Abstract
Targeted temperature management (TTM) in today's modern era, especially in intensive care units represents a promising multifaceted therapy for a variety of conditions. Though hypothermia is being used since Hippocratic era, the renewed interest of late has been since early 21st century. There have been multiple advancements in this field and varieties of cooling devices are available at present. TTM requires careful titration of its depth, duration and rewarming as it is associated with side-effects. The purpose of this review is to find out the best evidence-based clinical practice criteria of therapeutic hypothermia in critical care settings. TTM is an unique therapeutic modality for salvaging neurological tissue viability in critically ill patients viz. Post-cardiac arrest, traumatic brain injury (TBI), meningitis, acute liver failure and stroke. TTM is standard of care in post-cardiac arrest situations; there has been a lot of controversy of late regarding temperature ranges to be used for the same. In patients with TBI, it reduces intracranial pressure, but has not shown any favorable neurologic outcome. Hypothermia is generally accepted treatment for hypoxic ischemic encephalopathy in newborns. The current available technology to induce and maintain hypothermia allows for precise temperature control. Future studies should focus on optimizing hypothermic treatment to full benefit of our patients and its application in other clinical scenarios.
Keywords: Critical care, post-cardiac arrest, targeted temperature management, therapeutic hypothermia
Introduction
History of hypothermia dates back to Hippocratic era and in modern history to the 1950's when elective hypothermia (28–32°C) was practiced during general anesthesia for brain and heart protection.[1] In 1960's Peter Safar, father of modern cardio-pulmonary resuscitation (CPR) started using hypothermia, in post-cardiac arrest scenarios.[1] The initial enthusiasm in hypothermia was followed by subdued ebb in time zone of 1960–1980, due to complications arising from hypothermia, that is, patients were cooled longer and deeper which resulted in bleeding and septic complications. From 1980 to 2000 a lot of work on experimental animals showed that hypothermia leads to neuro-protection.[2] Two randomized controlled trials (RCT) which were published in 2002 showed the benefit of hypothermia in post-cardiac arrest situations, which changed the world regarding the use of hypothermia.[3,4] Therapeutic hypothermia (TM) is now-a-days popularly known as targeted temperature management (TTM). TTM is a unique modern era therapeutic modality for salvaging neurological tissue viability in critically ill patients.
Definitions
The normal body temperature in healthy individuals (measured in the oral cavity) is 36.8°C ± 0.4°C, with normal diurnal variations of 0.5°C.[5] Rectal temperatures are usually 0.4°C higher than oral readings.[5] The temperature of the blood measured with aid of pulmonary artery catheter is the accepted gold standard for “true” core temperature.[6] The lower esophageal temperature is the most rapid and accurate, noninvasive method of measurement of core temperature and is close to gold standard. The rectal temperature and bladder temperature also closely reflect core temperature. Clinically, tympanic temperature, which measures radiating heat from the tympanic membrane, is often used as a surrogate for deep brain temperature.[5]
Hypothermia
Hypothermia is defined as core temperature of <36.0°C.[5] This can be further classified as mild, moderate or severe. Temperature's range of 33–36°C is referred as mild hypothermia, moderate hypothermia is temperature in range of 28–32°C and deep hypothermia is temperature <28°C.
Induced hypothermia
It is reduction of a patient's core body temperature below 36.0°C which is intentional.[5]
Therapeutic hypothermia
It is induced hypothermia with side effects such as shivering, being controlled or suppressed.[5] This term therapeutic hypothermia is incomplete as it does not include interventions intended to maintain temperature near 37°C. The new term TTM was coined which covered all the deficiencies in the above term. It comprises of three distinct phases:[7]
Induction phase
This phase is characterized by change from the current temperature to a lower temperature which is the desired target temperature. This phase lasts for 60–80 min as cooling has to be initiated rapidly.
Maintenance phase
In this phase patient is kept at desired target temperature for a prolonged period of time. This phase lasts for 24–28 h with minimal or no fluctuations in temperature.
Rewarming phase
In this patient is slowly rewarmed to near normal temperature range from target temperature. The rewarming has to be done very slowly with side effects of vasodilatation caused by rewarming on various organ systems. In patients with traumatic brain injury (TBI), rate of rewarming should be very slow, that is, 0.1–0.2°C h and 0.25°C/h in patients with postcardiac arrest.
The side-effects of each phase of TTM are described in Table 1.
Table 1.
Side effects as per phases of TTM
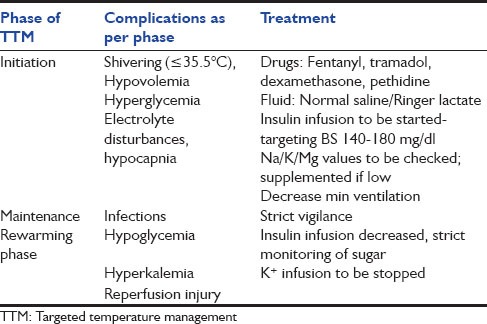
Methods of Cooling
Convection, conduction, radiation, and evaporation are four common mechanisms of heat loss. All methods of cooling basically aim at increasing convective or conductive heat loss. The convective heat loss is most common of all the mechanisms as it accounts for 20–30% of heat loss. A list of cooling methods is illustrated in Figure 1.
Figure 1.
Methods of cooling
Mechanism of Action of Hypothermia
Whenever there is neuronal injury either directly or indirectly it leads to increased free oxygen radicals formation, excess glutamate release, ion pump dysfunction ultimately leading to excess calcium influx and excess leakage through capillaries leading to cytotoxic edema. Hypothermia leads to neuroprotection basically by blunting all the four mechanisms.[8] Apart from these for every 1°C fall in temperature, the cerebral metabolism falls by 8%, which in turn leads to decrease in cerebral metabolic requirement of oxygen (CMRO2) and hence decreased cerebral blood flow.
Physiology of Thermoregulation
Thermoregulation is an important aspect of human homeostasis. Humans have been able to adapt to a great diversity of climates. High temperatures pose serious stresses for the human body, placing it in great danger of injury or even death. If the heat production is greater than heat loss; the heat concentration will increase and the body temperature will rise. If the heat production is less than heat loss; the heat concentration will decrease and the body temperature will drop. In hot conditions; the sweat glands under the skin secrete sweat, which travels up the sweat duct, through the sweat pore and onto the surface of the skin. This causes heat loss by evaporation; however, a lot of essential water is lost. The tiny muscles under the surface of the skin called erector pili muscles, relaxes so follicles are not erect. These flat hairs increase the flow of air next to the skin increasing heat loss by convection. The arterioles vasodilatation occurs; this is the process of relaxation of smooth muscle in arteriole walls allowing increased blood flow through the artery. The flow of blood, into the superficial capillaries in the skin increasing heat loss by convection and conduction. In contrast in cold conditions, the sweat stops being produced. The arterioles, carrying blood from superficial capillaries under the surface of the skin towards the warmer core of the body vaso-constrict. The muscles also receive messages from the thermoregulatory center of the brain (the hypothalamus) to cause shivering.
Effects of Hypothermia on Various Organ Systems
Central nervous system
Hypothermia is known for its neuro-protective action; for every 1°C fall in temperature, cerebral metabolism falls by 8%.[8] The most problematic of all the side-effects is shivering. The shivering in turn leads to increased cerebral metabolism, which in turn leads to increased cerebral blood flow and thus increase in intracranial pressure (ICP). Shivering starts at 35.5°C and stops at 33.5°C. The shivering is easy to control in patients on mechanical ventilation but is problematic to control in spontaneously breathing patients. A range of drugs is available to control shivering viz., pethidine, fentanyl, dexamethasone etc.[5]
Cardiovascular system
Hypothermia has varied effect on cardiovascular system.
Heart rate
The heart rate decreases with fall in temperature. The decrease in heart rate leads to decrease in myocardial oxygen demand hence myocardial contractility increases, as measured by systolic function. As temperature decreases below 35.5°C, sinus bradycardia occurs and as core temperatures falls below 32°C, the heart rate further decreases to around 40–45 beats/min.[5] The increase in heart rate artificially through administration of chronotropic drugs or pacing wire, leads to decrease in myocardial contractility.[9]
Cardiac output
The decrease in heart rate during mild to moderate hypothermia decreases cardiac output (CO) by 25–40%.[5] The decrease in metabolic rate is equal to or greater than the decrease in CO.
Systemic vascular resistance
The hypothermia induced vasoconstriction of peripheral arteries or arteriole leads to an increase systemic vascular resistance (SVR), which in turn leads to rise in blood pressure (by ≥10 mm Hg). This vasoconstrictive effect is absent or negligible in cerebral circulation.[10,11,12,13,14] The increase in SVR could lead to increased afterload of injured heart but patients who have reperfusion following cardiac arrest develop a systemic inflammatory response syndrome. In this condition an increase in SVR due to vasoconstriction will be beneficial, and will also increase coronary perfusion.
Blood pressure
The blood pressure is the product of CO and SVR.[5] A fall in heart rate leads to decrease in CO whereas hypothermia induced vasoconstriction leads to increased SVR. The net rise in blood pressure (by ≥10 mm Hg) is due to more pronounced effect of hypothermia induced increase in SVR.[15,16]
Central venous pressure
The central venous pressure which symbolizes increased preload due to vasoconstriction of peripheral arterioles and arteries as a result of hypothermia.[15,16]
Electrocardiographic changes
Characterized by prolonged PR interval, widening of the QRS complex, increased QT interval, finally resulting into Osborne waves.[5] Electrocardiographic changes, as described above, do not require treatment, and at a temperature of 32°C the heart rate of 40/min is perfectly normal. The increase in heart rate artificially through the administration of chronotropic drugs or pacing wire, leads to decrease in myocardial contractility.[9] Atropine is ineffective in this condition. The risk of arrhythmia is low as long as the core temperature is above 30°C. If the temperature is brought below 30°C then the risks of arrhythmia increases. Atrial fibrillation (AF) is most common arrhythmia seen in moderate to deep hypothermia, but this rhythm can change to ventricular tachycardia (VT) or ventricular fibrillation (VF) if temperature is allowed to go below 28°C.[5] If temperature is below 28°C and chest compressions are performed then it can easily be converted from AF to VF.[17] At temperature below 28°C myocardium is least sensitive to antiarrhythmic drugs and to defibrillation. These problems are least encountered above 30°C, hence target should be keep the temperature above that.[18,19,20,21]
Volume status
Hypovolemia is a common feature which occurs at the time of initiation of hypothermia and is multifactorial. Hypothermia leads to increased venous return, which in turn leads to decreased release of antidiuretic hormone along with increased release of atrial natriuretic factor thus contributing to cold diuresis.[22,23,24] This is accompanied by tubular dysfunction, which also leads to volume loss and thus hypovolemia. Thus careful attention at time of initiation of hypothermia should be paid to fluid balance and volume status of these patients.
Metabolic changes
At the time of initiation of hypothermia, hyperglycemia occurs due to decrease insulin sensitivity and reduced insulin secretion by pancreatic islet cells.[25] Similarly during induction phase, loss of volume and intracellular shifts lead to loss of electrolytes leading to hypokalemia, hyponatremia along with hypomagnesaemia. During rewarming phase the insulin gets functional and thus leads to hypoglycemia. There is also shift of electrolytes from intracellular compartment to extracellular compartment during rewarming phase and thus leads to rise in K+ levels. Strict monitoring of sugar and electrolytes is warranted.
Gastrointestinal
Targeted temperature management decreases gastrointestinal motility and eventually patients will require pro-kinetics to avoid delays in enteral feeding. Serum amylase and liver enzymes are commonly raised. The metabolic acidosis occurs as a result of increased production of free fatty acids, ketones and glycerol and increase in lactate concentrations.[26]
Ventilation
As hypothermia leads to decreased metabolic demand, this in turn leads to decreased carbon dioxide production. Normal mechanical minute ventilation at this stage leads to hypocapnia and cerebral vasoconstriction which will further aggravate brain injury.[5] The minute ventilation should be decreased to avoid developing hypocapnia during induction phase. Another point to consider is that value of blood gases are temperature dependent. The analyzers warm blood to 37°C before analysis and patient is actually hypothermic so both PaO2 and PaCO2 are overestimated.
Coagulation abnormalities
Coagulation abnormalities are witnessed at temperature <33°C whereas platelet dysfunction are noted at temperature <35°C.[27,28,29,30,31,32,33] The coagulation cascade is dependent on enzymes; due to hypothermia the enzymes are inactivated which in turn affects the coagulation cascade.[5] The risk of clinically significant bleeding is too low despite of coagulation defects.
Current Evidence and Practices in Critical Care
We used the search titled “therapeutic hypothermia” in PubMed search engine and applied filters: RCT, systematic reviews and meta-analyses in English literature and human subjects 1192 articles were retrieved. A detailed summary is described in Figure 2.
Figure 2.
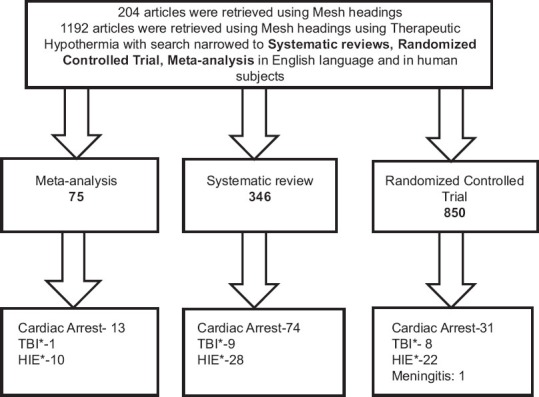
Schematic presentation of literature search strategy .TBI*: Traumatic brain injury, HIE*: Hypoxic ischemic encephalopthy
The inclusion criteria were conditions for which hypothermia is commonly being used in critical care settings such as for neuroprotection viz. In post-cardiac arrest, TBI, stroke, meningitis, acute liver failure (ALF) and other conditions such as hypoxic ischemic encephalopathy (HIE), spinal cord injury, trauma and myocardial infarction (MI). A detailed list is described in Figure 2. The references from identified studies were also searched for additional citations.
We also specifically looked for articles featuring therapeutic hypothermia and post-cardiac arrest in English literature and human subjects. Around 2839 articles were found which are shown in Figure 3. In the year 2002, two articles published in NEJM changed the world regarding the use of TTM in the post-cardiac arrest and there have been a plethora of articles post 2002 as shown in Figure 3. There has been International Liason Committee on Resuscitation (ILCOR) updates, Cochrane reviews and meta-analyses in concerned area. In the following review we will be discussing current evidence and practices of TTM in critical care settings.
Figure 3.
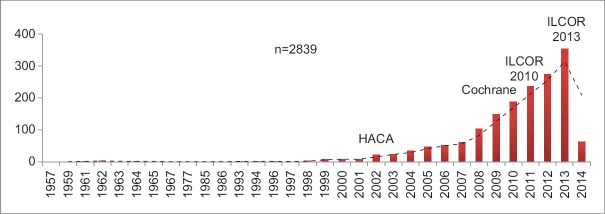
Use of therapeutic hypothermia and postcardiac arrest studies in literature
Outside hospital cardiac arrest
In early 1960's, the use of CPR increased survival for patients who had cardiovascular collapse. There was a gradual improvement in the number of survivors as more and more medical staff got trained and above all increased availability of defibrillators. Most patients who had return of spontaneous circulation (ROSC) did not survive to leave the hospital and even if they did so, that too in a neurologically devastated state due to the neuronal injury, which begins within minutes after cardiac arrest. The neurological injury was so severe that it led to research into this area so that more and more people can be neurologically restored. In this research, only one thing that came out to be feasible and functional was the use of therapeutic hypothermia.
Significant positive studies
Hypothermia after cardiac arrest trial
This trial was conducted at nine European centers and overall included 273 patients with out-of-hospital cardiac arrest (OHCA) due to VF or VT. The use of therapeutic hypothermia, that is, temperature of 32–34°C led to improved survival at 6 months (59% in hypothermic group vs. 45% in normothermic group; P = 0.009) and better neurologic outcome (55% in hypothermic group vs. 39% in normothermic group; P = 0.046).[4]
Bernard et al.
In contrast to multicenteric hypothermia after cardiac arrest (HACA) trial, this one was a single center trial, conducted in Australia. This trial included overall 77 patients with out-of-hospital cardiac arrest with presenting rhythm as VF. In this study, 49% of the patients in hypothermic group (33°C) survived with a good neurologic outcome as compared to 26% of normothermic group patients (P = 0.046).[3]
The Cochrane review
Included five RCT (Bernard et al. 2002; HACA et al. 2002; Hachimi-Idrissi et al. 2001; Laurent et al. 2005; Mori et al. 2000) which included a total of 481 patients. The Cochrane review summarizes that mild therapeutic hypothermia in patients with ROSC after cardiac arrest seemed to improve survival and neurologic outcome.[34]
International Liason Committee on Resuscitation 2010 statement
Therapeutic hypothermia was advocated by ILCOR, thereafter adopted by European Resuscitation Council, American Heart Association, and by the National Institute for Health and Clinical Excellence. Three important facts inferred from ILCOR statement are: One that patients with out-of-hospital cardiac arrest (OHCA) with presenting rhythm VF/VT should be cooled. Patients with in-hospital cardiac arrest (IHCA) or presenting rhythm as a systole or pulseless electrical activity (PEA) may be cooled. Third point is that the temperature of 32–34°C should be targeted, and duration of hypothermia should be at least for 12–24 h.[35,36]
Criticism of cooling and negative studies
Hypothermia after cardiac arrest trial
This trial had a very low inclusion rate that is, 8%, of all the 3551 screened only 275 patients were randomized. This low inclusion rates of around 8% make it difficult to generalize results to usual clinical practice. An average of just over one patient per week from nine centers was recruited over a period of 5 years. The study was abandoned because of a variety of reasons, which included slow recruitment, lack of funding and most importantly, there was no predefined power calculation. The level of Glasgow Coma Score (GCS) before randomization was not reported and withdrawal of care was not standardized leading to potential biases to the primary outcome measures of neurological outcome and death. The reporting of adverse effects was also inconsistent, making it difficult to assess the harm from this treatment.[4]
Bernard et al.
In the trial by Bernard et al. the patients in normo-thermia group, that is, 37.4°C were actually having temperatures of 35.5°C on admission in emergency and these patients were passively warmed in Intensive Care Unit (ICU) settings. It was rise in temperature of 2°C in normo-thermia group that was causing more harm than the benefit of therapeutic hypothermia in 32°C group.[3]
Cochrane review
The Cochrane review included trials by HACA group, Bernard et al., Hachimi-Idrissi et al., Laurent et al. and Mori et al. which were all conducted in time period between 2001 and 2005. The shortcomings of HACA trial are described above. In two trials, that is, by Hachimi Idrissi and Mori et al. there was baseline difference between groups. Similarly in trials by Bernard et al. and Laurent et al. there was no description of sequence generation or blinding.[34]
Trial sequential analysis by Nielsen et al.
The benefits and harms of the TTM were systematically evaluated, taking into account the risk of systematic bias and random errors. The treatment effects were quantified using trial sequential analysis, which reduced the risk of type I errors in contrast to cumulative meta-analysis.[37] This trial concluded that there was a lack of firm evidence of a beneficial effect of TTM and that too the quality of evidence was quite low.
Nielsen et al. 2013
It was a multicenteric trial conducted over a period of 3 years in between 2010 and 2013 at 36 centers both in Europe and Australia.[38] The inclusion criteria included patients with OHCA with presenting rhythm either as VF/VT or asystole (80% of patients had shockable rhythm, that is, VF, 12% had asystole and 8% PEA). The patients were randomized to receive TTM to either 33° or 36°C, for at least 28 h and were continued on fever-reduction methods for 72 h postarrest. Post 72 h, a neurologist who was blinded to initial treatment recommended continued care or withdrawal based on predictors of poor neurological outcome, that is, presence of myoclonus, bilateral absence of N20 response on SSEP, absence of motor response or pupillary response. TTM to 33°C did not improve outcome in any of the measurable way. 50% of the patients (33°C group), as compared to 48% of the patients (36°C group) died (Hazard ratio with 1.06; 95% confidence interval [CI], 0.89–1.28; P = 0.51). Similarly at 6 months follow-up, 54% of the patients (33°C group) had died or had poor neurologic function, as compared to 52% of patients (36°Cgroup) (Risk ratio, 1.02; 95% CI, 0.88–1.16; P = 0.78). When the analysis was restricted only to the 80% of subjects with shockable rhythms, that is, VF, then also there was no benefit of TTM. The comparison between the three major trials (HACA, Bernard et al. and Nielsen et al.) is shown in Table 2.
Table 2.
Summary of postcardiac arrest trials
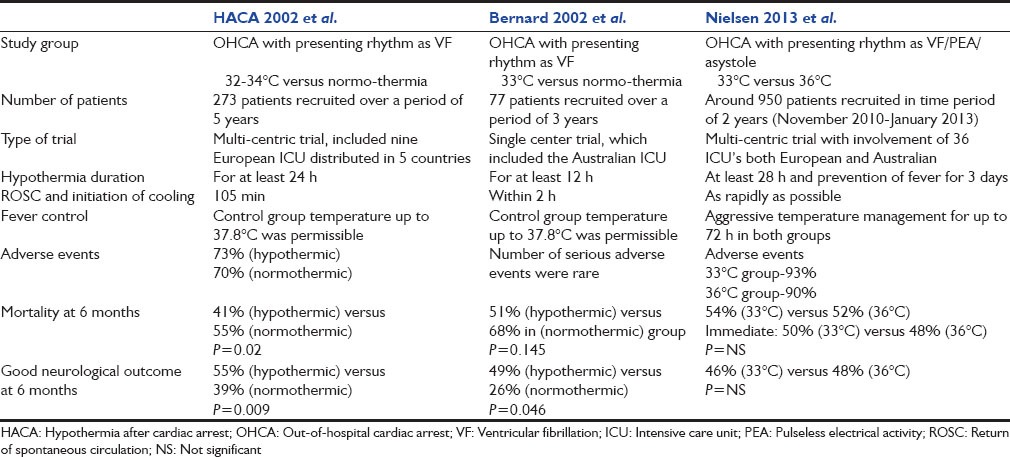
The trial by Nielsen et al. had some shortcomings: Higher dose of hypothermia was used in previous TTM trials; there was about 4–4.5°C average temperature difference between two groups, as opposed to 3°C in the present trial. In this trial, the patient population included patients with shockable rhythms and nonshockable rhythms as compared to previous trials which included patients with shockable rhythms only. Despite therapeutic hypothermia's proven physiologic benefits and success in experimental animals, the same success couldn't be extrapolated in human settings or real life scenarios. This proves that healthy experimental animal is different from real life individual with multiple comorbidities. Above all there have been advancements in the field of critical care in last 20 years and improvements in patient care may have reduced the potential benefits of any single intervention.
In patients with OHCA when the presenting rhythm is either VF/VT, these patients should be cooled as early as possible, that is, within 4 h of arrest not after 8 h.[39] The patients have to be cooled for at least 24–48 h. The trial by Nielsen et al. found similar outcomes (survival/neurological) with a new regimen targeting a temperature of 36°C. A word of caution is that we should not abandon therapeutic hypothermia in favor of strict fever management on the basis of one study, until all relevant issues have been satisfactorily addressed. Most importantly the patient should not be allowed to develop a fever for at least 72 h post-cardiac arrest.
Prehospital induction of hypothermia
Around 1359 adults with prehospital cardiac arrest (583 with VF and 776 without VF) were randomized into two groups - intervention and control.[40] Patients in the intervention group received prehospital cooling by infusing 2 L of 4°C normal saline following ROSC. The intervention reduced the time to achieve a temperature of <34°C by about 1 h. The intervention was neither associated with improved neurological status nor survival. Overall, the patients in the intervention group had more re-arrest in prehospital setup than control (26% vs. 21% respectively; P = 0.008), as well as increased diuretic use and pulmonary edema. The use of prehospital cooling reduced the time to reach a temperature of 34°C; it did not improve survival or neurological status.
Debaty et al. conducted a randomized, multicenter study in three prehospital emergency medical services and four critical care units in France, to study the impact of intra-arrest therapeutic hypothermia (IATH) on neurological injury and inflammation following OHCA.[41] The OHCA patients, irrespective of the initial rhythm, received either an infusion of cold saline and external cooling during cardiac arrest (IATH group) or TH was started after hospital admission (hospital-cooling group). The primary endpoint was serum neuron-specific enolase (NSE) concentrations at 24 h. The secondary end-points included interleukin-6 (IL-6), IL-8, and IL-10 concentrations and clinical outcome. 245 patients were included, 123 were analyzed in the IATH group and 122 in the hospital-cooling group. Levels of NSE and inflammatory biomarkers were not different between the two groups (median NSE at 24 h: IATH 96.7 μg/l (interquartile range (IQR): 49.9–142.8) vs. hospital cooling 97.6 μg/l (IQR: 74.3–142.4), P = 0.64). No difference in survival and cerebral performance were found at 1-month. The major criticism of this study was the patient population selected in this study was 80% of the patients with presenting rhythm as asystole and the NSE levels in this study were too high. Thus, the results of this study cannot be generalized as the results of this trial pertain to a specific patient population who already has a poor prognosis.[42]
Use of therapeutic hypothermia after in-hospital cardiac arrest
A multicenteric, prospective cohort study was conducted in a total of 538 hospitals which included 67,498 patients who had ROSC after IHCA.[43] The basic aim of this study was to evaluate, that in how many patients post IHCA therapeutic hypothermia was initiated and if so whether the desirable target temperature was achieved or not. Therapeutic hypothermia was initiated in total of 1,367 of the total 67,498 patients (2.0%) only. Target temperature of 32–34°C was not achieved in 44.3% of these patients within 24 h and 17.6% were overcooled. Younger age (P < 0.001), arrest in a non-ICU location (P < 0.001), on a weekday (P = 0.005), and in a teaching hospital (P = 0.001) were associated with an increased likelihood of therapeutic hypothermia being initiated. Post IHCA, therapeutic hypothermia is initiated rarely and that too if initiated, the target temperature was commonly not achieved.
Condition associated with cerebral edema
Traumatic brain injury
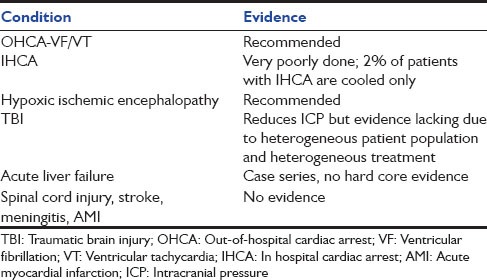
Therapeutic hypothermia as per its mechanism of action by decreasing CMRO2 is effective in reducing ICP. 16 RCT evaluating favorable neurological outcomes and fifteen evaluating survival were evaluated by a jury comprising of experts in field of critical care from various critical care societies, that is, SCCM, ESICM etc.[7] The spectrum of head injury varied in these trials from diffuse brain edema, diffuse axonal injury to subdural hematoma, extradural hematoma, and contusion. The first two conditions are treated medically while the latter conditions are treated surgically. Hence, hypothermia in each of these conditions is combined with adjuvant modality. The outcome depends upon underlying spectrum of head injury, the basic treatment given and whether it is given within appropriate time limit or not matters the most. The depth and duration of hypothermia applied in each of these trials have varied widely, as have the use of medical or surgical modalities. The better results were achieved in centers with expertise in applied hypothermia. Five published meta-analyses indicate a trend toward improved neurologic outcome and mortality when hypothermia was used, but definitive statistical significance is lacking.[7,44] The recommendation from the TTM group is as follows:
TTM may be considered in patients with TBI and increased ICP
Early initiation, duration of >48 h, and slow rewarming with tight monitoring of ICP appear to be of paramount importance
Patients hypothermic on admission should possibly be maintained hypothermic or very slowly rewarmed
However, despite superior ICP control, the favorable neurologic outcome could not be achieved in all patients.
The current data neither support nor discourage's the use of TTM in patients with TBI.
Stroke
Majority of stroke patients are spontaneously breathing patients and any attempt of cooling will lead to shivering. The shivering will lead to increased cerebral metabolic requirement and hence further aggravate brain injury. Thus mechanical ventilation becomes mandatory, if hypothermia has to be initiated in these patients. The current published literature contains no RCT to support or refute an assertion of TTM benefit.[7] Furthermore, the use of early thrombolysis has itself changed the outcomes in patients suffering from acute ischemic stroke. Whether TTM as adjunctive therapy would further improve the outcome is questionable. The use of therapeutic hypothermia will increase the need for endotracheal intubation and hence mechanical ventilation. As we all know both intubation and mechanical ventilation are associated with harmful effects, these must be acknowledged too before planning a trial.
Acute liver failure with cerebral edema
Therapeutic hypothermia alone has been used as a modality to reduce ICP and mortality in patients with ALF. It has also been used as a bridge for patients waiting orthotropic liver transplant (OLT) and has been used to decrease ICP during OLT. There is a case series suggesting a favorable effect of TTM in patients with ALF with cerebral edema.[7] No RCT exists till date in this field. This is an area of research where RCT can be planned regarding the use of TTM alone or in combination with hepatic dialysis strategies in patients with ALF.
Bacterial meningitis
A multicenteric, randomized clinical trial was conducted at 49 ICU in France, over a period of 2 years. Ninety-eight patients with bacterial meningitis with GCS score of <8 for <12 h were randomized.[45] All patients received appropriate antibiotics and adequate organ support. The patients in hypothermic group were cooled to 32–34°C for 48 h; on the other hand control group received standard care. After inclusion of 98 patients, the trial was terminated early because of excess mortality in the hypothermic group (25 of 49 patients [51%]) vs. the control group (15 of 49 patients [31%]; relative risk, 1.99; 95% CI, 1.05–3.77; P = 0.04). In severe bacterial meningitis use of mild-moderate hypothermia did not improve outcome but on the other hand proved to be more harmful.
Spinal cord injury
Due to high incidence of motor vehicle accidents the incidence of spinal cord injuries is on a rise. No RCT exists to support or criticize TTM as effective treatment.[7] Animal studies and little human success point to the importance of designing and executing an RCT evaluating benefit of TTM for this common and devastating condition.
Myocardial infarction
Hypothermia was sought as therapy in patients with MI as it decreases myocardial oxygen demand. With the advent of thrombolytic therapy, percutaneous coronary intervention and surgery, the outcome in patients with MI has improved so much over the last two decades.[7] In current scenario the use of any cooling intervention must not delay the proven effective treatments. Furthermore, with advancement in fields of medicine, mortality is now so rare that proof of further reduction in mortality would likely require a very large RCT.
Trauma
Spontaneous hypothermia is common posttrauma. The cause of hypothermia in this scenario is multifactorial. The injury itself leads to heat loss viz., bleeding which is accompanied by iatrogenic heat loss, which is due to exposure of injured part, cold environment and pumping of cold intravenous fluids to these patients. No RCT exists that support cooling of trauma patients and it is a known fact that uncontrolled hypothermia is associated with substantially worse outcomes.[7] The current practice at present aims at prevention of hypothermia, that is, preventing heat loss via conductive, convective mechanisms.
Hypoxic-ischemic encephalopathy [Table 3]
Table 3.
Summary of evidence
Hypoxic ischemic encephalopathy from asphyxial insults is associated with high mortality and long-term neurodevelopmental disability especially in infants and children. The injury is two staged. A certain amount of damage results from acute, primary neuronal death. This often is followed by a secondary, delayed period of neuronal loss. This secondary injury provides a therapeutic window in which further damage might be prevented. In a recent Cochrane review; the data from eleven RCT comprising 1505 term and late preterm infants were summarized.[46] The review concludes that TTM was of benefit to term newborns with HIE. Death or major disability in form of neuro-developmental disability were all reduced in term newborns born with HIE.
Conclusion
Targeted temperature management in the ICU is a promising multifaceted therapy for quite a few medical conditions. In patients with OHCA, if the presenting rhythm is either VF or VT these patients should be cooled as early as possible for at least 24–48 h. The use of TTM in IHCA is less widely practiced and prehospital use of hypothermia has not shown any benefit. TTM is generally accepted treatment for HIE in newborns. TBI represents heterogeneous patient population with heterogeneous treatments offered hence TTM alone has not shown any benefit. Future studies should focus on optimizing hypothermic treatment and assess its value in other clinical settings for the full benefit of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Drabek T, Kochanek PM. Evidence Based Practice of Critical Care. Ist ed. USA: Saunders, An Imprint of Elveiser; 2010. Is hypothermia useful in managing critically Ill patients? Which ones? Under what conditions? pp. 437–44. [Google Scholar]
- 2.Busto R, Dietrich WD, Globus MY, Ginsberg MD. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett. 1989;101:299–304. doi: 10.1016/0304-3940(89)90549-1. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: Practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37:1101–20. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- 6.Akata T, Setoguchi H, Shirozu K, Yoshino J. Reliability of temperatures measured at standard monitoring sites as an index of brain temperature during deep hypothermic cardiopulmonary bypass conducted for thoracic aortic reconstruction. J Thorac Cardiovasc Surg. 2007;133:1559–65. doi: 10.1016/j.jtcvs.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Nunnally ME, Jaeschke R, Bellingan GJ, Lacroix J, Mourvillier B, Rodriguez-Vega GM, et al. Targeted temperature management in critical care: A report and recommendations from five professional societies. Crit Care Med. 2011;39:1113–25. doi: 10.1097/CCM.0b013e318206bab2. [DOI] [PubMed] [Google Scholar]
- 8.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 9.Lewis ME, Al-Khalidi AH, Townend JN, Coote J, Bonser RS. The effects of hypothermia on human left ventricular contractile function during cardiac surgery. J Am Coll Cardiol. 2002;39:102–8. doi: 10.1016/s0735-1097(01)01694-1. [DOI] [PubMed] [Google Scholar]
- 10.Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–30. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- 11.Hägerdal M, Harp J, Nilsson L, Siesjö BK. The effect of induced hypothermia upon oxygen consumption in the rat brain. J Neurochem. 1975;24:311–6. doi: 10.1111/j.1471-4159.1975.tb11881.x. [DOI] [PubMed] [Google Scholar]
- 12.Palmer C, Vannucci RC, Christensen MA, Brucklacher RM. Regional cerebral blood flow and glucose utilization during hypothermia in newborn dogs. Anesthesiology. 1989;71:730–7. doi: 10.1097/00000542-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich MP, McCullough JN, Zhang N, Weisz DJ, Juvonen T, Bodian CA, et al. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg. 2002;73:191–7. doi: 10.1016/s0003-4975(01)03273-8. [DOI] [PubMed] [Google Scholar]
- 14.Aoki M, Nomura F, Stromski ME, Tsuji MK, Fackler JC, Hickey PR, et al. Effects of pH on brain energetics after hypothermic circulatory arrest. Ann Thorac Surg. 1993;55:1093–103. doi: 10.1016/0003-4975(93)90014-9. [DOI] [PubMed] [Google Scholar]
- 15.Thoresen M, Whitelaw A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics. 2000;106:92–9. doi: 10.1542/peds.106.1.92. [DOI] [PubMed] [Google Scholar]
- 16.Reuler JB. Hypothermia: Pathophysiology, clinical settings, and management. Ann Intern Med. 1978;89:519–27. doi: 10.7326/0003-4819-89-4-519. [DOI] [PubMed] [Google Scholar]
- 17.Nesemann ME, Busch HM, Jr, Gundersen AL, Gundersen AE, Newcomer KL. Asystolic cardiac arrest in hypothermia. Wis Med J. 1983;82:19–20. [PubMed] [Google Scholar]
- 18.Kirby CK, Jensen JM, Johnson J. Defibrillation of the ventricles under hypothermic conditions. AMA Arch Surg. 1954;68:663–5. doi: 10.1001/archsurg.1954.01260050665012. [DOI] [PubMed] [Google Scholar]
- 19.Martinez JB, Kass I, Hoffman MS. Factors involved in the recovery of a patient after prolonged ventricular fibrillation during hypothermia. J Thorac Surg. 1958;36:749–56. [PubMed] [Google Scholar]
- 20.Covino BG, Beavers WR. Changes in cardiac contractility during immersion hypothermia. Am J Physiol. 1958;195:433–6. doi: 10.1152/ajplegacy.1958.195.2.433. [DOI] [PubMed] [Google Scholar]
- 21.Covino BG, Hegnauer AH. Hypothermic ventricular fibrillation and its control. Surgery. 1956;40:475–80. [PubMed] [Google Scholar]
- 22.Polderman KH, Peerdeman SM, Girbes AR. Hypophosphatemia and hypomagnesemia induced by cooling in patients with severe head injury. J Neurosurg. 2001;94:697–705. doi: 10.3171/jns.2001.94.5.0697. [DOI] [PubMed] [Google Scholar]
- 23.Pozos RS, Danzl D. Human physiological responses to cold stress and hypothermia. In: Pandolf KB, Burr RE, editors. Medical Aspects of Harsh Environments, Vol Textbooks of Military Medicine. Washington, DC: Borden Institute, Office of the Surgeon General, US Army Medical Department; 2001. pp. 351–82. [Google Scholar]
- 24.Polderman KH, Tjong Tjin Joe R, Peerdeman SM, Vandertop WP, Girbes AR. Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med. 2002;28:1563–73. doi: 10.1007/s00134-002-1511-3. [DOI] [PubMed] [Google Scholar]
- 25.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–69. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 26.Luscombe M, Andrzejowski JC. Clinical applications of induced hypothermia. Contin Educ Anaesth Crit Care Pain. 2006;6:23–7. [Google Scholar]
- 27.Michelson AD, MacGregor H, Barnard MR, Kestin AS, Rohrer MJ, Valeri CR. Hypothermia-induced reversible platelet dysfunction. Thromb Haemost. 1994;71:633–40. [PubMed] [Google Scholar]
- 28.Watts DD, Trask A, Soeken K, Perdue P, Dols S, Kaufmann C. Hypothermic coagulopathy in trauma: Effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998;44:846–54. doi: 10.1097/00005373-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Valeri CR, MacGregor H, Cassidy G, Tinney R, Pompei F. Effects of temperature on bleeding time and clotting time in normal male and female volunteers. Crit Care Med. 1995;23:698–704. doi: 10.1097/00003246-199504000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Patt A, McCroskey BL, Moore EE. Hypothermia-induced coagulopathies in trauma. Surg Clin North Am. 1988;68:775–85. doi: 10.1016/s0039-6109(16)44585-8. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara A, MacArthur JD, Wright HK, Modlin IM, McMillen MA. Hypothermia and acidosis worsen coagulopathy in the patient requiring massive transfusion. Am J Surg. 1990;160:515–8. doi: 10.1016/s0002-9610(05)81018-9. [DOI] [PubMed] [Google Scholar]
- 32.Reed RL, 2nd, Bracey AW, Jr, Hudson JD, Miller TA, Fischer RP. Hypothermia and blood coagulation: Dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141–52. [PubMed] [Google Scholar]
- 33.Valeri CR, Feingold H, Cassidy G, Ragno G, Khuri S, Altschule MD. Hypothermia-induced reversible platelet dysfunction. Ann Surg. 1987;205:175–81. doi: 10.1097/00000658-198702000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128. doi: 10.1002/14651858.CD004128.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Morrison LJ, Deakin CD, Morley PT, Callaway CW, Kerber RE, Kronick SL, et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S345–421. doi: 10.1161/CIRCULATIONAHA.110.971051. [DOI] [PubMed] [Google Scholar]
- 36.Deakin CD, Morrison LJ, Morley PT, Callaway CW, Kerber RE, Kronick SL, et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2010;81(Suppl 1):e93–174. doi: 10.1016/j.resuscitation.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen N, Friberg H, Gluud C, Herlitz J, Wetterslev J. Hypothermia after cardiac arrest should be further evaluated - a systematic review of randomised trials with meta-analysis and trial sequential analysis. Int J Cardiol. 2011;151:333–41. doi: 10.1016/j.ijcard.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 39.Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011;39:1423–30. doi: 10.1097/CCM.0b013e318212020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: A randomized clinical trial. JAMA. 2014;311:45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 41.Debaty G, Maignan M, Savary D, Koch FX, Ruckly S, Durand M, et al. Impact of intra-arrest therapeutic hypothermia in outcomes of prehospital cardiac arrest: A randomized controlled trial. Intensive Care Med. 2014;40:1832–42. doi: 10.1007/s00134-014-3519-x. [DOI] [PubMed] [Google Scholar]
- 42.Saigal S, Sharma JP. Comment on Debaty et al.: Impact of intra-arrest therapeutic hypothermia in outcomes of prehospital cardiac arrest: A randomized controlled trial. Intensive Care Med. 2015;41:171. doi: 10.1007/s00134-014-3556-5. [DOI] [PubMed] [Google Scholar]
- 43.Mikkelsen ME, Christie JD, Abella BS, Kerlin MP, Fuchs BD, Schweickert WD, et al. Use of therapeutic hypothermia after in-hospital cardiac arrest. Crit Care Med. 2013;41:1385–95. doi: 10.1097/CCM.0b013e318287f2c4. [DOI] [PubMed] [Google Scholar]
- 44.Sinclair HL, Andrews PJ. Bench-to-bedside review: Hypothermia in traumatic brain injury. Crit Care. 2010;14:204. doi: 10.1186/cc8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mourvillier B, Tubach F, Wolff M. Induced hypothermia in severe bacterial meningitis: A randomized clinical trial. J Am Med Assoc. 2013;310:2174–83. doi: 10.1001/jama.2013.280506. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]


