with significant apical curvature.
Selected teeth were decoronated with a low-speed diamond cutting disc to create a standardized length of 14 mm. The root canal preparation was done with ProTaper rotary system (Dentsply) and irrigation was done with 0.5% sodium hypochlorite during preparation, followed by AH Plus (Dentsply India Pvt., Ltd.) sealer application within canal and obturation with laterally condensed GP After obturation, teeth were stored in 100% humidity for 48 h to prevent fragility. Then, 3-mm segment of root-end was resected perpendicular to the long axis of root using a diamond bur, followed by root-end cavity preparation (3 mm depth and 1 mm diameter) with a #170 fissure bur under water spray, followed by drying with paper points.
Sixty teeth were randomly divided into three-group of 20 teeth each and named as Group G (GMTA), Group W (WMTA) and Group P (PC). In all groups, the material were mixed according to manufacturer's instructions and placed in the cavity by a carrier and condensed thoroughly with a plugger, leaving a little additional material above the cavity margins and finally, used the burnisher for removal of excess material. Then, teeth were stored in 100% humidity at 37°C for 24 h to allow complete setting of the materials.
Now, each root was resected longitudinally with the help of low-speed diamond disc into 2-halves (40 specimens in each group). After mounting each specimen on aluminum stub sputter coated with gold with the help of equipment, they were assessed using SEM at ×100 and ×400. The higher magnification was used to make the measurements more convenient. The maximum value of width and length of gaps in both sides of each half of the specimen was measured and recorded separately. Finally, the mean overall gap was calculated for each group separately by taking the mean of their combined (additive) mean length and mean width.
Descriptive statistical analysis was performed to calculate the means with corresponding standard errors, median, and ranges. Also, one-way analysis of variance (ANOVA), followed by Tukey's Test was performed with the help of Critical Difference (CD) or least significant difference at 5% and 1% level of significance to compare the mean values. P < 0.05 was considered to be statistically significant.
RESULTS
The SEM observation of the longitudinal sections of the root-end-filled teeth revealed the existence of gaps between the retrograde material and dentinal wall in the majority of cases. Variations were observed in these specimens in terms of the width and length of gaps.
Gap (in terms of length) on both left and right side of the groups
The mean length of the gap in Group G was 414.28 μm. This was less than Group W (645.46 μm) and Group P (608.54 μm). Although, GMTA had a better length-wise adaptation than other materials, there was no significant difference between the three groups (P > 0.05).
Gap (in terms of width) on both left and right side of the groups
The mean width of gap in Group W was 15.88 μm, which was less than Group G (19.54 μm) and Group P (26.00 μm), but there was no significant difference between the three groups (P > 0.05).
Overall gap (in terms of combined length and width) in the groups
The mean of the overall gap in Group G was 216.91 μm. In Group W, it was 330.67 μm and in Group P, it was 317.27 μm. Although, GMTA exhibited a better marginal adaptation than the other materials, there was no significant difference between the three groups (P > 0.05).
The distribution of mean overall gap, ANOVA test, CD, and difference of means and level of significance as shown in Tables 1–4 respectively. Also, the graph for distribution of mean gap (in terms of the overall gap) has been shown in the Graph 1.
Table 1.
Distribution of overall gap (in μm) in the three groups
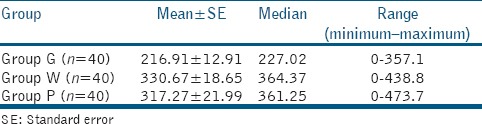
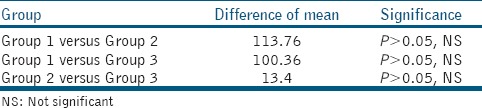
Table 4.
Difference of means and level of significance
Graph 1.
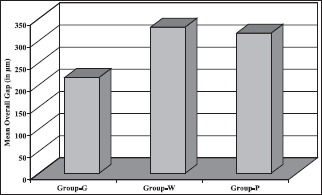
Distribution of mean overall gap in the groups
Table 2.
ANOVA table for mean overall gap (in μm)
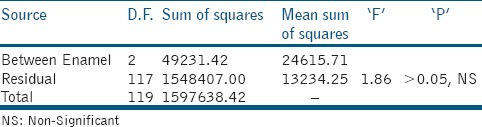
Table 3.
Critical difference (CD)

DISCUSSION
The surgical endodontics is often chosen when it would otherwise be difficult or impossible to clean and shape the root canal system or where there was irreversible change in the periradicular tissues that could not be resolved by a nonsurgical approach.[8] The main objective of a root-end filling material is to provide an apical seal that prevents the egress of bacteria and the diffusion of bacterial products from the root canal system into the periapical tissues.
It has been shown that MTA allows regeneration of the periradicular tissues rather than repair.[9] In-vivo studies have shown that, as a root-end filling material, it has the capacity to induce bone, periodontal ligament and cementum formation.[10] MTA also has potential bactericidal effects due to its release of hydroxyl ions and the production of a high pH environment.[11] Another advantage of MTA is its ability to set in the presence of moisture. It has been suggested that MTA has many of the properties of the ideal root-end filling material.[12]
PC contains the same chemical elements as MTA, except that MTA also contains bismuth.[13,14,15] This has generated interest in the evaluation of PC as an alternative to MTA. The biocompatibility of MTA and PC has been compared, and both materials were found to be biocompatible.[16,17]
Both MTA and PC are bioactive materials. The biocompatibility of the materials had originally been attributed to the chemical similarity to tooth hard tissues namely calcium phosphate. The similarity of action of both MTA and PC to calcium hydroxide had been postulated.[18]
In a study conducted by Holland et al.[19] showed that MTA and PC have similar comparative results when used in direct pulp protection after pulpotomy. They revealed that tissue reaction to MTA and PC was the same. On histomorphological analysis, there was a complete tubular hard tissue bridge in almost all specimens.
Several studies have indicated that MTA exhibits significantly less leakage than the other materials.[20,21,22] Because of the similarity between the components of MTA and PC, it would be expected that these materials have similar properties and effects.
The quality of apical seal obtained by root-end filling materials has been assessed by studies using dye penetration, radioisotope penetration, bacterial penetration, electrochemical means, fluid filtration technique, confocal microscopy and SEM, but none of these studies confirmed a correlation between marginal adaptation and micro-leakage.[23] Yoshimura et al.,[24] Abdal and Retief[25] and Xavier et al.[26] found a lack of such a correlation, whereas Shani et al.,[27] Stabholz et al.[28] and Torabinejad et al.[23] agree with this correlation.
Marginal adaptation of retrograde materials has been investigated in several studies. Moodnik et al.[29] in a SEM study in 1975 reported that the gap between amalgam and dentinal walls was 6–150 μm. As per a study by Torabinejad et al.,[23] they compared the marginal adaptation of various retrograde materials (MTA, Amalgam, Super-EBA, and IRM) and concluded that MTA has the best adaptation.
In the present ex-vivo study, the SEM observation (at ×100 and ×400) of the longitudinal sections of the root-end-filled teeth of three-different groups for the marginal adaptation revealed the existence of gaps between retrograde material and dentinal wall in the majority of cases [Figure 1].
Figure 1.
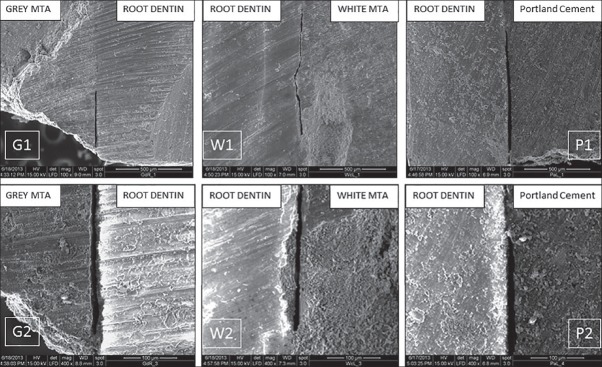
Scanning electron microscopy pictures of G1 and G2 (Group G specimen showing gap at ×100 and ×400 respectively), W1 and W2 (Group W specimen showing gap at ×100 and ×400 respectively) and P1 and P2 (Group P specimen showing gap at ×100 and ×400 respectively)
The present study exhibited a larger mean of gap size than the study of Shipper et al.,[30] possibly because of the difference in the method of sectioning. Shipper et al. sectioned the specimens after blocking, but in the current study sectioning was done without blocking. The force and vibration due to sectioning may cause increases in the mean gap size.
As per study of Xavier et al.[26] who evaluated the root-end sealing ability through dye leakage and the marginal adaptation through SEM of some root-end filling materials (MTA-Angelus, Super-EBA and Vitremer), showed significant differences among the three materials in relation to the sealing ability. Concerning marginal adaptation, MTA-angelus presented the best results.
Matt et al.[31] also showed GMTA demonstrated significantly less leakage than WMTA. Conflicting results have been reported by Islam et al.[7] who compared in-vitro sealing ability of GMTA, WMTA, grey PC (GPC) and white PC (WPC) when used as root-end filling materials. None of the teeth in any of the test groups showed leakage beyond the retrofilling and they concluded that considering low cost of PC and apparently similar properties, it is reasonable to consider PC as a possible substitute for MTA in endodontic application. However, they suggest that further tests, especially in-vivo biocompatibility tests, need to be conducted before recommendation of PC as clinical use.
A study by Shahi et al.,[32] compared the marginal adaptation of four root-end filling materials (WMTA, GMTA, WPC and GPC) using SEM they used the ultrasonic tips to prepare the root-end cavity and after root-end filling, they decoronated the teeth samples. After that, they assessed the gap (distance between the material and root dentinal wall) at four corners of each sample under SEM with ×16 power. Their result demonstrated statistically significant difference just between GMTA and GPC groups. Although, the marginal adaptation in WMTA group was better than WPC, but there was not statistically significant difference between them.
Here, in this study, the gap (in terms of maximum length and width) was measured between material and dentinal wall on both the sides (right and left) of specimen after longitudinal sectioning of prepared teeth under SEM at ×100 and ×400. The higher magnifications were used to record the measurements more conveniently.
The results as per observed by Shahi et al.[32] is in support with the results of this study as there is no significant difference between the marginal adaptation of three groups, that is, GMTA, WMTA, and WPC, although, they demonstrated statistically significant difference just between GMTA and GPC groups.
However, most studies were carried out in the controlled laboratory conditions without any contamination. A recent study by Milani et al.[33] was carried out to evaluate the effect of exposure to blood on marginal adaptation and surface microstructure of MTA and they concluded that exposure to blood during MTA setting had a negative effect on marginal adaptation of MTA.
The interaction of MTA with different types of storage media should be considered in studies on the physico-chemical properties of biomaterials. Several investigations showed the formation of apatite crystalline structures as a result of the interaction between MTA and synthetic tissue fluids such as phosphate-buffered saline solution (PBS) which might increase the sealing ability of the biomaterials.[34,35,36]
Considering the marginal adaptation of MTA to root dentin, a recent study[37] using microcomputed tomographic analysis has shown that the type of application technique (either orthograde or retrograde) do not significantly improve the dentin-MTA adaptation, instead with the use of 17% EDTA, a significant improvement could be achieved. In this study, a significant difference in the gap volumes was observed between retrograde MTA (RMTA filling without etching) and etched RMTA (ERMTA) (etching before retrograde filling) where etching significantly improved the MTA-dentin adaptation.
Besides marginal adaptation, when considering other physico-chemical properties, a recent study by Dorileo et al.[38] resulted that WPC had the highest solubility, while MTA-based cement had lowest values, and there were no differences among the pH and electrical conductivity analyses. Also, WPC showed highest dimensional alteration value and only MTA-based cement met the ANSI/ADA recommendations regarding radiopacity. Therefore, it can be simply stated that cheaper PC is having inferior physicochemical properties in some aspects than costlier MTA and inferior marginal adaptation than GMTA yet nonsignificant as shown in the present study.
CONCLUSION
Within the limitations of this study, GMTA showed the best adaptation to root dentinal wall compared to PC and WMTA. But still, other factors which may enhance the marginal adaptation of these materials should also be considered. Being biocompatible and cheaper, the PC can be an alternative but not a substitute for MTA.
It would be better to perform some additional in-vivo studies to prove the efficacy of these materials, especially the PC since, under natural surroundings, these may have some effects over their long-term marginal adaptation.
Financial support and sponsorship
Department of Conservative Dentistry and Endodontics, Dr. R. Ahmed Dental College and Hospital, Kolkata - 700 014 and Central Instrument Facility, Main Campus, Bose Institute, Kolkata - 700 009.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fahey T, Connor NO, Walker T, Chin-Shong D. Surgical endodontics: A review of current best practice. Oral Surg. 2011;4:97–104. [Google Scholar]
- 2.Torabinejad M, Pitt Ford TR. Root end filling materials: A review. Endod Dent Traumatol. 1996;12:161–78. doi: 10.1111/j.1600-9657.1996.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 3.Gartner AH, Dorn SO. Advances in endodontic surgery. Dent Clin North Am. 1992;36:357–78. [PubMed] [Google Scholar]
- 4.Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005;31:665–8. doi: 10.1097/01.don.0000157993.89164.be. [DOI] [PubMed] [Google Scholar]
- 5.Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent Mater. 2008;24:149–64. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: A review of the constituents and biological properties of the material. Int Endod J. 2006;39:747–54. doi: 10.1111/j.1365-2591.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- 7.Islam I, Chng HK, Yap AU. Comparison of the root-end sealing ability of MTA and Portland cement. Aust Endod J. 2005;31:59–62. doi: 10.1111/j.1747-4477.2005.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 8.Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–81. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- 9.von Arx T, Salvi GE, Janner S, Jensen SS. Gingival recession following apical surgery in the esthetic zone: A clinical study with 70 cases. Eur J Esthet Dent. 2009;4:28–45. [PubMed] [Google Scholar]
- 10.Baek SH, Plenk H, Jr, Kim S. Periapical tissue responses and cementum regeneration with amalgam, SuperEBA, and MTA as root-end filling materials. J Endod. 2005;31:444–9. doi: 10.1097/01.don.0000148145.81366.a5. [DOI] [PubMed] [Google Scholar]
- 11.Fridland M, Rosado R. MTA solubility: A long term study. J Endod. 2005;31:376–9. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Kratchman S. Modern endodontic surgery concepts and practice: A review. J Endod. 2006;32:601–23. doi: 10.1016/j.joen.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri J. The chemical composition of mineral trioxide aggregate. J Conserv Dent. 2008;11:141–3. doi: 10.4103/0972-0707.48834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrela C, Bammann LL, Estrela CR, Silva RS, Pécora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J. 2000;11:3–9. [PubMed] [Google Scholar]
- 15.Funteas UR, Wallace JA, Fochtman EW. A comparative analysis of Mineral Trioxide Aggregate and Portland cement. Aust Endod J. 2003;29:43–4. doi: 10.1111/j.1747-4477.2003.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah D, Ford TR, Papaioannou S, Nicholson J, McDonald F. An evaluation of accelerated Portland cement as a restorative material. Biomaterials. 2002;23:4001–10. doi: 10.1016/s0142-9612(02)00147-3. [DOI] [PubMed] [Google Scholar]
- 17.Saidon J, He J, Zhu Q, Safavi K, Spångberg LS. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:483–9. doi: 10.1067/moe.2003.20. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri J. A review of the methods used to study biocompatibility of Portland cement-derived materials used in dentistry. Malta Med J. 2006;18:09–14. [Google Scholar]
- 19.Holland R, de Souza V, Nery MJ, Faraco Júnior IM, Bernabé PF, Otoboni Filho JA, et al. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, Portland cement or calcium hydroxide. Braz Dent J. 2001;12:3–8. [PubMed] [Google Scholar]
- 20.Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root end filling materials: Effects of blood contamination. J Endod. 1994;20:159–63. doi: 10.1016/S0099-2399(06)80326-2. [DOI] [PubMed] [Google Scholar]
- 21.Torabinejad M, Rastegar AF, Kettering JD, Pitt Ford TR. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J Endod. 1995;21:109–12. doi: 10.1016/s0099-2399(06)80433-4. [DOI] [PubMed] [Google Scholar]
- 22.Tang HM, Torabinejad M, Kettering JD. Leakage evaluation of root end filling materials using endotoxin. J Endod. 2002;28:5–7. doi: 10.1097/00004770-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Torabinejad M, Smith PW, Kettering JD, Pitt Ford TR. Comparative investigation of marginal adaptation of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995;21:295–9. doi: 10.1016/S0099-2399(06)81004-6. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura M, Marshall FJ, Tinkle JS. In vitro quantification of the apical sealing ability of retrograde amalgam fillings. J Endod. 1990;16:5–12. [PubMed] [Google Scholar]
- 25.Abdal AK, Retief DH. The apical seal via the retrosurgical approach. I.A. preliminary study. Oral Surg Oral Med Oral Pathol. 1982;53:614–21. doi: 10.1016/0030-4220(82)90351-6. [DOI] [PubMed] [Google Scholar]
- 26.Xavier CB, Weismann R, de Oliveira MG, Demarco FF, Pozza DH. Root-end filling materials: Apical microleakage and marginal adaptation. J Endod. 2005;31:539–42. doi: 10.1097/01.don.0000152297.10249.5a. [DOI] [PubMed] [Google Scholar]
- 27.Shani J, Friedman S, Stabholz A, Abed J. A radionuclide model for evaluating sealability of retrograde filling materials. Int J Nucl Med Biol. 1984;11:46–52. doi: 10.1016/0047-0740(84)90031-7. [DOI] [PubMed] [Google Scholar]
- 28.Stabholz A, Friedman S, Abed J. Marginal adaptation of retrograde fillings and its correlation with sealability. J Endod. 1985;11:218–23. doi: 10.1016/s0099-2399(85)80063-7. [DOI] [PubMed] [Google Scholar]
- 29.Moodnik RM, Levey MH, Besen MA, Borden BG. Retrograde amalgam filling: A scanning electron microscopic study. J Endod. 1975;1:28–31. doi: 10.1016/S0099-2399(75)80247-0. [DOI] [PubMed] [Google Scholar]
- 30.Shipper G, Grossman ES, Botha AJ, Cleaton-Jones PE. Marginal adaptation of mineral trioxide aggregate (MTA) compared with amalgam as a root-end filling material: A low-vacuum (LV) versus high-vacuum (HV) SEM study. Int Endod J. 2004;37:325–36. doi: 10.1111/j.0143-2885.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- 31.Matt GD, Thorpe JR, Strother JM, McClanahan SB. Comparative study of white and gray mineral trioxide aggregate (MTA) simulating a one-or two-step apical barrier technique. J Endod. 2004;30:876–9. doi: 10.1097/01.don.0000136213.93171.45. [DOI] [PubMed] [Google Scholar]
- 32.Shahi S, Yavari HR, Eskandarinezhad M, Kashani A, Rahimi S, Sadrhaghighi H. Comparative investigation of marginal adaptation of mineral trioxide aggregate (MTA) and Portland cement as root-end filling materials: A scanning electron microscopy (SEM) study. Afr J Biotechnol. 2011;10:16084–8. [Google Scholar]
- 33.Salem Milani A, Rahimi S, Froughreyhani M, Vahid Pakdel M. Effect of blood contamination on marginal adaptation and surface microstructure of mineral trioxide aggregate: A SEM study. J Dent Res Dent Clin Dent Prospects. 2013;7:157–63. doi: 10.5681/joddd.2013.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shokouhinejad N, Nekoofar MH, Razmi H, Sajadi S, Davies TE, Saghiri MA, et al. Bioactivity of EndoSequence root repair material and bioaggregate. Int Endod J. 2012;45:1127–34. doi: 10.1111/j.1365-2591.2012.02083.x. [DOI] [PubMed] [Google Scholar]
- 35.Ghorbanzadeh A, Shokouhinejad N, Fathi B, Raoof M, Khoshkhounejad M. An in vitro comparison of marginal adaptation of MTA and MTA-like materials in the presence of PBS at one-week and two-month intervals. J Dent (Tehran) 2014;11:560–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white portland cement with dentin in a phosphate-containing fluid. J Endod. 2009;35:731–6. doi: 10.1016/j.joen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Al Fouzan K, Awadh M, Badwelan M, Gamal A, Geevarghese A, Babhair S, et al. Marginal adaptation of mineral trioxide aggregate (MTA) to root dentin surface with orthograde/retrograde application techniques: A microcomputed tomographic analysis. J Conserv Dent. 2015;18:109–13. doi: 10.4103/0972-0707.153069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorileo MC, Pedro FL, Bandeca MC, Guedes OA, Villa RD, Borges AH. Comparative analysis of physicochemical properties of root perforation sealer materials. Restor Dent Endod. 2014;39:201–9. doi: 10.5395/rde.2014.39.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]


