Abstract
Background and Aims:
Extracorporeal membrane oxygenation (ECMO) is used during pediatric resuscitation in case of refractory hypoxemia or septic shock under maximum therapy. Previous studies describe calcium homeostasis dysregulation. The aim of this study was to confirmed of calcium homeostasis dysregulation in neonates under ECMO and supposed news explanation.
Subjects and Methods:
From November 2012 to July 2013, we performed a prospective single center observational study. Eleven neonatal patients were included. Blood was obtained before and during ECMO (day 7, 14 and 21) for parathyroid hormone (PTH), protein adjusted serum calcium, ionized calcium, magnesium, and calcitriol levels. All surviving patients underwent a consultation up to 6 months after ECMO weaning.
Results:
During ECMO PTH was inadequately high with normal serum calcium on day 7 (PTH: 73.54 ± 40 ng/l; calcemia: 2.33 ± 0.21 mmol/l), day 14 (PTH: 57.63 ± 29.57 ng/l; calcemia: 2.44 ± 0.43 mmol/l) and day 21 (PTH: 54.93 ± 8.43 ng/l; calcemia: 2.13 ± 0.09 mmol/l). The absence of correlation between serum calcium and PTH levels seem to confirm the dysregulation of PTH - serum calcium metabolism during ECMO. Six months after ECMO weaning, we noticed hypercalcemia with normal PTH.
Conclusions:
We confirmed the existence of severe disturbances of calcium homeostasis in neonates on ECMO and supposed the possible damage of calcium regulation. We did not succeed in finding clear explanations of these disturbances.
Keywords: Calcium, extracorporeal membrane oxygenation, neonates, parathyroid, phosphorus
Introduction
Extracorporeal membrane oxygenation (ECMO) is used as a last resort during pediatric resuscitation in the case of persistent refractory hypoxemia or refractory septic shock under maximum conventional therapy.[1,2] These techniques can lead to major complications such as hemorrhage, thromboembolism, or oxygenator failure.[3,4] However, previous studies also describe recurrent metabolic disturbances such as calcium homeostasis dysregulation.[5,6] The mechanism of these abnormalities remains unclear, but severe consequences of hypercalcemia with nephrocalcinosis are anecdotally observed in surviving children.[7,8] The aim of this study was to confirm and precise calcium homeostasis disturbances during neonatal ECMO.
Subjects and Methods
Patients
From November 2012 to November 2013, we carried out a nonrandomized single-center prospective observational study on newborn patients hospitalized for ECMO in our Paediatric Intensive Care Unit. The type of ECMO to be administered (veno-venous [V-V] ECMO with single or double cannulae, or veno-arterial [V-A] ECMO) was determined according to each child's pathology.
Extracorporeal membrane oxygenation set up
ECMO was set up by trained vascular surgeons at the bedside because of hemodynamic instability. For respiratory indications, ECMO was performed via a single lumen canula inserted in the jugular vein. A nonocclusive roller pump with a stretched raceway tubing (Sofracob®) and an alternative clamp allowed alternative drainage and re-injection.[9] In case of persistent hypoxemia with a need of higher flow, the femoral vein was also cannulated in order to switch from single lumen canula to V-V ECMO. Jugular vein and carotid artery were cannulated for all neonates suffering from septic shock. The membrane oxygenators used were the Medos Hilite 800LT® and Maquet Quadrox-iD pediatric®. The main difference between these two types of membrane oxygenators lies in their conformation, surface, and cost.[10] The ECMO circuit is primed with 165 ml washed packed red blood cells and 85 ml fresh frozen plasma. Finally, 20 mEq of sodium bicarbonates and 330 mg of calcium chloride are added to the circuit prior initiation of ECMO in order to limit the risk of hypocalcemia due to the massive transfusion.
Patient management under extracorporeal membrane oxygenation
Unfractionated heparin was administered to obtain an activated partial thromboplastin time at 1.5–2 times normal for V-V ECMO and 2–3 times normal for V-A ECMO. Weaning from ECMO was based on the patient's capacity to maintain stable hemodynamics, as well as efficient CO2 removal (PaCO2 of 40–50 mmHg), and efficient oxygenation with minimum support.
Data collected
Prior to cannulation, we recorded the following information: Age, gender, neonatal disease, inotrope score (defined as dose of dobutamine μ/kg/mn + dose of epinephrine μ/kg/mn + dose of norepinephrine μ/kg/mn × 100), PaO2 /FiO2 ratio, oxygenation index, the alveolar-arterial difference for oxygen, blood lactate and pH, urine output, diuretic treatment, renal replacement, parathyroid hormone (PTH), 1,25 dihydroxyvitamin D (1,25(OH)2D3) vitamin, 25-hydroxyvitamin D (25(OH)D) vitamin levels, total calcium, ionized calcium, bicarbonate, magnesium, phosphorus, concentrations of albumin and protein in plasma. Urinary calcium, phosphorus, albumin were also collected. All these data were also collected at day 7, day 14, day 21, and day 28 of ECMO. All surviving patients underwent a consultation with a pediatric nephrologist and PTH, 1,25(OH)2D3 vitamin, 25(OH)D vitamin levels, total calcium, ionized calcium, bicarbonate, magnesium, phosphorus, concentrations of albumin and protein in plasma, urinary calcium, phosphorus, albumin were collected, 6 months after intensive care discharge. PTH, 1,25(OH)2D3 vitamin, 25(OH)D vitamin levels were defined by immunoenzymatic assay. Maternal 1,25(OH)2D3 vitamin deficiency was not screened.
Statistics
All of the statistics cited in this study were produced through a Pearson's test. The significance level was set at P < 0.05.
This study was approved by the Institutional Review Board of our hospital as an observational study.
Results
Patients
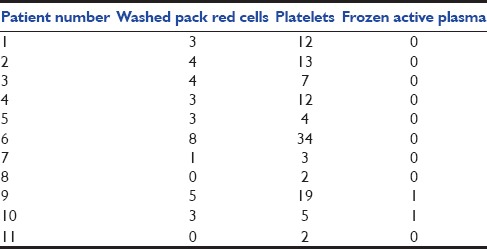
Thirteen patients were enrolled in our study, and 2 patients were excluded because of an early death in the 1st week of ECMO (patients 7 and 8). Indication for ECMO is described in Table 1. Included birth weight was 3.36 ± 0.25 kg (range 3–3.7 kg), and gestational age was 40.4 ± 1.3 weeks (range 37–42). Seven patients were girls and four were boys. Ages at cannulation were 3.5 ± 4 days (range 1–15). Oxygenation index was 64.9 cm d’H2 O/mmHg ± 70 (range 22–263), the alveolar-arterial difference for oxygen was 603.8 ± 15.9 (range 571–623). Inotrope score was 138.18 μ/kg/mn ± 170 (range 0–520). Lactate prior cannulation was 6.1 ± 3.7 mmol/l (range 1.7–11), pH prior cannulation was 7.12 ± 0.16 (range 6.80–7.36). All results are described in Table 2. All patients were treated with inhaled nitric oxide. Four patients received V-A ECMO, 3 patients received V-V ECMO and 4 patients received single lumen V-V ECMO. Six patients suffered from acute renal failure at day 0 (54%) [Table 2]. Continuous renal replacement therapy was used for one patient during 15 days (9%) (patient 6). Duration of ECMO was 7.6 ± 2.7 days (range 5–14). Ten of the 11 patients survived from ECMO. Six of the 10 survivors underwent a consultation 6 months post-ECMO. The total daily doses of calcium, phosphorus, and magnesium are shown in Table 3. Total blood transfusion is shown in Table 4.
Table 1.
Initial pathologies requiring ECMO
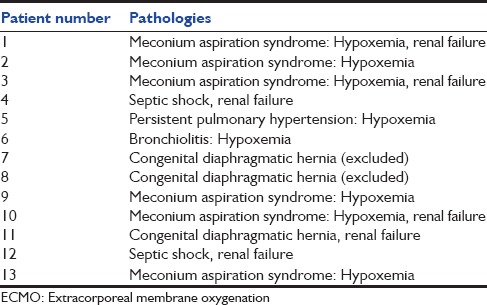
Table 2.
Patient's characteristics before ECMO implantation (11 patients)
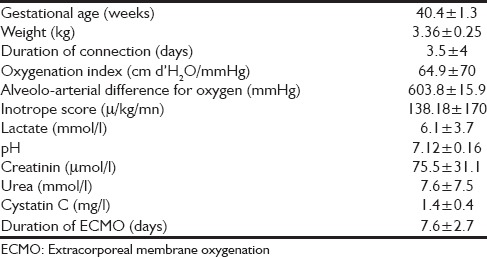
Table 3.
Intakes of calcium, phosphorus, and magnesium before ECMO and on day 7, 14, and 21 post-ECMO implantation (11 patients)
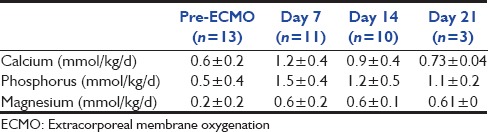
Table 4.
Amount of washed pack red cells, frozen active plasma and platelets transfused
Parathyroid hormone: Calcemia correlation
Prior cannulation, 7 patients presented hypercalcemia associated with a high PTH [Table 5]. There was no correlation between PTH and calcemia on day 7 (r = 0.79), day 14 (z = 0.13) and day 21 (z = 0.2).
Table 5.
Calcium homeostasis parameters pre-ECMO and on day 7, 14, 21, and 6 months post-ECMO weaning (11 patients)
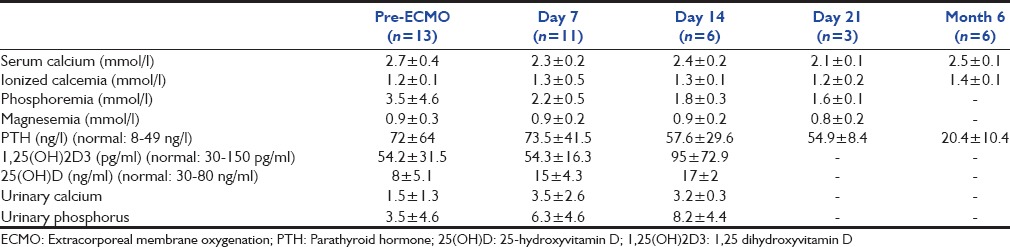
Furosemide was used before and during ECMO. Mean dose before ECMO was 1 mg/kg/d and 5.7 mg/kg/d at day 7, 5.2 mg/kg/d at day 14, and 4.7 mg/kg/d at day 21. Urinary calcium increased during ECMO, which could be due to furosemide exposure. Six months after ECMO weaning, serum calcium, ionized calcium, and PTH were collected for surviving patients. Six patients underwent a consultation. All patients had moderate hypercalcemia (2.55 ± 0.06 mmol/l) with normal PTH (20 ± 10 ng/l). Serum ionized calcium were 1.34 mmol/l, 1.35 mmol/l, 1.36 mmol/l, 1.39 mmol/l, and 1.41 mmol/l, respectively, (1.37 ± 0 mmol/l). One patient had nephrocalcinosis diagnosed by ultrasound, and two of them required treatment with a 1-α hydroxylase inhibitor (oral fluconazol) for hypercalcemia. Mothers’ calcium levels, concentrations of albumin and protein in plasma were normal.
Parathyroid hormone: Magnesemia correlation
During ECMO PTH was inadequately high with normal serum magnesium There was no correlation between serum magnesium and PTH levels on day 7 (z = 1) and day 14 (z = 0.82) [Table 5].
Parathyroid hormone: 1,25 dihydroxyvitamin D vitamin correlation
There was no correlation between serum 1,25(OH) 2D3 vitamin and PTH levels at day 7 (r = 0.55) and day 14 (r = 0.43) [Table 5].
Discussion
This prospective study enabled us to confirm the existence of severe disturbances of calcium homeostasis in neonates on ECMO.
Calcium homeostasis is a complex mechanism often dysregulated in critically ill neonates and children.[11,12,13] Hypocalcemia is well-described but partially unexplained in critically ill neonates.[14,15] Unlike these reports, our patients had a normal or high serum calcium level before ECMO implantation. In a retrospective study, Ruiz Magro et al.[16] reported several metabolism disturbances in critically ill children such as hypocalcemia (24.5%) and hypercalcemia (5.8%).[1]
Calcium homeostasis disturbances in neonates under ECMO have first been suspected in 2001 by Fridriksson, who described hypercalcemia during ECMO in neonates.[5] In these patients, hypercalcemia was associated with an increased mortality and longer duration of ECMO. In 2005, Hak reported an increase of PTH levels and a decrease of calcitriol during neonatal ECMO.[6] In our study, we confirmed the absence of correlation between PTH and serum calcium which could be due to pathologically high PTH secretion.
Furthermore, increase in PTH is supposed to stimulate the renal transformation from 25(OH)D to 1,25(OH)2D3. During ECMO, there was no correlation between PTH and 1,25(OH)2D3. Indeed, despite high PTH, 1,25(OH)2D3 remained below normal value. This dysregulation could be explained by renal dysfunction (defined in our study by a concentration of creatinine in plasma higher than 80 μmol/l at day 1 and 50 μmol/l at day 3) which was reported in eight of eleven patients before ECMO.[17] However, normalization of renal function for nine of the 11 patients at day 7 did not result in a correlation between PTH and 1,25(OH)2D3.
PTH secretion also depends on magnesium as a cofactor. In our study, there was no correlation between PTH and magnesium. Indeed, serum magnesium was not increased despite high PTH levels.
The reasons for dysregulation of calcium homeostasis remain unknown. A review of the literature did not result in a clear explanation for this disturbance. Personal data on asphyxiated neonates reveal a normal PTH levels with a normal ionized calcium levels. Our study might suggest long-term abnormalities which imply possibilities of irreversible damage of calcium regulation. Injuries to the parathyroid gland due to the jugular vein or carotid artery cannulas were one of our hypotheses. However, the existence of almost four parathyroid glands may cast doubts on this hypothesis.
The second hypothesis was an alteration of local parathyroid gland blood flow due to jugular cannulation that is the most often used drainage site. It can be responsible for back pressure in the superior vena cava territory and abnormal parathyroid gland perfusion such as cerebral perfusion.[17]
Other hypotheses were an associated dysregulation between PTH and calcitonin or the effect of heparin on the osteoclastic activity. Heparin effect on ionized calcium remained unclear.[18,19,20,21] Both hypotheses were not tested in our study.[22]
Lastly, calcium-sensing receptors of the parathyroid gland may be affected by metabolic disturbances that occur in these particularly ill neonates who need ECMO (e.g., pH, albuminemia).[20]
In this study, blood samples were taken before implantation of the ECMO and every seven days to avoid possible effects on calcium levels in the blood due to preservatives in the washed packed red cells and fresh frozen plasma.
Despite the small number of patients and the lack of power, our study highlights the possible long-term abnormalities of phosphocalcic metabolism even 6 months after ECMO weaning. Hypercalcemia was related to nephrocalcinosis for one patient and required treatment for 2 patients. As a consequence of these results, we decided to implement a consultation with a pediatric nephrologist for all patient weaned from ECMO to prevent complications of hypercalcemia.
Conclusion
We confirmed the existence of severe disturbances of calcium homeostasis in neonates on ECMO and detected possibly irreversible damage of calcium regulation.
In order to explain, the mechanism of this dysregulation, further studies of calcium homeostasis in critically ill neonates, who do not undergo ECMO, could clarify the impact of ECMO in calcium homeostasis disturbances.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lequier L. Extracorporeal life support in pediatric and neonatal critical care: A review. J Intensive Care Med. 2004;19:243–58. doi: 10.1177/0885066604267650. [DOI] [PubMed] [Google Scholar]
- 2.Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev. 2008;16:CD001340. doi: 10.1002/14651858.CD001340. [DOI] [PubMed] [Google Scholar]
- 3.Hervey-Jumper SL, Annich GM, Yancon AR, Garton HJ, Muraszko KM, Maher CO. Neurological complications of extracorporeal membrane oxygenation in children. J Neurosurg Pediatr. 2011;7:338–44. doi: 10.3171/2011.1.PEDS10443. [DOI] [PubMed] [Google Scholar]
- 4.Niebler RA, Punzalan RC, Marchan M, Lankiewicz MW. Activated recombinant factor VII for refractory bleeding during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2010;11:98–102. doi: 10.1097/PCC.0b013e3181b0620b. [DOI] [PubMed] [Google Scholar]
- 5.Fridriksson JH, Helmrath MA, Wessel JJ, Warner BW. Hypercalcemia associated with extracorporeal life support in neonates. J Pediatr Surg. 2001;36:493–7. doi: 10.1053/jpsu.2001.21608. [DOI] [PubMed] [Google Scholar]
- 6.Hak EB, Crill CM, Bugnitz MC, Mouser JF, Chesney RW. Increased parathyroid hormone and decreased calcitriol during neonatal extracorporeal membrane oxygenation. Intensive Care Med. 2005;31:264–70. doi: 10.1007/s00134-004-2543-7. [DOI] [PubMed] [Google Scholar]
- 7.Kenny J, Lees MM, Drury S, Barnicoat A, Van’t Hoff W, Palmer R, et al. Sotos syndrome, infantile hypercalcemia, and nephrocalcinosis: A contiguous gene syndrome. Pediatr Nephrol. 2011;26:1331–4. doi: 10.1007/s00467-011-1884-z. [DOI] [PubMed] [Google Scholar]
- 8.Canpolat N, Özdil M, Kurugoglu S, Çaliskan S, Sever L. Nephrocalcinosis as a complication of subcutaneous fat necrosis of the newborn. Turk J Pediatr. 2012;54:667–70. [PubMed] [Google Scholar]
- 9.Léger PL, Guilbert J, Isambert S, Le Saché N, Hallalel F, Amblard A, et al. Pediatric single-lumen cannula venovenous extracorporeal membrane oxygenation: A French center experience. Artif Organs. 2013;37:57–65. doi: 10.1111/aor.12024. [DOI] [PubMed] [Google Scholar]
- 10.Rambaud J, Guilbert J, Guellec I, Renolleau S. A pilot study comparing two polymethylpentene extracorporeal membrane oxygenators. Perfusion. 2013;28:14–20. doi: 10.1177/0267659112457970. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier B, Trachtman H, Di Carmine F, Urivetsky M, Tobash J, Chasalow F, et al. Hypocalcemia and hypercalcitoninemia in critically ill children. Crit Care Med. 1990;18:1215–9. doi: 10.1097/00003246-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Tsang RC, Steichen JJ, Chan GM. Neonatal hypocalcemia mechanism of occurrence and management. Crit Care Med. 1977;5:56–61. [PubMed] [Google Scholar]
- 13.Hyman SJ, Novoa Y, Holzman I. Perinatal endocrinology: Common endocrine disorders in the sick and premature newborn. Endocrinol Metab Clin North Am. 2009;38:509–24. doi: 10.1016/j.ecl.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Serret-Montoya J, Villegas-Silva R, Muro-Flores R, Peña LA, Villasís-Keever MA. Evaluation of diagnostic tests for hypocalcemia in critically ill newborns. Rev Invest Clin. 1998;50:471–6. [PubMed] [Google Scholar]
- 15.Broner CW, Stidham GL, Westenkirchner DF, Tolley EA. Hypermagnesemia and hypocalcemia as predictors of high mortality in critically ill pediatric patients. Crit Care Med. 1990;18:921–8. doi: 10.1097/00003246-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz Magro P, Aparicio López C, López-Herce Cid J, Martínez Campos M, Sancho Pérez L. Metabolic changes in critically ill children. An Esp Pediatr. 1999;51:143–8. [PubMed] [Google Scholar]
- 17.Skarsgard ED, Salt DR, Lee SK. Extracorporeal Life Support Organization Registry. Venovenous extracorporeal membrane oxygenation in neonatal respiratory failure: Does routine, cephalad jugular drainage improve outcome? J Pediatr Surg. 2004;39:672–6. doi: 10.1016/j.jpedsurg.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MR, Boerckel JD, Dupont KM, Guldberg RE. Functional restoration of critically sized segmental defects with bone morphogenetic protein-2 and heparin treatment. Clin Orthop Relat Res. 2011;469:3111–7. doi: 10.1007/s11999-011-2012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban P, Scheidegger D, Buchmann B, Skarvan K. The hemodynamic effects of heparin and their relation to ionized calcium levels. J Thorac Cardiovasc Surg. 1986;91:303–6. [PubMed] [Google Scholar]
- 20.Soong WJ, Wang HZ, Hwang B. Heparinization of blood decreases ionized calcium concentration. Zhonghua Yi Xue Za Zhi (Taipei) 1991;47:331–5. [PubMed] [Google Scholar]
- 21.Haverstick DM, Brill LB, 2nd, Scott MG, Bruns DE. Preanalytical variables in measurement of free (ionized) calcium in lithium heparin-containing blood collection tubes. Clin Chim Acta. 2009;403:102–4. doi: 10.1016/j.cca.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Breitwieser GE, Miedlich SU, Zhang M. Calcium sensing receptors as integrators of multiple metabolic signals. Cell Calcium. 2004;35:209–16. doi: 10.1016/j.ceca.2003.10.013. [DOI] [PubMed] [Google Scholar]


