Abstract
Context:
Few malnutrition screening tests are validated in the elderly Intensive Care Unit (ICU) patient.
Aim:
Having previously established malnutrition as a cause of higher mortality in this population, we compared two screening tools in elderly patients.
Subjects and Methods:
For this prospective study, 111 consecutive patients admitted to the ICU and > 65 years underwent the Malnutrition Universal Screening Tool (MUST), and the Geriatric Nutrition Risk Index (GNRI) screening tests.
Statistical Analysis:
Standard definition of malnutrition risk was taken as the gold standard to evaluate the sensitivity, specificity and predictive values of the tools. The k statistic was calculated to measure the agreement between the tools. The Shrout classification was used to interpret its values.
Results:
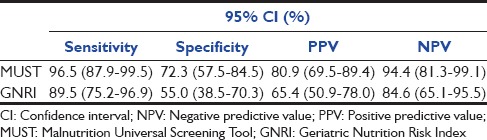
The mean age of the patients screened was 74.7 ± 8.4 (65–97 years). The standard definition, MUST and GNRI identified 52.2%, 65.4%, and 64.9% to be malnourished, respectively. The sensitivity and specificity of the tests were 96.5% computed tomography (CI) (87.9–99.5%) and 72.3% CI (57.5–84.5%) for MUST and 89.5% CI (75.2–96.7%) and 55.0% CI (75.2–96.9%) for GNRI, respectively. Screening was not possible by GNRI and MUST tool in 31% versus 4% of patients, respectively. The agreement between the tools was moderate for Standard-MUST k = 0.65 and MUST-GNRI k = 0.60 and fair for Standard-GNRI k = 0.43.
Conclusions:
The risk of malnutrition is high among our patients as identified by all the tools. Both GNRI and MUST showed a high sensitivity with MUST showing a higher specificity and greater applicability.
Keywords: Critical care outcome, elderly critically ill, malnutrition, nutrition screening tools
Introduction
Malnutrition among patients admitted to the Intensive Care Unit (ICU) is known to be associated with greater mortality and morbidity.[1] The elderly patient is known to have reduced appetite, longer periods of illness, duration of hospital stay, higher infection rates, and delayed wound healing.[2] Malnutrition is not readily recognizable or distinguishable from the changes of the aging process, which means that a significant percentage of cases are undiagnosed.[3] In a previous study, we have observed that up to 68% of critically ill elderly patients in our ICUs may be at a risk of being malnourished and that this significantly increases long-term mortality.[4]
Nutritional status assessment of the critically ill patient is performed to classify nutritional status, identify nutritional risk, and to serve as a baseline for monitoring nutrition support adequacy. Many scores or assessment tools are available for the assessment of nutrition risk.[5,6,7,8,9,10] Having mostly been developed and validated in outpatient or inpatient settings,[8] there are few scores available for being used among the geriatric critically ill population where it may be the most beneficial. We aimed to compare the applicability, effectiveness, and utility of two easy to use nutrition screening tests in this population.
Subjects and Methods
Patient selection
All patients >65 years of age admitted consecutively to the 12 bedded mixed medical-surgical ICU of a tertiary care hospital were included in this study. Duration of ICU stay had to be more than 24 h. The ICU caters to all patients except postcardiac and neurosurgery under a certified intensivist cover. Average annual patient admission rate is >700 and the average length of stay 3.5 days. The nurse to patient ratio is 1:1 for ventilated and 1:2–3 for non-ventilated patients. Patients opting for care limitation/withdrawal within 24 h, admitted after elective surgery for <24 h, and those admitted after cardiac arrest were excluded. After approval by the hospital ethical committee and review board, the patient or a close family member gave consent to participate in a larger study from of which this data were a part.[4]
Nutritional status
The prevalence of risk of malnutrition was measured by using a preset definition of malnutrition; this definition has been used widely in similar studies involving other patient groups and allows of comparability among studies and scores.[11,12,13,14] Patients were defined to be at a severe risk of malnutrition when the following conditions were present: Body mass index (BMI) <20 kg/m2 and an unintentional weight loss of more than 5% during the last 3 months. Patients were defined to be at a moderate risk if they had more than 5–10% unintentional weight loss during the last 6 months, independent of BMI.
Patients were weighed by a few trained members of the nursing staff. The ICU has its own set of scales, which are calibrated annually or sooner if needed, by an external agent. Patients were asked to recall their height or were measured by the admitting nurse using a free-standing measuring rod. In patients who were unable to stand or be weighed, height and weight were estimated using ulnar length or knee lengths measurements, as per protocol. On admission, patients or relatives were asked to report any unplanned weight loss.
Using these data, the scores were calculated by the admitting nurse, as part of normal admissions procedure within the first 24 h of admission to the ICU. Each patient was categorized into a low, medium, or high-risk group of malnutrition by the investigator.
Screening tests
Malnutrition Universal Screening Tool (MUST) is a five-step screening tool to identify adults, who are malnourished, at risk of malnutrition (undernutrition), or obese. It also includes management guidelines which can be used to develop a care plan.[10] It is for use in hospitals, community and other care settings and can be used by all care workers. A considerable amount of work has been undertaken to validate MUST. The practicality and predictive validity for mortality and length of stay of “MUST” in a group of acutely ill hospitalized elderly patients and its ability to screen all of the elderly patients including those who can and cannot be weighed has been demonstrated.[15,16,17]
The Geriatric Nutrition Risk Index (GNRI) index is a modification of the Nutritional Risk Index in which the value of “normal weight patients” replaces the original formula of “ideal weight patients” (calculated from Lorentz's formula) to be applied in the geriatric population.[9,18] This index takes into account two main parameters: Serum albumin and the ratio between the current weight and ideal weight of the individual.
Statistical analysis
Standard descriptive statistical methods were used to express means, standard deviations, percentages, frequencies, and minimum and maximum values. Cross-tabulations were used to present sensitivity, specificity and positive and negative predictive values. A 95% confidence interval was assessed. Multiple imputations by assuming missing at random was applied to predict missing values. Statistical analyses were performed using the SPSS-system for Windows, version 21.0 (SPSS, Chicago, IL, USA). MUST and GNRI classify patients into three categories. To compare the tools and their agreements, the results were organized into two variables: No risk for malnutrition (MUST - 0 and GNRI - low risk) and at risk of malnutrition (MUST - 1, 2 and GNRI - medium and high). The predefined standard definitions of malnutrition risks were taken as the gold standard to evaluate the sensitivity, specificity, and predictive values of the tools. The k statistic was calculated to measure the agreement between the tools and the Shrout classification was used to interpret its values as follows - 0–0.1: Virtually none; 0.11–0.4: Slight; 0.41–0.6: Fair; 0.61–0.8: Moderate; and 0.81–1.0: Substantial.[19,20]
Results
A total of 250 patients >65 years of age were admitted to the ICU during this period of which 111 (80 males, 31 females) were included in the analyses. The exclusion was by predefined criteria (105), or when the data required for assessing nutrition status by the preset definition was incomplete (24). The mean age was 74.6 ± 8.2 years (65–97 years), mean Acute Physiology and Chronic Health Evaluation II (APACHE) II was 19.1 ± 6.5 SD, and mean BMI was 22.7 ± 2.2 kg/m2 (range 15–34 kg/m2). The mean length of stay was 7.2 ± 3.3 SD. There was a predominance of medical over surgical indications for admission [Figure 1].
Figure 1.
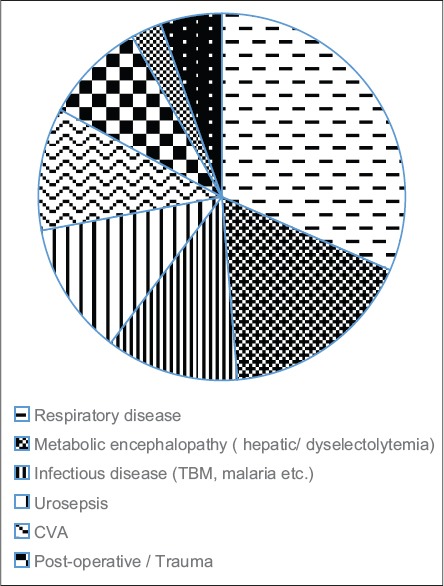
Distribution of disease n = 111
The data for MUST was complete for 96% and GNRI in 70.3% of the patients. The large percentage of missing GNRI data were due to missing weight measurements. 52.3%, 63%, and 65% were at risk of malnutrition by the preset definition, MUST and GNRI, respectively. Patients at nutritional risk had a longer length of stay in the ICU (7.5 vs. 6.11 days). The nutrition status significantly affected the long-term mortality at 1-year: Log-rank (Mantel-Cox) P = 0.010 [Figure 2].[4] Table 1 shows the sensitivities, specificities, positive and negative predictive values of the comprehensive malnutrition screening tools. The agreement between the tools was moderate for Standard-MUST k = 0.65 and MUST-GNRI k = 0.60 and fair for Standard-GNRI k = 0.43.
Figure 2.
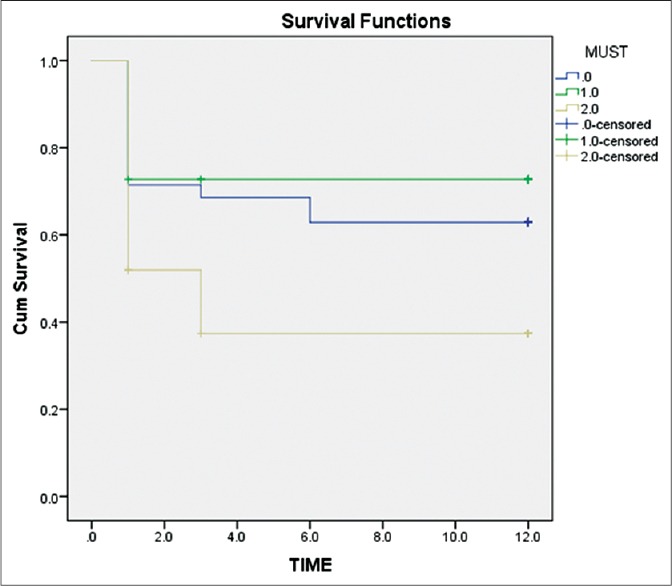
Test of survival distributions for the different levels of Malnutrition Universal Screening Tool.[4] Log-rank (Mantel-Cox) P = 0.010
Table 1.
Sensitivity, specificity, and predictive values of the nutrition screening tools
Discussion
In what we understand to be the first study of its type, we compared two easy to use nutrition screening tools in critically ill elderly patients. We found the prevalence of malnutrition to be high, and both MUST and GNRI to have a high sensitivity with the former performing better and being easier to implement (lesser missing values). The greater incidence of missing values in the GNRI tool stems from an inherent difference in weight calculation of the patient by the two tools: MUST provide a nomogram for the estimation of BMI from mid arm circumference if weight cannot be measured in a critically sick patient - GNRI has no alternatives to actual weight measurement. In ICUs with beds having inbuilt weighing scales, this problem may be mitigated.
There is a wide variation in the methods of nutrition screening of acutely ill patients: Most have not been evaluated for their sensitivity or specificity, and the comparison has usually been with another screening tool.[21] Like Neelemaat et al. we have compared the tools with a preset definition to enable better generalization.[22] We found that the prevalence of malnutrition was high by all three methods, clearly indicating the need for routine screening for risk of malnutrition.
For a screening tool, high sensitivity will ensure that patients at a true risk of malnutrition will not remain undiagnosed. Both tools showed a high sensitivity in our population. A low specificity (as we got for both tests) however, may result in over diagnosis of the risk of malnutrition.
The implications of an overdiagnosis of a malnourished state are difficult to predict with the current controversies regarding the appropriate amount of calories and proteins to be administered to ICU patients. A large prospective study by Alberda et al. involving more than 2770 patients concluded that increased protein and caloric intake improves outcomes in patients with when BMI is <25 or ≥35.[23] Heyland et al. in an elegant multicentric prospective observational audit demonstrated that the statistical method employed in calculating the effect of nutrition on ICU patients affects the results - they concluded that an attempt to meet the estimated caloric requirements of the critically ill patient may result in improved outcomes.[24] The EPaNIC, EDEN, and other large trials, on the other hand, suggest that early administration of larger protein or caloric loads to critically ill patients may be of no benefit or even harmful.[25,26,27,28] Whether aggressive nutrition replacement should be done for critically ill patients screened as malnourished is not clear as these patients are not well represented in randomized clinical trials with the EPaNIC excluding patients with BMI <17, and Doig et al. having only 46 patients with a BMI <18.5.[29] The results of Alberda et al., however, suggest that the effect of different levels of caloric intake may differ in patients with different underlying nutritional state; an easy to use, accurate assessment of the risk of malnutrition would be useful. Apart from the tools used by us, more recently, the NUTRIC tool has been described - it incorporates age, APACHE II score, Sequential Organ Failure Assessment score, number of comorbidities, days of hospital stay prior to ICU admission, and IL6 levels. It is used to quantify the risk of critically ill patients developing adverse events that may be modified by aggressive nutrition therapy.[30]
Apart from helping to identify the patient at a greater risk of preadmission malnutrition and related complications, a simple to use validated screening test tailored to a particular ICU and incorporated into its daily practise is also helpful in characterizing patients for research protocols and in increasing the awareness among medical personnel about nutritional issues.[31]
Conclusion
An easy to use screening test when used in the ICU to screen for malnutrition risk may alert the treating, team. Malnutrition, especially in the geriatric patients, has been associated with poorer long-term outcomes; in our population the MUST and GNRI screening tests may both be implemented successfully, with the MUST being better and easier to use.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The MUST is reproduced here with the kind permission of British Association for Parenteral and Enteral Nutrition. For further information on “MUST” see www.bapen.org.uk.
References
- 1.Sungurtekin H, Sungurtekin U, Okke D. Nutrition assessment in critically ill patients. Nutr Clin Pract. 2008;23:635–41. doi: 10.1177/0884533608326137. [DOI] [PubMed] [Google Scholar]
- 2.Feldblum I, German L, Castel H, Harman-Boehm I, Bilenko N, Eisinger M, et al. Characteristics of undernourished older medical patients and the identification of predictors for undernutrition status. Nutr J. 2007;6:37. doi: 10.1186/1475-2891-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durán Alert P, Milà Villarroel R, Formiga F, Virgili Casas N, Vilarasau Farré C. Assessing risk screening methods of malnutrition in geriatric patients: Mini Nutritional Assessment (MNA) versus Geriatric Nutritional Risk Index (GNRI) Nutr Hosp. 2012;27:590–8. doi: 10.1590/S0212-16112012000200036. [DOI] [PubMed] [Google Scholar]
- 4.Tripathy S, Mishra JC, Dash SC. Critically ill elderly patients in a developing world - Mortality and functional outcome at 1 year: A prospective single-center study. J Crit Care. 2014;29:474.e7–13. doi: 10.1016/j.jcrc.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? 1987. Classical article. Nutr Hosp. 2008;23:400–7. [PubMed] [Google Scholar]
- 6.Malnutrition Advisory Group. A consistent and reliable tool for malnutrition screening. Nurs Times. 2003;99:26–7. [PubMed] [Google Scholar]
- 7.Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15:458–64. doi: 10.1016/s0899-9007(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 8.Anthony PS. Nutrition screening tools for hospitalized patients. Nutr Clin Pract. 2008;23:373–82. doi: 10.1177/0884533608321130. [DOI] [PubMed] [Google Scholar]
- 9.Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–83. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 10.Malnutrition Universal Screening Tool. [Last accessed 24/3/15]. Available from: http://www.bapen.org.uk/pdfs/must/must_full.pdf .
- 11.Detsky AS, Smalley PS, Chang J. The rational clinical examination. Is this patient malnourished? JAMA. 1994;271:54–8. doi: 10.1001/jama.271.1.54. [DOI] [PubMed] [Google Scholar]
- 12.Kruizenga HM, Wierdsma NJ, van Bokhorst-de van der Schueren MA, Hollander HJ, Jonkers-Schuitema CF, Van der Heijden E, et al. Screening of nutritional status in The Netherlands. Clin Nutr. 2003;22:147–52. doi: 10.1054/clnu.2002.0611. [DOI] [PubMed] [Google Scholar]
- 13.Stratton RJ, Green CJ, Elia M. Scientific criteria for defining malnutrition. In: Richards G, editor. Disease Related Malnutrition: An Evidence-Based Approach to Treatment. Cambridge: CABI Publishing; 2003. pp. 1–34. [Google Scholar]
- 14.FAO/WHO/UNU. Energy and Protein Requirements, 724. Geneva: WHO; 1985. (Technical Report Series). [PubMed] [Google Scholar]
- 15.Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87:106–13. doi: 10.1093/ajcn/87.1.106. [DOI] [PubMed] [Google Scholar]
- 16.Henderson S, Moore N, Lee E, Witham MD. Do the Malnutrition Universal Screening Tool (MUST) and Birmingham nutrition risk (BNR) score predict mortality in older hospitalised patients? BMC Geriatr. 2008;8:26. doi: 10.1186/1471-2318-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratton RJ, King CL, Stroud MA, Jackson AA, Elia M. ‘Malnutrition Universal Screening Tool’ predicts mortality and length of hospital stay in acutely ill elderly. Br J Nutr. 2006;95:325–30. doi: 10.1079/bjn20051622. [DOI] [PubMed] [Google Scholar]
- 18.Cereda E, Pedrolli C. The Geriatric Nutritional Risk Index. Curr Opin Clin Nutr Metab Care. 2009;12:1–7. doi: 10.1097/MCO.0b013e3283186f59. [DOI] [PubMed] [Google Scholar]
- 19.Buzby GP, Knox LS, Crosby LO, Eisenberg JM, Haakenson CM, McNeal GE, et al. Study protocol: A randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr. 1988;47(2 Suppl):366–81. doi: 10.1093/ajcn/47.2.366. [DOI] [PubMed] [Google Scholar]
- 20.Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res. 1998;7:301–17. doi: 10.1177/096228029800700306. [DOI] [PubMed] [Google Scholar]
- 21.Chima CS, Dietz-Seher C, Kushner-Benson S. Nutrition risk screening in acute care: A survey of practice. Nutr Clin Pract. 2008;23:417–23. doi: 10.1177/0884533608321137. [DOI] [PubMed] [Google Scholar]
- 22.Neelemaat F, Meijers J, Kruizenga H, van Ballegooijen H, van Bokhorst-de van der Schueren M. Comparison of five malnutrition screening tools in one hospital inpatient sample. J Clin Nurs. 2011;20:2144–52. doi: 10.1111/j.1365-2702.2010.03667.x. [DOI] [PubMed] [Google Scholar]
- 23.Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med. 2009;35:1728–37. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 24.Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: Depends on how you slice the cake! Crit Care Med. 2011;39:2619–26. doi: 10.1097/CCM.0b013e318226641d. [DOI] [PubMed] [Google Scholar]
- 25.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–17. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 26.Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: A post hoc analysis. Am J Respir Crit Care Med. 2013;187:247–55. doi: 10.1164/rccm.201206-0999OC. [DOI] [PubMed] [Google Scholar]
- 27.Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: A randomized controlled trial. JAMA. 2013;309:2130–8. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 28.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, et al. Initial trophic vs. full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014;370:2450–1. doi: 10.1056/NEJMc1404896. [DOI] [PubMed] [Google Scholar]
- 30.Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit Care. 2011;15:R268. doi: 10.1186/cc10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preiser JC. Do we need an assessment of the nutrition risk in the critically ill patient? Crit Care. 2012;16:101. doi: 10.1186/cc10572. [DOI] [PMC free article] [PubMed] [Google Scholar]


