Abstract
Hydrogel scaffolds serve as semi synthetic or synthetic extra cellular matrix to provide an amenable environment for cellular adherence and cellular remodeling in three dimensional structures mimicking that of natural cellular environment. Additionally, hydrogels have the capacity to carry small molecule drugs and/or proteins, growth factors and other necessary components for cell growth and differentiation. In the context of drug delivery, hydrogels can be utilized to localize drugs, increase drugs concentration at the site of action and consequently reduce off-targeted side effects. The current review aims to describe and classify hydrogels and their methods of production. The main highlight is chitosan-based hydrogels as biocompatible and medically relevant hydrogels for drug delivery.
Keywords: Chitosan, Crosslinking, Drug delivery, Hydrogel, Tissue engineering
1. INTRODUCTION
There has been an increasing interest in developing medically relevant hydrogels. Such an interest is due to the wide range of suitable characteristics and preparation methods for hydrogels in medical and pharmaceutical industries.
Various natural and synthetic polymers have been studied in hydrogel researches. Chitosan is a natural cationic copolymer that presents well deal of interests for hydrogel structures. This polymer has hydrophilic nature with ability of degradation via human enzymes which result in biocompatibility and biodegradability, the two biological properties commonly needed for biological devices. Chitosan-based hydrogels are potentially engineering scaffolds to obtain tissue repair achievements.
Furthermore, they have been applied as delivery systems for the controlled release of therapeutic ingredients. Many polymers used in hydrogel lattices, like chitosan, exert their mucoadhesive characteri-stics via interactions between opposite charges. This specific feature can provide the ability of tissue binding for the aim of specific drug delivery (1,2). In order to improve feasibility of chitosan for medical and pharmaceutical applications, several derivatives of chitosan with different ligands such as 4-azidobenzoic acid, methyl acroloyl glycine and poly ethylene glycol have been synthesized and studied. In this review, hydrogel structure and characterization of the systems compromised from chitosan derivatives prepared using different ligands will be briefly explained. Then, specifically chitosan-based hydrogels with biomedical and biopharmaceutical applications will be discussed and their cons and pros in comparison with each other would be interpreted.
2. Hydrogels
2.1. Definition and structure of hydrogels
Hydrogels are cross-linked networks of the same or different types of polymerswith high capacity for water absorption.
Hydrogel forming polymers have hydrophilic functional groups in their polymeric structure such as amine (NH2), hydroxyl [-OH], amide (-CONH-, -CONH2) and sulphate (-SO3 H)(3). The hydrophilic groups enable the hydrogel to absorb water and watery fluids that results in hydrogel expansion and occupation of larger volume, the process which is known as swelling. During swelling, the cross-linked structure of hydrogels prevents the dissolution and destruction of the hydrogel cross-links (4). A schematic representation of hydrogel swelling is shown in Fig. 1.
Fig. 1.
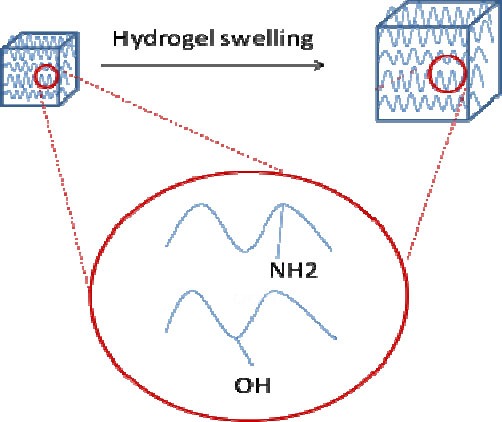
A schematic representation of hydrogel construct and hydrogel swelling in water. Circle depicts some of the possible functionalities responsible for water absorption.
There are three states of water molecules in polymeric hydrogel, free, intermediate and bound water molecules. Free water molecules undergo freezing process at freezing points since no bond exists between free water and polymer functional groups. The amount of free water molecules is dependent on the hydrogel structure that ultimately influences the swelling ratio. Thereby, a compact hydrogel structure contains lower quantity of free water. Second state of water or intermediate water can form weak interactions with functional groups in polymeric chains. Hydrogen bonding between polymeric chains and water molecules forms bound water. These water molecules are nonfreezing (5).
The amount of water absorption in different types of hydrogel is varied from trivial to significant volumes. Quantity and speed of water absorption is dependent on the following factors: i) cross-linking density, ii) chemical structure of the polymers and iii) environmental conditions.
Cross-linking density, the amount of cross-linked chains, governs the hydrogel swelling ratio and is inversely proportional to water quantity. In addition, presence of hydrophilic or hydrophobic functional groups on the polymer chain determines the swelling ratio (6). Hydrogels have ability to swell in water or aqueous solutions. Because of high amount of water absorption, these structures can be similar to human body tissues.
Hydrophobic hydrogels with hydrophobic chains such as poly (Lactic acid) (PLA) or poly (Lactide-co-glycolide) (PLGA) (7) or those prepared via polymeric modifications to enhance polymer hydrophobicity (8) have lower water capacity than hydrophilic lattices. Swelling ratio of hydrophilic hydrogels varies with polymer hydrophilic density. Environmental conditions such as pH, temperature, certain chemicals (9), light, pressure and electrical field (3) are influential on hydrogel swelling. The environmental factors control the swelling kinetics and could be modified to modulate the swelling properties of the hydrogels. For example protein-based systems are susceptible to acidic gastric environment. Therefore, pH sensitive swelling hydrogels that are swollen in intestinal pH are useful devices for the purpose of oral delivery.
2.2. Classification of hydrogels
Hydrogels are classified into natural or synthetic polymeric based networks.
Natural hydrogel constructs are often made of polysaccharide or protein chains. Polysaccharides have hydrophilic structure which is a favorable property of hydrogel preparation (10). Some examples of polysaccharide-based hydrogels are hydrogels made of alginate (11), cellulose (12), chitin, chitosan (13), dextran, hyaluronic acid (14), pectin (15), starch (16) and xanthan gum.
Collagen (17), silk, keratin, elastin, resilin (18) and gelatin (19) are protein chains that form natural hydrogel lattices.
Synthetic polymers such as poly (vinyl alcohol), polyacrylamide, poly (ethylene oxide) and poly (ethylene glycol) have been used for hydrogel formation (1). Natural polymers usually present higher biocompatibility compared to synthetic polymers, as they undergo enzyme controlled biodegradation by human enzymes like lysozyme and produce biocompatible byproducts (20). On the other hand, synthetic polymers are chemically stronger than natural ones, because of hydrolysable moieties with slower degradation rate. This feature provides more prolonged lifetime in human body (21).
2.3. Formation of hydrogels
Hydrogels are prepared via chemical (permanent bonds) or physical cross-linking. Methods for chemical cross-linking of hydrogels include i) radical polymerization (22), ii) photopolymerization (23), iii) enzymatic reactions, iv), and covalent cross-linking via linkers such as aldehydes (24). In contrast, physical cross-linking forms a nonpermanent network with physical interactions such as hydrogen or electrostatic bonds, physical entanglements (25,26) and crystal formation (27). So the physically cross-linked hydrogels can be formed via ion interactions, using graft copolymers (28), crystallization and stereocomplex formation (29).
2.4. Applications of hydrogels in pharmaceutical sciences
Hydrogels are widely used in agriculture (30), food industry (31) and pharmaceutical fields (32).
In pharmaceutical area, they are applied for systemic and localized drug delivery and tissue engineering.
2.4.1. Drug delivery
Hydrogels are used as platforms for both drugs and gene delivery (33). Hydrogels can encapsulate macromolecule drugs especially proteins (34) into their polymeric chains. Polymeric network of hydrogels protects drugs from fast dissolution (35) and control release rate from matrices (36).
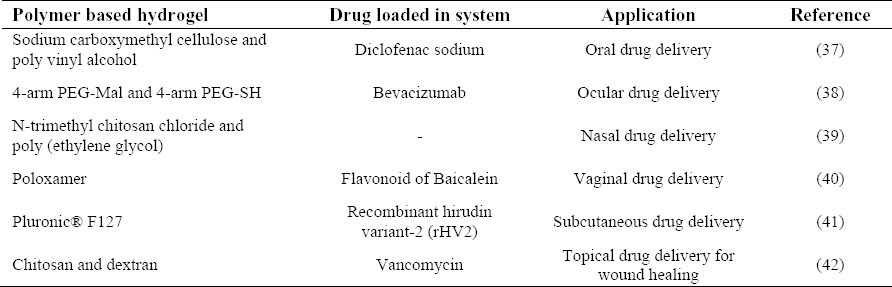
Hydrogels can be administered via oral, ocular, nasal, vaginal and subcutaneous routes. Table 1 summarizes some of studies on applying hydrogels for drug delivery.
Table 1.
Examples of applying hydrogels as drug delivery systems.
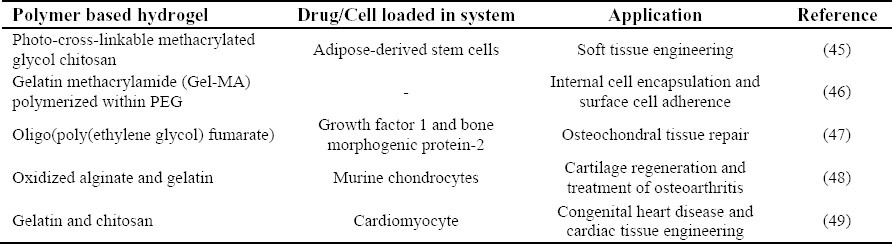
Hydrogels are also utilized extensively in tissue repairs (43,44). Some examples of this application of hydrogels are presented in Table 2.
Table 2.
Hydrogel applications in tissue engineering.
Hydrogels have also been developed as artificial cartilages (50), contact lenses, artificial corneas (5), biosensors (51) and surgical aids. Synthetic materials are also applied as alternative to extracellular matrix.
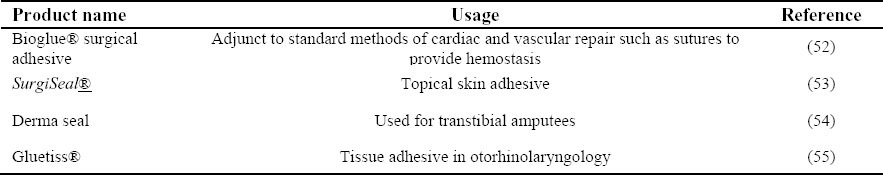
Table 3 lists some of the hydrogels marketed by different companies for their clinical applications.
Table 3.
Current marketed hydrogels and their applications.
3. Chitosan-based hydrogels
3.1. Chitosan
Chitosan is obtained by partial deacetylation (56) of insoluble naturally available chitin, obtained from exoskeletons of crustaceans (57), fungi and insects (58). Chitin has rigid crystalline structure due to hydrogen interactions between acetamide groups and hydroxyl groups (59). Chitin is not readily applicable due to its high level of acetylated groups and rigid structure as well as poor solubility in aqueous solutions. When chitin is partially deacetylated and converted to chitosan (Fig. 2), the amount of amino groups and its aqueous solubility is enhanced. There is a proportional increase in chitosan deacetylation, and enhancement of bio-compatibility and biodegradability.
Fig. 2.
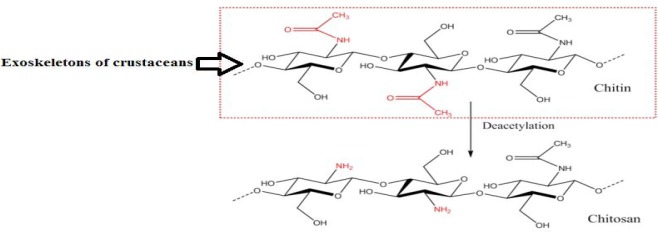
Chitin is extracted from crab shell from which chitosan is made by N-deacetylation.
The polysaccharide structure of chitosan is made of glucosamine and N-acetylglucosamine. Glucosamine is generated from glucose in body and it can produce glucosaminoglycans (GAGs), which is a part of extracellular matrix and cartilage tissue (60).
The charge density of chitosan depends on degree of deacetylation which represents the amino group density. Indeed, pH of the chitosan solution represents the quantity of ionized amino groups (61).
Chitosan is a weak base with pka 6.5 which can be dissolved in dilute acidic medium. Because of the presence of amine and hydroxyl groups, chitosan molecules can form hydrogen bonds leading to the crystalline structure of the polymer (62).
Chitosan exists in different molecular weights and degree of acetylation. The average molecular weight of chitosan lies between 50-2000 KD. Hydrophilic polymers such as chitosan may undergo systemic absorption in human body, so the polymers should have proper molecular weight to eliminate by renal filtration. In vitro studies showed that chitosan can be degraded via several enzymes such as β-N-acetylhexosaminidase, chitosanase, chitinase and chitin deacetylase. In human body, chitosan can be biodegraded by lysozyme, acid, gastrointestinal enzymes and colon bacteria (63).
3.2. Hydrogel preparation via chitosan cross-linking
The intermolecular forces between polysaccharide chains of chitosan are hydrogen, hydrophobic and ionic interactions. These interactions are influenced by molecular weight and ionic strength (64).
Cross-linking of chitosan polymers is necessary to improve chitosan properties such as stability and durability for the aim of drug delivery. Chitosan based hydrogel networks are categorized based on the method of chitosan cross-linking and preparation.
3.2.1. Preparation of chitosan hydrogels via chemical cross-linking
Chemically cross-linked hydrogels are formed by covalent linking of the chitosan macromers, where the bond formation is irreversible. Chemical cross-linked hydrogels are found in four states of formation, a) chitosan cross-linked system, b) hybrid polymer networks (HPN), c) interpenetrating polymer networks (IPN), and d) semi interpenetrating polymer networks (SIPN). Fig. 3 shows schematic representation of these four states.
Fig. 3.
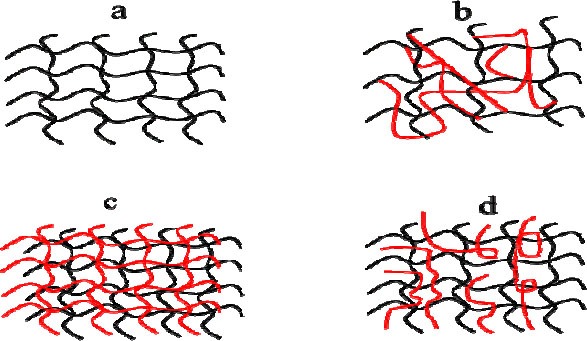
Structure of chitosan-based hydrogel prepared by covalent cross-linking. Chitosan-based hydrogels include a; only chitosan chain cross links, b; chitosan is cross linked via a different polymer, c; chitosan and another polymer are entangled and each polymer type is cross-linked, d; another polymer entangles with chitosan, where chitosan macromers are crosslinking.
The simplest type of chemical hydrogel formation occurs when chitosan undergoes cross-linking reaction with another polymeric chain of its own. Second chain can be similar to or different from first structural unit in derivation.
Amines and hydroxyl groups situated on chitosan chains are responsible for chemical cross-linking. Chemical cross-linking can occur via cross-linkers or photopolymerization reaction.
3.2.1.1. Cross-linking via cross-linkers
Cross-linking can be formed between polymers themselves or between polymers and a cross-linker (65). Cross-linkers initiate cross-linking reaction between chitosan chains (4,26).
A few of customary cross-linkers include dialdehyde compounds such as glutaraldehyde (66) and other reagents like genipine (67), palladium cation (68), diisocyanate (69), and acrylic acid (70).
Glutaraldehyde has been extensively used for chemical cross-linking of chitosan. Glutaraldehyde is mainly used for cross-linking when a second polymer is added to chitosan for modification of its properties. In an attempt, Pluronic F127 has been used for modification of chitosan and this hybrid was cross-linked by glutaraldehyde for controlled delivery of 5-FU (71). Genipine is a natural, water soluble and bifunctional cross-linking agent made of a glucoside named geniposide by β-glucosidase enzyme. Genipine is isolated from gardenia fruits and as shown in Fig. 4, it could react with amines, proteins and amino groups of chitosan (72).
Fig. 4.
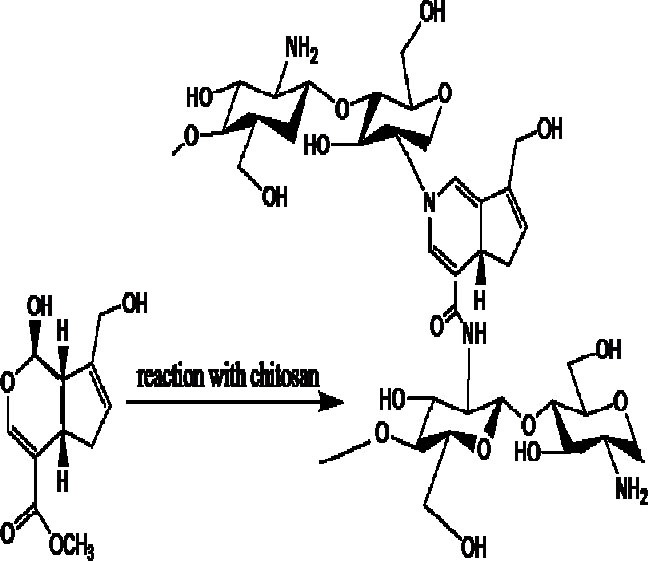
Chemical cross-linking between chitosan chains in the presence of genipin.
Genipine is widely used as cross-linker in tissue fixation, food industries and drug delivery which is due to its, lower toxicity and higher biocompatibility versus other cross-linkers especially glutaraldehyde (73). There are a large number of studies on cross-linking of chitosan by genipine. Chitosan/gelatin networks cross-linked by genipine have been developed for articular cartilage tissue repair (71). Genipine-cross-linked chitosan hydrogels present slower degradation rate in comparison to glutaraldehyde-cross-linked hydrogels and higher biocompatibility, but there is the risk of incompatibility with the therapeutic agent loaded into the hydrogel (65).
3.2.1.2.Cross-linking via photopolymerization
The other method of forming covalently cross-linked chitosan hydrogels is photopolymerization(74). Photopolymerization is the process of changing liquid precursor solution to gel with the help of photoinitiators and visible or UV irradiation. This technique is used in vivo and in vitro (75,77). The polymeric reaction is controlled by adjusting the distance and duration of exposure. UV or visible light in reaction with molecules called photoinitiators, produce free radicals which initiates radical polymerization and forms cross-linked hydrogel. Photopolymerization has a distinct advantage over general methods of polymerization and that is in situ formation of hydrogels which can be used in vivo for several applications like in laparoscopic devices, following subcutaneous injection or in different surgeries (75).
By introducing azide and lactose moieties to chitosan, a photocrosslinkable derivative of this polymer has been synthesized. This modified chitosan can be used as a tissue adhesive in punctures.
Azide modified chitosan and vinyl benzoic acid derivatives of chitosan can also provide photo cross-linked networks for different applications (78).
3.2.2. Physical cross-linking
Physical cross-linking to form chitosan-based hydrogel networks is another class of crosslinking. Physical interactions can be ionic interactions, as in ionically cross-linked chitosan hydrogels and polyelectrolyte complexes, or can be secondary interactions such as networks named grafted chitosan hydrogels and entangled chitosan hydrogels (26).
3.2.2.1. Ionically cross-linked chitosan hydrogel
Since chitosan is a cationic polyelectrolyte polymer with ionizable amine groups (56), anions are often employed as ionic cross-linkers to engineer ionically cross-linked chitosan hydrogels (Fig. 5). One of the examples is the multivalent counter ions such as phosphate bearing molecules like tripolyphosphate (TPP). This ionic cross-linking process which is also called ionic gelation of chitosan is mostly used for loading of low molecular weight drugs, but recently has been used for macromolecules as well (79).
Fig. 5.
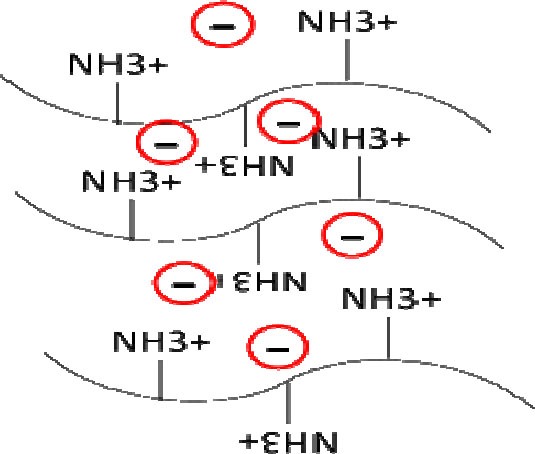
Structure of chitosan-based hydrogel prepared by ionic interactions.
3.2.2.2. Polyelectrolyte complexed chitosan hydrogel
Polyelectrolyte complex networks are formed via ionic interactions between two opposite charged polymers. Polysaccharides are good choices for preparing polyelectrolyte complex because of biocompatibility and biodegradability (61).
Oppositely charged polysaccharides interact with each other in the solution spontaneously and form polyelectrolyte complexes. Chitosan is positively charged, thereby, a negatively charged natural polymer like alginate, pectin, carrageenan, xanthan gum, chondroitin sulfate, dextran sulfate or hyaluronic acid or a synthetic one like polylactic acid, polyacrylic acid or polyphosphoric acid are suitable candidates for such interactions (80,81).
Formation of these polyelectrolyte complexes depends on a number of variables including the charge density of the polymers, mixing ratio, amount of each polymer, etc. Solubility of the resulting complex also depends on the net charge. If the net charge is zero then the complex will usually be insoluble and precipitates (80).
3.2.3. Chemical versus physical cross-linking
Type of cross-linking determines the stability of hydrogels. Covalently cross-linked hydrogels with covalent cross-linkers have permanent feature that show resistance to environmental variables. However these systems need extra process of purification to remove toxic unreacted cross-linkers.
Physically cross-linked hydrogels are more biocompatible due to the lack of chemical cross-linkers and well tolerated compared to covalently systems.
However they may have not high mechanical stability and they may react to environmental changes such as pH, temperature or ionic strength (26). This especial feature of physically cross-linked hydrogels is very useful for preparation of stimuli responsive systems that are sensitive to environmental conditions and can be used for drug delivery in specific conditions (9).
3.3. Molecules used for modification of chitosan properties
In order to improve chitosan based hydrogel properties, chitosan derivatives have been synthesized and evaluated. The functional amino groups on chitosan chains help the polymer to enter chemical reactions which generate derivatives with improved properties such as muco-adhesion, high drug loading and ability for gene transfer (82). Some other chemical modifications have gained interest to prepare photopolymerizable chitosan derivatives or to improve water solubility of chitosan.
3.3.1. Chitosan and 4-azido-benzoic acid (Az-CS)
Azido-benzoic acid is one of the cross-linking agents and photoinitiators with two functional groups, azide and carboxylic acid.
The carboxylic acid group of benzoic acid enters in the reaction with amino group of chitosan which results in preparation of photosensitive chitosan derivative. Upon UV irradiation, the azide functional group is changed into nitrene and further process causes cross-linking for gel formation. Fig. 6 shows the reaction of chitosan with 4-azido-benzoic acid and resulting photo-cross-linking. Study on Az-CS derivatives showed that the solution made gel in less than a minute under UV light and the resulting gel was very adhesive similar to fibrin glue. The resulting gel was also non-toxic in acute and chronic exposures (83).
Fig. 6.
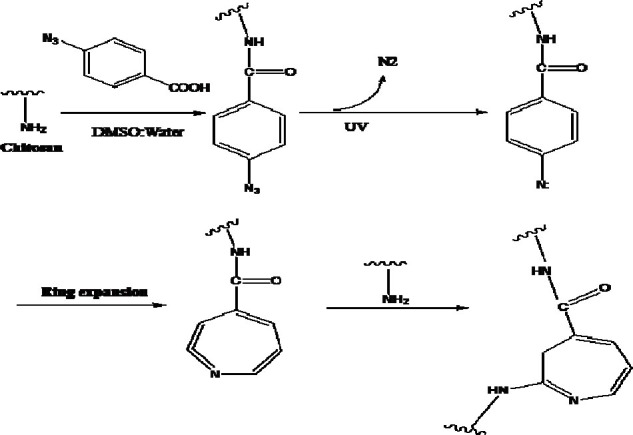
Conjugation of chitosan and 4-azidobenzoic acid and photo-cross-linking of chitosan-4-azidobenzoic acid chains (ref. 75).
3.3.2. Chitosan and methyl acroloyl glycine
The conjugation of chitosan and methyl acroloyl glycin (CS-MAG) is obtained by reaction of amino group in chitosan with carboxyl group of methyl acroloyl glycine. Compared with crystallized form of chitosan, CS-MAG is an amorphous compound with lower intermolecular forces and thermal stability. The precursor can be gelled via photopolymerization in presence of photoinitiator (84).
3.3.3. Carboxylation of chitosan
As biodegradability is one of the essential features of biocompatible polymers such as chitosan, the extent of degradability is of importance. Dependent on chitosan chain length and its application, sometimes it is needed to enhance the degradation rate of chitosan.
One of the approaches is to synthesize carboxyl methyl chitosan with higher dissolution rate in aqueous environments and faster degradation in the presence of enzymes such as lysozyme (85,86). Fig. 7 depicts the chemical structure of carboxy methyl chitosan. Carboxy methyl chitosan is water soluble in a wide range of pH along with high viscosity, gel forming ability, low toxicity and acceptable biodegradability which makes it a good option for use in food and cosmetic products (87).
Fig. 7.
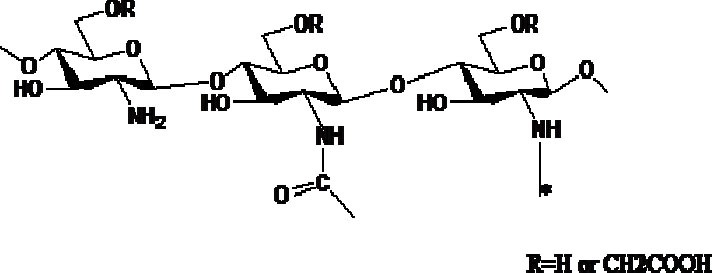
Structure of carboxymethyl chitosan.
The carboxy methyl derivative of chitosan can also be modified by photoinitiators and provide photopolymerizabale derivatives. Low molecular weight carboxy methyl chitosan modified by photoreactive 4-azido benzoyloxy succinimide has been synthesized as antiadhesive agent in surgeries and tissue regeneration (88).
3.3.4. Chitosan and poly (ethylene glycol)
Poly (ethylene glycol) (PEG) is a polymer that is dissolved in aqueous and oily phases (89). This polymer is biocompatible, non-toxic and non-immunogenic material and has FDA approval for biomedical applications.
Chitosan conjugated with PEG has been used for sustained drug release in several drug delivery applications (90) like gastro-intestinal drug delivery (91). Results indicate that, the rate of drug release form PEG-chitosan carriers is greatly slower than that of chitosan based systems (92). PEG could improve the solubility of chitosan. Investigations have shown that pegylated chitosan has significantly higher solubility than unmodified chitosan (93). One of the benefits of increasing water solubility of chitosan via pegylation is increasing its transfection efficiency as a gene carrier (94).
The use of PEG can also reduce the elimination of carrier by reticulo-endothelial system as proved for pegylated derivatives of other polymers (95).
3.3.5. Chitosan and sugar
Sugar bearing chitosan derivatives have been synthesized to increase its water solubility in a wide range of pH. These derivatives are formed through an amide bond between chitosan and hydrophilic sugar moieties (96). Modification of chitosan with monosaccharides and disaccharides can occur via chitosan N-alkylation. One important application of this kind of chitosan derivative is recognition of specific cells, viruses or bacteria. Sugar moieties can recognize specific cells and bind to the specific receptors on cell surface. This interesting characteristic is very applicable for designing targeted drug delivery systems. For example fucosylated chitosan was synthesized for its specific interaction with lectins on the cells or galactosylated chitosan was used for targeting the delivery system to the hepatocytes (97).
3.3.6. Thiolated chitosan
One of important modifications on chitosan to increase its mucoadhesiveness is thilation of chitosa. By thiolation, -SH groups are introduced to the chitosan chain which readily can form disulfide bond with cystein-rich parts in the mucus glycoproteins. Besides increasing of mucoadhesion, thilation could also increase the solubility and permeation properties of chitosan. All these changes together would have positive effect on bioavailability of drugs encapsulated in thiolated chitosan systems (98,99).
3.3.7. Chitosan and alkyltrimethylammonium
Chtiosan is a natural polymer with activity against fungi and bacteria. Antifungal activity of chitosan is mostly related to the interaction of positively charged polysaccharide with the negatively charged residues on the fungi cell wall. Substitution of alkyltrimethyl-ammonium groups such as propyl and pentyltrimethylammonium improves the antifungal activity of chitosan. Antifungal activity and interactions between derivate chitosan and cells are related to hydrocarbon chain length. As a result the activity of chitosan-pentyl derivative is higher than chitosan-propyl activity (100).
4. Chitosan-based hydrogel applications
Chitosan is known to be biocompatible and biodegradable (89,101) and its degradation products are non-toxic and non-immunogenic. Chitosan is bioadhesive and bacteriostatic (102), acts as chelating agent (103), hemostatic agent and antioxidant (104). This polymer can control bleeding via incorporating a procoagulant that helps accelerated clotting (105). Chitosan has found attention in many different fields including pharmaceutical, medical, cosmetics, agricultural and food industries (106). The pharmaceutical applications of chitosan include drug and gene delivery (107,108), wound dressing (109), tissue repair (77,110), and tissue engineering (111).
4.1. Drug delivery
Hydrogels based on chitosan and its chemical modified forms are investigated in several drug delivery applications (71).
Chitosan has cationic nature due to the presence of amine group and mucosal glycoproteins are negatively charged (112,113).
Therefore, it can adhere to negatively charged biological surfaces as a bioadhesive material. The use of bioadhesive polymers like chitosan prolongs the residence time of drug-loaded system and provides localized drug delivery (114). Chitosan also mediates paracellular transportation of drugs that greatly influences efficiency of drug delivery systems (115). As chitosan is a biocompatible and biodegradable with a structure which could be modified easily, it has been used as drug carrier for different routes of administration. Some of the most important chitosan-based drug delivery routes are discussed in the next sections.
4.1.1. Oral drug delivery
Hydrogel scaffolds can be used for drug delivery to oral cavity, stomach, intestine and colon. Delivery of drugs to oral cavity can be used to alleviate mouth diseases without the risk of first pass effect. The pH sensitive hydrogels allocate drug delivery to specified sites such as stomach or intestine and increase drug bioavailability. Colon drug delivery systems of chitosan based hydrogels can be designed for relief of diseases such as irritable or inflammatory bowel diseases (116,117).
Mucoadhesion of chitosan is an important property for improving oral absorption of drugs. Buccal tablets of nifedipine and propranolol have been formulated with chitosan as the mucoadhesive layer to enhance the systemic bioavailability of the drugs (118).
Interpenetrating networks (IPN) of chitosan and polyethylene oxide has been developed as stomach-specific drug delivery system for treatment of Helicobacter pylori. This IPN network presents pH dependent swelling and drug release properties (119).
Chitosan-poly acrylic acid hydrogels have been tested for colon specific drug delivery. Biodegradability of chitosan by colonic normal flora along with pH sensitivity of the polyacrylic acid segment, provide a potential suitable carrier for release of drug in the colonic region (120).
4.1.2. Ocular drug delivery
The major drawback of conventional ocular formulations is their short retention in the affected area. Administration of drugs in hydrogel systems could increase the retention of drug in the site, thereby increasing the chance of higher bioavailability. Thermosensitive chitosan-gelatin based hydrogel loaded with latanoprost has been used for controlling ocular hypertension (121). In an attempt diclofenac micelles loaded into nano-composite hydrogel improved drug residual time compared to diclofenac eye drop (122). A thermosensitive chitosan-glycerophosphate hydrogel increased the permeation and corneal bioavailability of ofloxacin compared to the aqueous solution. In-situ thermosensitive hydrogel of chitosan and isopropyl acrylamide was used for ocular delivery of timolol and the system doubled the drug release (71).
4.1.3. Nasal drug delivery
Chitosan is capable of opening tight junctions between epithelial cells of mucosal membranes and improving drug molecules transportation (123). Furthermore, the high water absorption and mucoadhesive potential of chitosan facilitate nasal drug delivery (124). A thermo sensitive hydrogel was prepared with chitosan and PEG. After spraying of formulation into nasal cavity, the solution formed gel at body temperature. This hydrogel system presented lower mucosal clearance and sustained drug release in site (125). Nasal administration using chitosan hydrogels was promising for delivery of vaccines and peptide drugs which oral drug delivery is not practically useful (126,127).
4.2. Wound healing
Chitosan in topical form is used for wound healing. The probable mechanism of healing is infiltration of inflammatory cells such as polymorphonuclear leukocytes, secretion of inflammatory mediators like tumor necrosis factor-α, migration of macrophages and increase in the amount of collagen. The binding of GlcNac (N-acetyl-D-glucoseamine), a part of chitosan, to specific receptors in body increases macrophage activation that results in further events such as release of biological mediators (128). Additionally, chitosan activates the complement system (129) and stimulates fibroblasts to release IL-(8) and other cytokines (130).
The major use of chitosan hydrogels for wound healing is using these systems as wound dressing and hemostatic agent to promote the process of wound healing. One of the commercially available chitosan based hemostatic products is HemCon bandage. HemCon can stop severe bleeding by attaching to negatively charged cells of tissue as well as attracting negatively charged red blood cells and forming a tight seal over the wound (131) A schematic representation of this interaction is presented in Fig. 8.
4.3. Tissue engineering
Chitosan hydrogels were used as scaffolds for tissue engineering in the past two decades. The foundation of these systems relies on two components, cells and polymeric chains of hydrogel. Biodegradability is amongst advantages of chitosan as a scaffold. Chitosan can be degraded with human enzymes like lysozyme (132). Additionally, chitosan can be modified via N-acetylation to optimize biodegradability and biocompatibility properties needed in tissue engineering applications. Chitosan with high deacetylation degree near to 100 is reported to have higher rate of degradation, cell biocompatibility and higher opportunity for cell adhesion (133). The biodegradation rate of scaffold should conform to the time that malfunction tissue requires to be repaired.
For scaffolds used in tissue engineering, porosity of chitosan-based hydrogels presents a huge impact on properties such as swelling, cell adhesion and cell proliferation rate that are of importance in tissue growth. There are methods of forming porous hydrogels for tissue regeneration including i) freeze drying, ii) gas foaming and iii) salt leaching. The method of high pressure CO2 employs CO2 gas as a foaming agent and reduces the need of organic solvents (134). As shown in Fig. 9, channels formed in the hydrogel allow host cells migration and proliferation into the injured tissue and finally replacing the malfunction organs (57).
Chitosan scaffolds can be used for regeneration of various tissues such as bone (135), cartilage (136), skin (137) and nerves (138). The treatment of central nervous system disorders is challengeable because neural cells have lower ability for regeneration. Nerve tissue engineering requires neural stem cells such as embryonic, fetal or adult stem cells (139).
5. CONCLUSION
Numerous hydrogel structures have been prepared and characterized for biomedical and biopharmaceutical applications. The significant features of these networks for in vivo applications include swelling ability, similarity to host tissues and mechanical strength as well as biodegradability. In addition to owning biocompatibility and biodegradability, biopolymers like chitosan, has potential abilities for structural modifications, which results in formation of new applicable derivatives. In addition to inherent properties of chitosan like antibacterial and antifungal activities, biocompatibility and biodegradability, different strategies to prepare chitosan derivatives make it a good carrier for pharmaceuticals, cosmetics and food products. Hydrogel preparation, modified performance and cross-linking mechanism should be related to appointed aim, for example sustained release profile for drug delivery systems or porous structural appearance for tissue engineering applications.
REFERENCES
- 1.Li H, Koenig AM, Sloan P, Leipzig ND. In vivo assessment of guided neural stem cell differentiation in growth factor immobilized chitosan-based hydrogel scaffolds. Biomaterials. 2014;35:9049–9057. doi: 10.1016/j.biomaterials.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Zhang X, Wang Y, Wu Z, An J, Lu Z, et al. In situ cross-linked polysaccharide hydrogel as extracellular matrix mimics for antibiotics delivery. Carbohydr Polym. 2014;105:63–69. doi: 10.1016/j.carbpol.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 3.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Park SJ, Kim SI. Swelling behavior of interpenetrating polymer network hydrogels composed of poly (vinyl alcohol) and chitosan. React Funct Polym. 2003;55:53–59. [Google Scholar]
- 6.Peppas NA, Khare AR. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv Drug Deliv Rev. 1993;11:1–35. [Google Scholar]
- 7.Lin CC, Metters AT. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58:1379–408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Abdurrahmanoglu S, Can V, Okay O. Design of high-toughness polyacrylamide hydrogels by hydrophobic modification. Polymer. 2009;50:5449–5455. [Google Scholar]
- 9.Zhang J, Xie R, Zhang SB, Cheng CJ, Ju XJ, Chu LY. Rapid pH/temperature-responsive cationic hydrogels with dual stimuli-sensitive grafted side chains. Polymer. 2009;50:2516–2525. [Google Scholar]
- 10.Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym Int. 2008;57:397–430. [Google Scholar]
- 11.Gao C, Liu M, Chen J, Zhang X. Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym Degrad Stabil. 2009;94:1405–1410. [Google Scholar]
- 12.Chang C, Zhang L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr Polym. 2011;84:40–53. [Google Scholar]
- 13.Dodane V, Vilivalam VD. Pharmaceutical applications of chitosan. Pharm Sci Technol To. 1998;1:246–253. [Google Scholar]
- 14.Coviello T, Matricardi P, Marianecci C, Alhaique F. Polysaccharide hydrogels for modified release formulations. J Control Release. 2007;119:5–24. doi: 10.1016/j.jconrel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Wei R, Cheng J, Cai J, Zhou J. Synthesis and characterization of pectin/poly (sodium acrylate) hydrogels. Carbohydr Polym. 2011;86:313–319. [Google Scholar]
- 16.Yoshimura T, Yoshimura R, Seki C, Fujioka R. Synthesis and characterization of biodegradable hydrogels based on starch and succinic anhydride. Carbohydr Polym. 2006;64:345–349. [Google Scholar]
- 17.Park JW, Kang YDS, Kim JS, Lee JH, Kim HW. 3D microenvironment of collagen hydrogel enhances the release of neurotrophic factors from human umbilical cord blood cells and stimulates the neurite outgrowth of human neural precursor cells. Biochem Biophys Res Commun. 2014;447:400–406. doi: 10.1016/j.bbrc.2014.03.145. [DOI] [PubMed] [Google Scholar]
- 18.Silva R, Fabry B, Boccaccini A. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials. 2014;35:6727–6738. doi: 10.1016/j.biomaterials.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 19.Gaowa A, Horibe T, Kohno M, Sato K, Harada H, Hiraoka M, et al. Combination of hybrid peptide with biodegradable gelatin hydrogel for controlled release and enhancement of anti-tumor activity in vivo. J Control Release. 2014;176:1–7. doi: 10.1016/j.jconrel.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Sokker HH, Abdel Ghaffar AM, Gad YH, Aly AS. Synthesis and characterization of hydrogels based on grafted chitosan for the controlled drug release. Carbohydr Polym. 2009;75:222–229. [Google Scholar]
- 21.Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface. 2009;6:S311–S24. doi: 10.1098/rsif.2008.0448.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KH, Kim J, Ho Jo W. Preparation of hydrogel nanoparticles by atom transfer radical polymerization of N-isopropylacrylamide in aqueous media using PEG macro-initiator. Polymer. 2005;46:2836–2840. [Google Scholar]
- 23.Kýzýlel S, Sawardecker E, Teymour F, Pérez-Luna VH. Sequential formation of covalently bonded hydrogel multilayers through surface initiated photopolymerization. Biomaterials. 2006;27:1209–1215. doi: 10.1016/j.biomaterials.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Zhou Y, Ma G, Shi S, Yang D, Lu F, et al. A water-soluble photocrosslinkable chitosan derivative prepared by Michael-addition reaction as a precursor for injectable hydrogel. Carbohydr Polym. 2010;79:507–512. [Google Scholar]
- 25.Berger J, Reist M, Mayer JM, Felt O, Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57:35–52. doi: 10.1016/s0939-6411(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 26.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57:19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 27.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu LC, Yang J, Kopeèek J. Hybrid hydrogels self-assembled from graft copolymers containing complementary â-sheets as hydroxyapatite nucleation scaffolds. Biomaterials. 2011;32:5341–5353. doi: 10.1016/j.biomaterials.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jong S, Eerdenbrugh B, Nostrum C, Bosch J, Hennink W. Physically crosslinked dextran hydrogels by stereocomplex formation of lactic acid oligomers: degradation and protein release behavior. J Control Release. 2001;71:261–275. doi: 10.1016/s0168-3659(01)00228-0. [DOI] [PubMed] [Google Scholar]
- 30.Saxena AK. Synthetic biodegradable hydrogel (Pleura Seal) sealant for sealing of lung tissue after thoracoscopic resection. J Thorac Cardiovasc Surg. 2010;139:496–497. doi: 10.1016/j.jtcvs.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Shewan H, Stokes J. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J Food Eng. 2013;119:781–792. [Google Scholar]
- 32.Deligkaris K, Tadele T, Olthuis W, Berg A. Hydrogel-based devices for biomedical applications. Sensor Actuat B-Chem. 2010;147:765–774. [Google Scholar]
- 33.Brandl F, Kastner F, Gschwind RM, Blunk T, Teßmar J, Göpferich A. Hydrogel-based drug delivery systems: Comparison of drug diffusivity and release kinetics. J Control Release. 2010;142:221–228. doi: 10.1016/j.jconrel.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Censi R, Di Martino P, Vermonden T, Hennink WE. Hydrogels for protein delivery in tissue engineering. J Control Release. 2012;161:680–692. doi: 10.1016/j.jconrel.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Geever LM, Cooney CC, Lyons JG, Kennedy JE, Nugent MJD, Devery S, et al. Characterisation and controlled drug release from novel drug-loaded hydrogels. Eur J Pharm Biopharm. 2008;69:1147–1159. doi: 10.1016/j.ejpb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Uchiyama T, Kiritoshi Y, Watanabe J, Ishihara K. Degradation of phospholipid polymer hydrogel by hydrogen peroxide aiming at insulin release device. Biomaterials. 2003;24:5183–5190. doi: 10.1016/s0142-9612(03)00441-1. [DOI] [PubMed] [Google Scholar]
- 37.Banerjee S, Siddiqui L, Bhattacharya S, Kaity S, Ghosh A, Chattopadhyay P, et al. Interpenetrating polymer network (IPN) hydrogel microspheres for oral controlled release application. Int J Biol Macromol. 2012;50:198–206. doi: 10.1016/j.ijbiomac.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Xu X, Yao F, Luo Z, Jin L, Xie B, et al. In situ covalently cross-linked PEG hydrogel for ocular drug delivery applications. Int J Pharm. 2014;470:151–157. doi: 10.1016/j.ijpharm.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 39.Nazara H, Fatouros D, Merwea SM, Bouropoulos N, Avgouropoulos G, Tsibouklis J, et al. Thermosensitive hydrogels for nasal drug delivery: The formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur J Pharm Biopharm. 2011;77:225–232. doi: 10.1016/j.ejpb.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Zhoua Q, Zhonga L, Weia X, Doua W, Choua G, Wang Z. Baicalein and hydroxypropyl-ã-cyclodextrin complex in poloxamer thermal sensitive hydrogel for vaginal administration. Int J Pharm. 2013;454:125–134. doi: 10.1016/j.ijpharm.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Lu WL, Wang J-C, Zhang X, Zhang H, Wang X-Q, et al. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic® F127 hydrogel for subcutaneous administration: In vitro and in vivo characterization. J Control Release. 2007;117:387–395. doi: 10.1016/j.jconrel.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Zhang X, Wang Y, Wu Z, An J, Lu Z, et al. In situ cross-linked polysaccharide hydrogel as extracellular matrix mimics for antibiotics delivery. Carbohydr Polym. 2014;105:63–69. doi: 10.1016/j.carbpol.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Ma G, Shi S, Yang D, Nie J. Photopolymerized water-soluble chitosan-based hydrogel as potential use in tissue engineering. Int J Biol Macromol. 2011;48:408–413. doi: 10.1016/j.ijbiomac.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Kong X, Zhang Z, Nan K, Li L, Wang X, et al. Cytotoxicity and biocompatibility evaluation of N,O-carboxymethyl chitosan/oxidized alginate hydrogel for drug delivery application. Int J Biol Macromol. 2012;50:1299–1305. doi: 10.1016/j.ijbiomac.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Cheung H, Han T, Marecak D, Watkins J, Amsden B, Flynn L. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cell. Biomaterials. 2014;35:1914–1923. doi: 10.1016/j.biomaterials.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 46.Daniele M, Adams A, Naciri J, North S, Ligler F. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials. 2014;35:1845–1856. doi: 10.1016/j.biomaterials.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Lu S, Lam J, Trachtenberg J, Lee E, Seyednejad H, Beuckend J, et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials. 2014;35:8829–8839. doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balakrishnan B, Joshi N, Jayakrishnan A, Banerjee R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014;10:3650–3663. doi: 10.1016/j.actbio.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 49.Pok S, Myers J, Madihally S, Jacot J. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013;9:5630–5642. doi: 10.1016/j.actbio.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobashi T, Tomita N, Maki Y, Chang CP, Yamamoto T. An analysis of anisotropic gel forming process of chitosan. Carbohydr Polym. 2011;84:709–712. [Google Scholar]
- 51.Peppas N. Vol. 3. Boca Raton, Florida: CRC Press; 1986. Hydrogels in medicine and pharmacy; pp. 20–30. [Google Scholar]
- 52.Chao HH, Torchiana DF. BioGlue: albumin/glutaraldehyde sealant in cardiac surgery. J Card Surg. 2003;18:500–503. doi: 10.1046/j.0886-0440.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 53.Singer AJ, Perry L. A comparative study of the surgically relevant mechanical characteristics of the topical skin adhesives. Acad Emerg Med. 2012;19:1281–1286. doi: 10.1111/acem.12009. [DOI] [PubMed] [Google Scholar]
- 54.Graf M, Freijah N. Early trans-tibial oedema control using polymer gel socks. Prosthet Orthot Int. 2003;27:221–226. doi: 10.1080/03093640308726685. [DOI] [PubMed] [Google Scholar]
- 55.Schneider G. Tissue adhesives in otorhinolaryngology. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2009;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 56.Martínez-Ruvalcaba A, Chornet E, Rodrigue D. Viscoelastic properties of dispersed chitosan/xanthan hydrogels. Carbohydr Polym. 2007;67:586–595. [Google Scholar]
- 57.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–2349. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 58.Merzendorfer H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur J Cell Biol. 2011;90:759–769. doi: 10.1016/j.ejcb.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 59.Murakami K, Aoki H, Nakamura S, Nakamura S, Takikawa M, Hanzawa M, et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials. 2010;31:83–90. doi: 10.1016/j.biomaterials.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 60.Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol. 2010;56:290–299. doi: 10.1016/j.yrtph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Sæther HV, Holme HK, Maurstad G, Smidsrød O, Stokke BT. Polyelectrolyte complex formation using alginate and chitosan. Carbohydr Polym. 2008;74:813–821. [Google Scholar]
- 62.Mi FL, Sung HW, Shyu SS, Su CC, Peng CK. Synthesis and characterization of biodegradable TPP/genipin co-crosslinked chitosan gel beads. Polymer. 2003;44:6521–6530. [Google Scholar]
- 63.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Qun G, Ajun W. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohydr Polym. 2006;64:29–36. [Google Scholar]
- 65.Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Z, Lin S, Yue T, Lee T. Adsorption of food dyes from aqueous solution by glutaraldehyde cross-linked magnetic chitosan nanoparticles. J Food Eng. 2014;126:133–141. [Google Scholar]
- 67.Pujan M, Pérez-Álvarez L, Iturbe L, Katime I. Biodegradable chitosan nanogels crosslinked with genipin. Carbohydr Polym. 2013;94:836–842. doi: 10.1016/j.carbpol.2013.01.082. [DOI] [PubMed] [Google Scholar]
- 68.Zeng M, Yuan X, Yang Z, Qi C. Novel macroporous palladium cation crosslinked chitosan membranes for heterogeneous catalysis application. Int J Biol Macromol. 2014;68:189–197. doi: 10.1016/j.ijbiomac.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 69.Zia K, Anjum S, Zubera M, Mujahid M, Jamil T. Synthesis and molecular characterization of chitosan based polyurethane elastomers using aromatic diisocyanate. Int J Biol Macromol. 2014;66:26–32. doi: 10.1016/j.ijbiomac.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen NT, Liu JH. A green method for in situ synthesis of poly (vinyl alcohol)/chitosan hydrogel thin films with entrapped silver nanoparticles. J Taiwan Inst Chem E. 2014;45:2827–2833. [Google Scholar]
- 71.Giri TK, Thakur A, Alexander A, Ajazuddin, Badwaik H, Tripathi DK. Modified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applications. Acta Pharmaceutica Sinica B. 2012;2:439–449. [Google Scholar]
- 72.Muzzarelli RAA. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr Polym. 2009;77:1–9. [Google Scholar]
- 73.Cui L, Jia J, Guo Y, Liu Y, Zhu P. Preparation and characterization of IPN hydrogels composed of chitosan and gelatin cross-linked by genipin. Carbohydr Polym. 2014;99:31–38. doi: 10.1016/j.carbpol.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 74.Chen R, Chen Q, Huo D, Ding Y, Hu Y, Jiang X. In situ formation of chitosan-gold hybrid hydrogel and its application for drug delivery. Colloids Surf B Biointerfaces. 2012;97:132–137. doi: 10.1016/j.colsurfb.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 76.Hu J, Hou Y, Park H, Choi B, Hou S, Chung A, et al. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater. 2012;8:1730–1738. doi: 10.1016/j.actbio.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 77.Amoozgar Z, Rickett T, Park J, Tuchek C, Shi R, Yeo Y. Semi-interpenetrating network of polyethylene glycol and photocrosslinkable chitosan as an in-situ-forming nerve adhesive. Acta Biomater. 2012;8:1849–1858. doi: 10.1016/j.actbio.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue eng. 2007;13:2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 79.Papadimitriou SA, Achilias DS, Bikiaris DN. Chitosan-g-PEG nanoparticles ionically crosslinked with poly (glutamic acid) and tripolyphosphate as protein delivery systems. Int J Pharm. 2012;430:318–327. doi: 10.1016/j.ijpharm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Hamman JH. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar Drugs. 2010;8:1305–1322. doi: 10.3390/md8041305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi T, Takayama K, Machida Y, Nagai T. Characteristics of polyion complexes of chitosan with sodium alginate and sodium polyacrylate. Int J Pharm. 1990;61:35–41. [Google Scholar]
- 82.Casettari L, Vllasaliu D, Lam J, Soliman M, Illum L. Biomedical applications of amino acid-modified chitosans: A review. Biomaterials. 2012;33:7565–7583. doi: 10.1016/j.biomaterials.2012.06.104. [DOI] [PubMed] [Google Scholar]
- 83.Rickett T, Amoozgar Z, Tuchek C, Park J, Yeo Y, Shi R. Rapidly photo-cross-linkable chitosan hydrogel for peripheral neurosurgeries. Biomacromolecules. 2011;12:57–65. doi: 10.1021/bm101004r. [DOI] [PubMed] [Google Scholar]
- 84.Qi Z, Xu J, Wang Z, Nie J, Ma G. Preparation and properties of photo-crosslinkable hydrogel based on photopolymerizable chitosan derivative. Int J Biol Macromol. 2013;53:144–149. doi: 10.1016/j.ijbiomac.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 85.Lu G, Kong L, Sheng B, Wang G, Gong Y, Zhang X. Degradation of covalently cross-linked carboxymethyl chitosan and its potential application for peripheral nerve regeneration. Eur Polym J. 2007;43:3807–3818. [Google Scholar]
- 86.Hjerde RJN, Vårum KM, Grasdalen H, Tokura S, Smidsrød O. Chemical composition of O-(carboxymethyl)-chitins in relation to lysozyme degradation rates. Carbohydr Polym. 1997;34:131–139. [Google Scholar]
- 87.Jimtaisong A, Saewan N. Utilization of carboxymethyl chitosan in cosmetics. Int J Cosmet Sci. 2014;36:12–21. doi: 10.1111/ics.12102. [DOI] [PubMed] [Google Scholar]
- 88.Na HN, Kim KI, Han JH, Lee JG, Son TI, Han DK, et al. Synthesis of O-carboxylated low molecular chitosan with azido phenyl group: Its application for adhesion prevention. Macromol Res. 2010;18:1001–1007. [Google Scholar]
- 89.Li Q, Yang D, Ma G, Xu Q, Chen X, Lu F, et al. Synthesis and characterization of chitosan-based hydrogels. Int J Biol Macromol. 2009;44:121–127. doi: 10.1016/j.ijbiomac.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 90.Wu J, Wei W, Wang L, Su Z, Ma G. A thermosensitive hydrogel based on quaternized chitosan and poly (ethylene glycol) for nasal drug delivery system. Biomaterials. 2007;28:2220–2232. doi: 10.1016/j.biomaterials.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 91.Buranachai T, Praphairaksit N, Muangesin N. Chitosan/polyethylene glycol beads crosslinked with tripolyphosphate and glutaraldehyde for gasterointestinal drug delivery. AAPS PharmSciTech. 2010;11:1128–1137. doi: 10.1208/s12249-010-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hassani Najafabadi A, Abdouss M, Faghihi S. Synthesis and evaluation of PEG-O-chitosan nanoparticles for delivery of poor water soluble drugs: Ibuprofen. Mater Sci Eng C Mater Biol Appl. 2014;41:91–99. doi: 10.1016/j.msec.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 93.Ito T, Yoshida C, Murakami Y. Design of novel sheet-shaped chitosan hydrogel for wound healing: A hybrid biomaterial consisting of both PEG-grafted chitosan and crosslinkable polymeric micelles acting as drug containers. Mater Sci Eng, C Mater Biol Appl. 2013;33:3697–3703. doi: 10.1016/j.msec.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 94.Chan P, Kurisawa M, Chung JE, Yang YY. Synthesis and characterization of chitosan-g-poly (ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials. 2007;28:540–549. doi: 10.1016/j.biomaterials.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 95.Stolnik S, Illum L, Davis SS. Long circulating microparticulate drug carriers. Adv Drug Deliv Rev. 1995;16:195–214. [Google Scholar]
- 96.Park JH, Cho YW, Chung H, Kwon IC, Jeong SY. Synthesis and characterization of sugar-bearing chitosan derivatives: aqueous solubility and biodegradability. Biomacromolecules. 2003;4:1087–1091. doi: 10.1021/bm034094r. [DOI] [PubMed] [Google Scholar]
- 97.Mourya VK, Inamdar NN. Chitosan-modifications and applications: Opportunities galore. React Funct Polym. 2008;68:1013–1051. [Google Scholar]
- 98.Kurniawan DW, Fudholi A, Susidarti RA. Synthesis of thiolated chitosan as matrix for the preparation of metformin hydrochloride microparticles. Res Pharm. 2012;2:26–35. [Google Scholar]
- 99.Zhu X, Su M, Tang Sh, Wang L, Liang X, Meng F, et al. Synthesis of thiolated chitosan and preparation nanoparticles with sodium alginate for ocular drug delivery. Mol vis. 2012;18:1973–1982. [PMC free article] [PubMed] [Google Scholar]
- 100.de Oliveira Pedro R, Takaki M, Gorayeb TCC, Del Bianchi VL, Thomeo JC, Tiera MJ, et al. Synthesis, characterization and antifungal activity of quaternary derivatives of chitosan on Aspergillus flavus. Microbiol Res. 2013;168:50–55. doi: 10.1016/j.micres.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 101.Tamura H, Furuike T, Nair SV, Jayakumar R. Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr Polym. 2011;84:820–824. [Google Scholar]
- 102.Xu T, Xin M, Li M, Huang H, Zhou S. Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. Carbohydr Polym. 2010;81:931–936. [Google Scholar]
- 103.Delben F, Stefancich S, Muzzarelli RAA. Chelating ability and enzymatic hydrolysis of water-soluble chitosans. Carbohydr Polym. 1992;19:17–23. [Google Scholar]
- 104.Ai H, Wang F, Xia Y, Chen X, Lei C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012;132:493–498. doi: 10.1016/j.foodchem.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 105.Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29:23–32. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 106.Peniche C, Argüelles-Monal W, Goycoolea F. Monomers, Polymers and Composites from Renewable Resources. In: Belgacem M, Gandini A, editors. Amsterdam: Elsevier; 2008. pp. 517–542. [Google Scholar]
- 107.Islam A, Riaz M, Yasin T. Structural and viscoelastic properties of chitosan-based hydrogel and its drug delivery application. Int J Biol Macromol. 2013;59:119–124. doi: 10.1016/j.ijbiomac.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 108.Buschmann M, Merzouki A, Lavertu M, Thibault M, Jean M, Darras V. Chitosans for delivery of nucleic acids. Adv Drug Deliv Rev. 2013;65:1234–1270. doi: 10.1016/j.addr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chena S, Tsaoa C, Chang C, Laid Y, Wu M, Chuang C, et al. Assessment of reinforced poly (ethylene glycol) chitosan hydrogels as dressings in a mouse skin wound defect model. Mater Sci Eng C Mater Biol Appl. 2013;33:2584–2594. doi: 10.1016/j.msec.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 110.Rickett TA, Amoozgar Z, Tuchek CA, Park J, Yeo Y, Shi R. Rapidly photo-cross-linkable chitosan hydrogel for peripheral neurosurgeries. Biomacromolecules. 2011;12:57–65. doi: 10.1021/bm101004r. [DOI] [PubMed] [Google Scholar]
- 111.Parka H, Choia B, Hua J, Lee M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013;9:4779–4786. doi: 10.1016/j.actbio.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 112.He P, Davis SS, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–88. [Google Scholar]
- 113.Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–48. [Google Scholar]
- 114.Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17:1553–1561. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 115.De Campos AM, Sánchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm. 2001;224:159–168. doi: 10.1016/s0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 116.Yang J, Chen J, Pan D, Wan Y, Wang Z. pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydr Polym. 2013;92:719–725. doi: 10.1016/j.carbpol.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 117.Mukhopadhyay P, Sarkar K, Bhattacharya S, Bhattacharyya A, Mishra R, Kundu PP. pH sensitive N-succinyl chitosan grafted polyacrylamide hydrogel for oral insulin delivery. Carbohydr Polym. 2014;112:627–637. doi: 10.1016/j.carbpol.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 118.Remuñán-López C, Portero A, Vila-Jato JL, Alonso MJ. Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery. J Control Release. 1998;55:143–152. doi: 10.1016/s0168-3659(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 119.Patel V, Amiji M. Preparation and characterization of freeze-dried chitosan-poly (ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm Res. 1996;13:588–593. doi: 10.1023/a:1016054306763. [DOI] [PubMed] [Google Scholar]
- 120.Gong S, Tu H, Zheng H, Xu H, Yin Y. Chitosan-g-PAA hydrogels for colon-specific drug delivery: Preparation, swelling behavior and in vitro degradability. J Wuhan Univ Technol Mater Sci Ed. 2010;25:248–251. [Google Scholar]
- 121.Chenga YHHung KHTsai TH, Lee CJ, Ku RY, Chiu AW, et al. Sustained delivery of latanoprost by thermosensitive chitosan-gelatin-based hydrogel for controlling ocular hypertension. Acta Biomater. 2014;10:4360–4366. doi: 10.1016/j.actbio.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 122.Li X, Zhang Z, Chen H. Development and evaluation of fast forming nano-composite hydrogel for ocular delivery of diclofenac. Int J Pharm. 2013;448:96–100. doi: 10.1016/j.ijpharm.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 123.Casettaria L, VIIasaliu D, Castagninoa E, Stolnikb S, Howdlec S, Illum L. PEGylated chitosan derivatives: Synthesis, characterizations and pharmaceutical applications. Prog Polym Sci. 2012;37:659–685. [Google Scholar]
- 124.Nazar H, Fatouros DG, Merwe SM, Bouropoulos N, Avgouropoulos G, Tsibouklis J, et al. Thermosensitive hydrogels for nasal drug delivery: The formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur J Pharm Biopharm. 2011;77:225–232. doi: 10.1016/j.ejpb.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 125.Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel based on quaternized chitosan and poly (ethylene glycol) for nasal drug delivery system. Biomaterials. 2007;28:2220–2232. doi: 10.1016/j.biomaterials.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 126.Dyer AM, Hinchcliffe M, Watts P, Castile J, Jabbal-Gill I, Nankervis R, et al. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm Res. 2002;19:998–1008. doi: 10.1023/a:1016418523014. [DOI] [PubMed] [Google Scholar]
- 127.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 128.Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105–115. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 129.Minami S, Suzuki H, Okamoto Y, Fujinaga T, Shigemasa Y. Chitin and chitosan activate complement via the alternative pathway. Carbohydr Polym. 1998;36:151–155. [Google Scholar]
- 130.Ishihara M, Ono K, Saito Y, Yura H, Hattori H, Matsui T, et al. Photocrosslinkable chitosan: an effective adhesive with surgical applications. Int Congr Ser. 2001;1223:251–257. [Google Scholar]
- 131. http://www.hemcon.com/
- 132.Pangburn SH, Trescony PV, Heller J. Lysozyme degradation of partially deacetylated chitin, its films and hydrogels. Biomaterials. 1982;3:105–108. doi: 10.1016/0142-9612(82)90043-6. [DOI] [PubMed] [Google Scholar]
- 133.Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26:5872–5878. doi: 10.1016/j.biomaterials.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 134.Ji C, Khademhosseini A, Dehghani F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials. 2011;32:9719–9729. doi: 10.1016/j.biomaterials.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 135.Cao L, Werkmeister J, Wanga J, Veronica Glattauerb V, McLean K, Liu C. Bone regeneration using photocrosslinked hydrogel incorporating rhBMP-2 loaded 2-N, 6-O-sulfated chitosan nanoparticles. Biomaterials. 2014;35:2730–2742. doi: 10.1016/j.biomaterials.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 136.Mirahmadi F, Tafazzoli-Shadpoura M, Shokrgozarb M, Bonakdar S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2013;33:4786–4794. doi: 10.1016/j.msec.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 137.Miguela S, Ribeiroa M, Brancala H, Coutinho P, Correia I. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr Polym. 2014;111:366–373. doi: 10.1016/j.carbpol.2014.04.093. [DOI] [PubMed] [Google Scholar]
- 138.Gnavi S, Barwig C, Freier T, Haastert-Talini K, Grothe C, Geuna S. The Use of chitosan-based scaffolds to enhance regeneration in the nervous system. Int Rev Neurobiol. 2013;109:1–62. doi: 10.1016/B978-0-12-420045-6.00001-8. [DOI] [PubMed] [Google Scholar]
- 139.Li X, Katsanevakis E, Liu X, Zhang N, Wen X. Engineering neural stem cell fates with hydrogel design for central nervous system regeneration. Prog Polym Sci. 2012;37:1105–1129. [Google Scholar]


