Abstract
Background and Aims:
Decreasing mortality in sick and ventilated neonates is an endeavor of all neonatologists. To reduce the high mortality in this group of neonates, identification of risk factors is important. This study was undertaken to find out the indications of ventilation and complications in ventilated neonates and also study possible predictors of outcome.
Subjects:
Age <1-month; mechanically ventilated; not having suspected metabolic disorders or congenital anomalies; excluding postoperative patients.
Methods:
Neonates consecutively put on mechanical ventilation during the study period (October 2011 to November 2013) enrolled. Primary disease of the neonates along with complications present listed. Clinical and laboratory parameters analyzed to find the predictors of mortality.
Results:
Total 300 neonates were ventilated. 52% were male. Mean age, weight, and gestational age were 21 ± 62 h, 2320 ± 846.2 g, and 35.2 ± 4.9 weeks, respectively. 130 (43%) neonates died. Respiratory distress syndrome (RDS) (31.1%), sepsis (22.7%), and birth asphyxia (18%) were the most common indications for ventilation. Mortality in ventilated patients with sepsis, pneumonia, RDS or birth asphyxia was 64.7%, 60%, 44.6%, and 33.3%, respectively. Weight <2500 g, gestation <34 weeks, initial pH <7.1, presence of sepsis, apnea, shock, pulmonary hemorrhage, hypoglycemia, neutropenia, and thrombocytopenia were significantly associated with mortality (P < 0.05). Resuscitation at birth, seizures, intra ventricular hemorrhage, pneumothorax, ventilator-associated pneumonia, PO2, or PCO2 did not have a significant association with mortality. On logistic regression, gestation <34 weeks, initial pH <7.1, pulmonary hemorrhage, or shock were independently significant predictors of mortality.
Conclusions:
Weight <2500 g, gestation <34 weeks, initial arterial pH <7.1, shock, pulmonary hemorrhage, apnea, hypoglycemia, neutropenia, and thrombocytopenia were significant predictors of mortality in ventilated neonates.
Keywords: Complications of ventilation, neonatal mechanical ventilation, predictors of mortality
Introduction
Advances in perinatal and neonatal care have significantly reduced neonatal morbidity and mortality rates. Outcome in sick infants has improved significantly, mostly due to more effective newborn intensive care and aggressive respiratory and cardiovascular support. It is the introduction of widespread mechanical ventilation in the neonatal Intensive Care Units (NICU) during 1960s and 1970s[1] and its judicious use since, which has revolutionized the outcome and survival of sick newborns.[2] A significant proportion of neonates admitted to NICU require mechanical ventilation; and mechanically ventilated neonates have a high fatality. Survival rate in artificially ventilated neonates is reported as 64% by Trotman[3] and 67.9% by Karthikeyan and Hossain[4] though survival of such neonates has been higher in developed countries.[5] This variation in the mortality has been attributed to more biomedical technological advancements in the developed countries. Various studies in developing countries have shown a mortality rate in the range of 40–60%[6,7] in ventilated babies. Despite the availability of mechanical ventilation, mortality is still high in sick neonates. So to improve the mortality in ventilated neonates, identification of bad prognostic factors, and their remedy becomes mandatory.
The primary aim of the present study was to find the risk factors responsible for poor outcome in ventilated neonates. We studied numerous clinical, biochemical, and hematological parameters in ventilated neonates and tried to relate them to outcome.
Methods
This prospective study was conducted from October 2011 to November 2013 in the NICU of A Tertiary Care Referral Hospital in North India. All neonates (aged 0–28 days) who required ventilation during the study period were eligible for inclusion in the study. Neonates who had congenital anomalies or suspected metabolic disorders or postsurgical babies were excluded from the study; all other ventilated neonates were included. Clearance was taken from the local ethical committee. Whenever a neonate is ventilated, his parents/guardians are informed as a routine in our NICU by the senior resident on duty. Informed consent was obtained at the same time from the parents/caregivers of every included neonate. For each ventilated neonate information, including age, sex, admission, weight (recorded by electronic weighing machine), gestational age (by modified Ballard's scoring), any maternal illness, type of delivery, any resuscitation at the time of birth, neonatal problems, primary diagnosis, hospital stay, and complications were recorded. The indications for initiation of mechanical ventilation were: (i) PaO2 <50, (ii) PaCO2 >60 mmHg, (iii) intractable or recurrent apnea, (v) gasping or poor respiration, (vi) O2 saturation <85% on supplemental oxygen, (vii) continuous positive airway pressure (CPAP) failure, defined as worsening respiratory distress, and/or hypoxemia (PaO2 <50 mmHg)/hypercarbia (PaCO2 >60 mmHg) despite CPAP pressure of 7–8 cm H2O and FiO2 of 0.8 or recurrent episodes of apnea.[8] Synchronized intermittent mandatory ventilation mode was the main mode of ventilation in the neonates in our study. Lung protective ventilator strategies were employed as a standard practice. Pressure limited time cycled ventilation was used and the different ventilator parameters were individualized depending upon the particular disease and the lung mechanics and compliance. Arterial blood gases were analyzed regularly. In general, the parameters were: Tidal volume 6–10 ml/kg, positive end-expiratory pressure 4–8 mmHg, peak inspiratory pressure 18–28 mmHg, inspiratory time 0.25–0.5 s, and FiO2 0.40–0.80. High frequency ventilation is not available at our hospital and was not used in any neonate. Relevant investigations such as complete blood counts, chest X-ray, kidney function test, liver function test, blood culture, cerebrospinal fluid analysis, blood sugar, serum calcium, cranial ultrasound, etc., were done and repeated as needed. All neonates with positive blood culture or diagnosed with pneumonia, meningitis, or urinary tract infection were considered to have sepsis. Shock was defined by clinical evidence of hypoperfusion in form of delayed capillary refill time (>3 s), oliguria (urine output <1 ml/kg/h), weak peripheral pulses, and hypotension in the presence of lactic acidosis. Ventilator-associated pneumonia (VAP) was defined as pneumonia that occurred 48–72 h or thereafter following endotracheal intubation and was characterized by the presence of a new or progressive infiltrate, symptoms and signs of infection, changes in sputum/endotracheal aspirate characteristics, and detection of a causative agent.[9]
Thrombocytopenia was defined as platelet count <150,000/μl. Absolute neutrophil count varies considerably in the immediate neonatal period and neutropenia was defined with respect to the normal reference ranges available from Manroe's charts.[10] For very low birth weight infants, the reference ranges available from Mouzinho's charts[11] were used. C-reactive protein (CRP) levels >6 mg/dl were taken as positive. Hypoglycemia was defined as blood sugar <50 mg/dl. The data was recorded on Microsoft Excel Worksheet and analyzed by GraphPad Instat 3.1 statistical program. Continuous variables were tested for normality using Kolmogorov and Smirnov method and Student's t-test, and Mann-Whitney U-test were used for comparison of normally distributed and nonparametric variables, respectively. Chi-square test and Fischer's exact test were used for categorical variables. P < 0.05 was considered significant. Those risk factors that were significant on univariate analysis (P < 0.05) were entered into a forward step-wise multivariable logistic regression model and independent risk factors were determined.
Results
During the study, 2980 neonates were admitted in total, out of which 300 were included in the study. One-hundred fifty-six (52%) were male and 144 (48%) were female. Respiratory distress syndrome (RDS) (31.1%), sepsis (22.7%), and birth asphyxia (18%) were the most common indications for ventilation [Table 1]. All the preterm neonates with gestational age <32 weeks or recurrent apneas or early features of RDS were given nasal CPAP therapy, and those who had a failure of nasal CPAP therapy (as defined above in methods section) were ventilated. Overall mortality in NICU during the study period was 12% and mortality in ventilated neonates was 43% (130/300). Disease-specific mortality was 33.3% in perinatal asphyxia, 44.7% in RDS, 26.7% in meconium aspiration syndrome (MAS), 60% in pneumonia, 64.7% in sepsis, and 54.5% in meningitis. Patients with sepsis had the highest disease-specific mortality (67.4%). The usual organisms isolated in cultures included Staphylococcus aureus, Klebsiella pneumonae, Enterobacter, and Candida species. Antibiotic usage was as per the antibiogram of our NICU generated by the Department of Microbiology on a regular basis.
Table 1.
Indications for ventilation
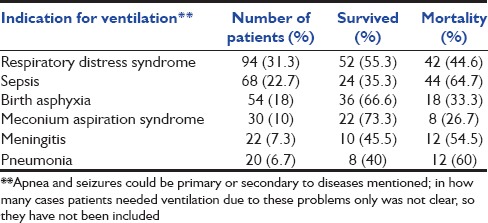
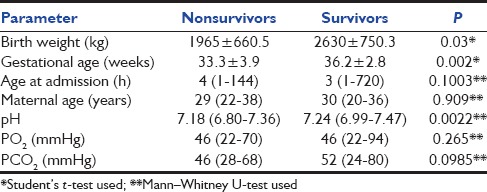
Among continuous variables studied, birth weight, gestational age, and initial pH differed significantly between ventilated neonates who survived and those who died (P < 0.05). Maternal age, age at admission, and initial blood gas values (PO2, PCO2) did not differ significantly in the two groups [Table 2].
Table 2.
Predictors of mortality in ventilated neonates
Among categorical parameters studied, birth weight <2.5 kg, prematurity <34 weeks, apnea, sepsis, shock, pulmonary hemorrhage, hypoglycemia, neutropenia, thrombocytopenia, and initial pH ≤7.1 were significantly associated with mortality. Resuscitation at birth, birth asphyxia, RDS, MAS, pneumonia, meningitis, seizures, intraventricular hemorrhage (IVH), VAP, pneumothorax, elevated CRP levels or hypoxemia, and hypercarbia had no significant relationship with mortality [Table 3].
Table 3.
Predictors of mortality in ventilated neonates
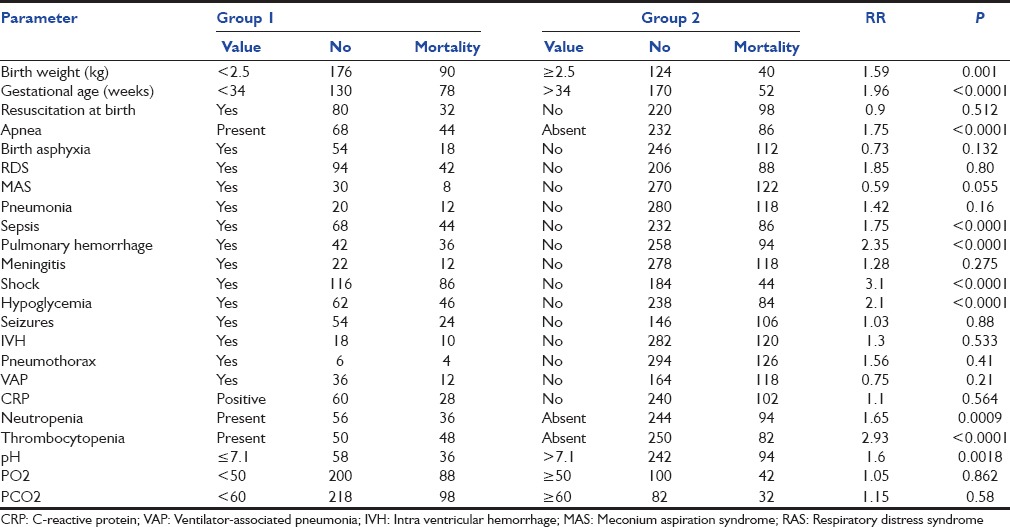
The complications recorded in patients were shock, VAP, pulmonary hemorrhage, hypoglycemia, IVH, and pneumothorax. Of these, only shock, pulmonary hemorrhage, and hypoglycemia were significantly associated with mortality. Pulmonary hemorrhage occurred in cases of shock, disseminated intravascular coagulation, and pneumonia and in cases of RDS after surfactant therapy. VAP was detected in 36 patients of whom 12 died. The common organisms isolated in neonates with VAP were S. aureus and K. pneumonae.
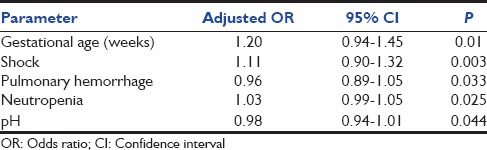
Multiple regression analysis was performed on data for 10 variables found significant on univariate analysis (gestational age <34 weeks, weight <2500 g, shock, apnea, sepsis, pulmonary hemorrhage, neutropenia, thrombocytopenia, and pH <7.1). Insignificant variables were eliminated to give final results as shown in Table 4. Gestational age <34 weeks, shock, neutropenia, pulmonary hemorrhage, and pH <7.1 were found to be significant independent predictors of fatality in mechanically ventilated neonates.
Table 4.
Multiple logistic regression analysis of variables
Discussion
Mortality among sick neonates in NICU is high,[12] but mortality among mechanically ventilated neonates and outborn neonates referred for ventilation is even higher.[2,13,14,15] In this study, mortality in ventilated neonates was 43.3%, which is comparable to mortality of 46% reported by Sangeeta et al.[16] Hossain et al.[17] and Mathur et al.[15] reported higher figures of 70.6% and 74%, respectively.
Sex and age at admission did not have a statistically significant association with the outcome as was found by Kollef[18] and Riyas et al.[6] The outcome was however affected by the gestational age and birth weight of the neonate. Impact of low birth weight and prematurity on survival of neonates is known and reported by Mathur et al.[15] and Hossain et al.[17] as well.
We did not find a statistically significant difference in the mortality of neonates who were resuscitated as compared to those who were not resuscitated. It is a known fact that only cases of severe perinatal asphyxia have a high mortality and outcome is good in cases of mild and moderate perinatal asphyxia. Improved resuscitative measures at the time of birth and early referral for such babies seem to have led to the improvement in survival of all resuscitated and subsequently ventilated neonates.
Survival rate in our neonates for perinatal asphyxia and RDS was comparable to Hossain et al.[17] and Anantharaj and Bhat[19] However, for diseases such as MAS and apnea survival rate in our NICU was slightly better as compared to Basu et al.,[20] Anantharaj and Bhat[19] and Riyas et al.[6]
We saw a dismal outcome in neonates with sepsis. We had a survival rate of merely 35.3%. Increasing emergence of drug resistant organisms is changing the equations of survival in NICUs. Anantharaj and Bhat[19] saw a survival rate of 46.1% in cases of sepsis. Respiratory failure and need for mechanical ventilation in a septic neonate signifies severe advanced disease and is a bad prognostic indicator.
Pulmonary hemorrhage is a life threatening event in neonates and prognosis was dismal in our patients with this complication. Karthikeyan and Hossain[4] and Anantharaj and Bhat[19] also found a very poor outcome in neonates following pulmonary hemorrhage.
Shock had a significant impact on mortality in ventilated neonates in our study. Anantharaj and Bhat[19] reported shock as the most common complication in ventilated neonates and also an important cause of mortality in their study. Shock represents an advanced stage of a disease process of varied etiologies and its relationship with mortality is understandable.
Hypoglycemia is common in sick neonates and predicted increased mortality in our study. Association of hypoglycemia with poor neurological outcome is well known, but we also saw poor survival in hypoglycemic ventilated neonates. It needs to be kept in mind that hypoglycemia was not the primary cause of death in all of these neonates. Survival in patients who developed IVH was better than what is reported by Basu et al.[20] (44% vs. 0%). Early suspicion, routine screening for IVH, bed-side cranial sonography, and adherence to proper preventive measures like sedation probably led to better outcome. Similarly, the outcome of patients who developed pneumothorax was better as compared to Anantharaj and Bhat[19] Judicious ventilator strategies, proper sedation, timely extubation, and readily available X-ray and surgical facilities help to control this problem.
Neutropenia and thrombocytopenia had a statistically significant correlation with mortality in our patients. Prognostic significance of these parameters has been recognized by others as well.[21,22,23] Acidosis (pH <7.1) at admission also predicted an increased mortality. A lower pH at admission indicates a larger time gap between the onset of events leading to the deterioration in the clinical status of the child and presentation to the healthcare facility. Thus, it will definitely have an impact on the outcome of the patients.
Limitations
Our study did not focus on the proportion of patients receiving noninvasive ventilation/CPAP who subsequently needed intubation and mechanical ventilation.
Furthermore, we were not able to study the factors determining the morbidity such as duration of NICU stay, mechanical ventilation, and hospital stay.
Conclusions
Identification of risk of fatality in ventilated neonates is compulsory in order to intervene early, decrease the mortality, and even for triage in resource limited settings. Among the numerous commonly available variables studied by us, weight <2500 g, gestation <34 weeks, initial arterial pH <7.1, shock, pulmonary hemorrhage, apnea, hypoglycemia, neutropenia, and thrombocytopenia were significant predictors of mortality in ventilated neonates.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Carlo WA, Martin RJ. Principles of neonatal assisted ventilation. Pediatr Clin North Am. 1986;33:221–37. doi: 10.1016/s0031-3955(16)34977-x. [DOI] [PubMed] [Google Scholar]
- 2.Singh M, Deorari AK, Paul VK, Mittal M, Shanker S, Munshi U, et al. Three-year experience with neonatal ventilation from a tertiary care hospital in Delhi. Indian Pediatr. 1993;30:783–9. [PubMed] [Google Scholar]
- 3.Trotman H. The neonatal intensive care unit at the University Hospital of the West Indies: The first few years' experience. West Indian Med J. 2006;55:75–9. doi: 10.1590/s0043-31442006000200002. [DOI] [PubMed] [Google Scholar]
- 4.Karthikeyan G, Hossain MM. Conventional ventilation in neonates: Experience from Saudi Arabia. Indian J Pediatr. 2002;69:15–8. doi: 10.1007/BF02723768. [DOI] [PubMed] [Google Scholar]
- 5.Richardson DK, Gray JE, Gortmaker SL, Goldmann DA, Pursley DM, McCormick MC. Declining severity adjusted mortality: Evidence of improving neonatal intensive care. Pediatrics. 1998;102(4 Pt 1):893–9. doi: 10.1542/peds.102.4.893. [DOI] [PubMed] [Google Scholar]
- 6.Riyas PK, Vijayakumar KM, Kulkarni ML. Neonatal mechanical ventilation. Indian J Pediatr. 2003;70:537–40. doi: 10.1007/BF02723151. [DOI] [PubMed] [Google Scholar]
- 7.Kambarami R, Chidede O, Chirisa M. Neonatal intensive care in a developing country: Outcome and factors associated with mortality. Cent Afr J Med. 2000;46:205–7. [PubMed] [Google Scholar]
- 8.Sankar M. Jeeva, Sankar Jhuma, Agarwal Ramesh, Paul Vinod, Deorari Ashok. Protocol for Administering Continuous Positive Airway Pressure in Neonates. AIIMS Neonatology Protocols 2014. Available from: URL: http://www.newbornwhocc.org/pdf/cpap_310508.pdf . [DOI] [PubMed]
- 9.Niederman MS, Craven DE. Guidelines for the management of adults with hospital acquired, ventilator associated, and healthcare associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. Available at: URL: https://www.thoracic.org/statements/resources/mtpi/guide1-29.pdf . [DOI] [PubMed] [Google Scholar]
- 10.Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophilic cells. J Pediatr. 1979;95:89–98. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 11.Mouzinho A, Rosenfeld CR, Sánchez PJ, Risser R. Revised reference ranges for circulating neutrophils in very-low-birth-weight neonates. Pediatrics. 1994;94:76–82. [PubMed] [Google Scholar]
- 12.Arafa MA, Alshehri MA. Predictors of neonatal mortality in the intensive care unit in Abha, Saudi Arabia. Saudi Med J. 2003;24:1374–6. [PubMed] [Google Scholar]
- 13.Nangia S, Saili A, Dutta AK, Gaur V, Singh M, Seth A, et al. Neonatal mechanical ventilation - Experience at a level II care centre. Indian J Pediatr. 1998;65:291–6. doi: 10.1007/BF02752306. [DOI] [PubMed] [Google Scholar]
- 14.Mathur NC, Kumar S, Prasanna AL, Sahu UK, Kapoor R, Roy S, et al. Intermittent positive pressure ventilation in a neonatal intensive care unit: Hyderabad experience. Indian Pediatr. 1998;35:349–52. [PubMed] [Google Scholar]
- 15.Mathur NB, Garg P, Mishra TK. Predictors of fatality in neonates requiring mechanical ventilation. Indian Pediatr. 2005;42:645–51. [PubMed] [Google Scholar]
- 16.Sangeeta ST, Rajesh KC, Anurakti S. Study of early predictors of fatality in mechanically ventilated neonates in NICU. Online J Health Allied Sci. 2009;8:3–9. Available from: URL: http://www.ojhas.org/issue31/2009-3-9.htm . [Google Scholar]
- 17.Hossain MM, Mahfuza S, Abdullah MA, Hassan MN, Sahidullah M. Predictors of mortality in ventilated neonates in intensive care unit. Bangladesh J Child Health. 2009;33:77–82. [Google Scholar]
- 18.Kollef MH. Do age and gender influence outcome from mechanical ventilation. Heart Lung. 1993;22:442–9. [PubMed] [Google Scholar]
- 19.Anantharaj A, Bhat BV. Outcome of neonates requiring assisted ventilation. Turk J Pediatr. 2011;53:547–53. [PubMed] [Google Scholar]
- 20.Basu S, Rathore P, Bhatia BD. Predictors of mortality in very low birth weight neonates in India. Singapore Med J. 2008;49:556–60. [PubMed] [Google Scholar]
- 21.Baley JE, Stork EK, Warkentin PI, Shurin SB. Neonatal neutropenia. Clinical manifestations, cause, and outcome. Am J Dis Child. 1988;142:1161–6. doi: 10.1001/archpedi.1988.02150110039016. [DOI] [PubMed] [Google Scholar]
- 22.Ballin A, Koren G, Kohelet D, Burger R, Greenwald M, Bryan AC, et al. Reduction of platelet counts induced by mechanical ventilation in newborn infants. J Pediatr. 1987;111:445–9. doi: 10.1016/s0022-3476(87)80477-8. [DOI] [PubMed] [Google Scholar]
- 23.Qazi I, Charoo B, Ahmad A, Sheikh M, Baba AR. Thrombocytopenia and other haematological parameters in culture positive neonatal sepsis and their impact. J Pediatric Infect Dis. 2013;8:25–9. [Google Scholar]


