Abstract
Glutamate neurotoxicity and pro-inflammatory cytokines have an important role in the central sensitization of neuropathic pain. The purpose of the present study was to evaluate anti-hyperalgesic effect of repeated administration of ceftriaxone, which selectively activates and increases the expression of glutamate transporter, as well as minocycline, a selective inhibitor of microglia activation, either alone or together in Wistar rats subjected to the chronic constriction injury (CCI) of sciatic nerve. Ceftriaxone (100, 150 and 200 mg/kg) and minocycline (25, 50 and 100 mg/kg) were administered intraperitoneally from the day of surgery for seven consecutive days. Thermal hyperalgesia was assessed by focal radiant heat source on the hind paw of animals one day before surgery and on 3, 5, 7, 10 and 14 days following that. Ceftriaxone dose dependently, attenuated thermal hyperalgesia in animals. None of the administered doses of minocycline affected the CCI induced-thermal hyperalgesia in neuropathic animals. A fixed dose of minocycline (50 mg/kg) combined with three different doses of ceftriaxone administered for 7 consecutive days yielded a potentiating effect in the enhancing latency time to noxious thermal stimulus remaining until the 14th day post-surgery. The results suggest that modulation of microglia activity could have a supportive role in the improvement of CCI-induced thermal hyperalgesia and combination of such classes of drugs which have no antibiotic effects could be a new and promising therapeutic strategy for treatment.
Keywords: Thermal hyperalgesia, Minocycline, Ceftriaxone, Neuropathic pain, Rat
INTRODUCTION
Injury to peripheral nerves is one of the most common causes of neuropathic pain. This kind of chronic pain is characterized by two hallmarks of allodynia and hyperalgesia subsequent to nerve injury (1,2). Neuropathic symptoms poorly respond to current drugs and still a large percentage of patients have been suffering from pain. Presently available therapies are non-steroidal anti-inflammatory drugs (NSAIDs), opioids, tricyclic anti-depressants, anticonvulsants, anti-arrhythmics and topical agents. Unfortunately all of these treatments are of limited efficacy due to the serious adverse effects restricting their usefulness (3). Other explanations for low satisfactory of patients may be due to unresolved exact cellular and molecular mechanisms involved as well as interaction of complex mechanisms in the induction of this kind of pain (4).
Many studies have demonstrated that microglia may have an important role in the pathogenesis of neuropathic pain (5,6). The neuroprotective role of minocycline, a second-generation tetracycline with good penetration into CNS, has been shown in neurodegenerative diseases such as multiple sclerosis, Parkinson's disease, spinal cord injury and stroke (7,8). However, there are few and inconsistent results on its effects on neuropathic pain.
Mechanical allodynia was attenuated by chronic intrathecal administration of minocycline in traumatic spinal cord injury in rats (9,10). Another study indicated that intraperitoneal (i.p.) administration of minocycline attenuates mechanical hyper-algesia and allodynia (11). Thermal anti-hyperalgesic effect of minocycline at doses of 10, 20 and 40 mg/kg in warm water (42 °C) was revealed in a study by Zanjani and co-workers (12).
It has been reported that repeated i.p. administration of minocycline attenuated mechanical hyperalgesia in chronic constriction injury (CCI) rats (13). In contrast with these results, anti-nociceptive effect of systemic administration of minocycline was not shown by Hua and co-workers (14). Tolerance to morphine decreased by minocycline and pentoxyfylline in CCI-exposed mice, while these drugs have no effect on mechanical allodynia and thermal (cold plate) hyperalgesia, individually (15).
On the other hand, the role of glutamate, the primary excitatory amino acid neuro-transmitter, has been established in the central sensitization of neuropathic pain and the development of hyperalgesia. Clearance of glutamate in neural synaptic space has been done via transporters, among these glutamate transporter 1 (GLT1) provides more than 90% of the total glutamate uptake which mostly occurs in astroglia, other major member of glia (16).
It has been indicated that downregulation of GLT1 has a major role in several neuro-degenerative diseases including neuropathic pain (17,18). It has recently been shown that the level of GLT1 is increased by β-lactam antibiotics (19). Antinociceptive effect of ceftriaxone as a member of β-lactam family has been recently reported (20).
We recently reported that co-administration of ceftriaxone and minocycline produces more anti-allodynic effect than that presented by each of the drugs alone (21). Therefore this investigation was set to evaluate the effect of ceftriaxone and minocycline alone and in combination on the CCI-induced thermal hyperalgesia (as the other behavioral response of neuropathic pain) in rats for assessing whether a low subeffective dose of minocycline would potentiate the anti-hyperalgesia effects of ceftriaxone.
MATERIALS AND METHODS
Animals
All animal procedures followed the ethical guidelines for investigation on experimental pain in conscious animals (22). Experiments were performed on healthy male Wistar rats weighting 220-260 g, maintained on standard conditions with free access to rat food and water. The experiments were done between 8 a.m to 13 p.m to prevent fluctuation in response. The experimental protocol was approved by Isfahan University of Medical Sciences and conducted in accordance with the internationally accepted principles for laboratory animal use and care (22).
Drugs and solutions
Ceftriaxone (JaberEbneHayyan Pharma-ceutical Co., Tehran, Iran) was dissolved in saline solution (0.9% NaCl) and injected in a dosage range of 100, 150 and 200 mg/kg/i.p. once daily, from the day of surgery for 7 continuous days. Minocycline (STADA, Arzneimittel, GmbH) was suspended in water using tween 80 and injected in a dosage range of 25, 50 and 100 mg/kg/i.p. once daily, from the day of surgery for 7 continuous days. Test doses were chosen with respect to earlier studies (15,20). Gabapentin was donated by Tehran Darou Pharmaceutical Co. (Tehran, Iran), dissolved in saline solution (0.9% NaCl) and injected at a dose of 100 mg/kg once daily for 7 continuous days.
Chronic constriction injury surgery of sciatic nerve
CCI, a classic model of neuropathic pain, originally described by Bennetand Xie, was induced in animals (23). Induction of anesthesia was performed by a cocktail consisting ketamine (64 mg/kg, i.p.) and xylazine (1.4 mg/kg, i.p.). After shaving the skin, the surgical area was scrubbed by povidone iodine solution. A blunt incision was made at the mid-thigh level of left hind paw through biceps femoris muscle to expose common sciatic nerve. The connective tissue was freed from nerve. Next, four ligatures by 4-0 chromic gut suture, proximal to the sciatic trifurcation, were loosely placed around the sciatic nerve until a slight twitching was observed in the expected hind paw with an intervals of 1-1.5 mm among sutures. In sham-operated rats, the same surgical procedure was followed but without any ligature around the nerve. Animals were then placed in a heated area for surgical recovery and after that kept in pairs.
Study Protocol
Rats were randomly divided into the following groups:
-
1)
Control group: The animals subjected to CCI surgery were treated with vehicle. Vehicle was 1% tween 80 in saline (v/v) and administered at a dose of 1 ml/kg.
-
2)
Sham groups: The animals without the CCI surgery were treated with vehicle and the highest dose of ceftriaxone (200 mg/kg) or minocycline (100 mg/kg), respectively.
-
3)
Groups I, II and III: CCI surgery animals were treated with three different doses of ceftriaxone alone (100, 150 and 200 mg/kg), respectively.
-
4)
Groups IV, V and VI: CCI surgery animals were treated with three different doses of minocycline alone (25, 50 and 100 mg/kg), respectively.
-
5)
Groups VII, VIII and IX: CCI surgery animals were treated with three combinations of minocycline (50 mg/kg) and ceftriaxone (100, 150 and 200 mg/kg), respectively.
-
6)
CCI surgery animals were treated with gabapentin (100 mg/kg) alone.
Dosage selection in combination therapies was based on the results from single administration of drugs. Administration of drugs was begun immediately following surgery and continued for 7 successive days. All of drugs were administered via intraperitoneal route.
Thermal withdrawal threshold
Thresholds for response to thermal stimulus was assessed with a plantar test apparatus (model 37370; Ugo Basile Biological Instruments, Comerio, Italy), according to Hargreaves methods (24). At first the animals were placed in the clear Plexiglas chambers of the apparatus on the top of a glass floor to accommodate to the new environment, for about 15 min. When the animals became calm, a movable infrared radiant heat source was focused behind the paw and activated. The time from initial heat source activation until paw withdrawal was recorded in seconds. Paw withdrawal latency was defined as the amount of time it took until hind paw began to shake or remove from the surface. The test was carried out 3-4 times every 1 min and the average was considered as the result of the test. A cut-off time of 30 s was chosen to prevent tissue injury.
The test was repeated if the withdrawal of the paw was suspected to be as locomotion or grooming. The experiments were conducted in a randomized and blinded manner by the same experimenter. Assessment of thermal hyperalgesia performed at different times, before (baseline latency) and after surgery (i.e., days of 3, 5, 7, 10 and 14 post-CCI). Experimenter was blinded with respect to drug administration. For comparison of the data, they were converted to percent maximum possible effect (%MPE) according to the following equation:
%MPE= [(AUC value of treatment) − (AUC value of control)/(ceiling AUC value of assay) − (AUC value of control)] ×100 (25).
Statistics
Time-courses of antinociceptive effects of drugs either individually or in combination were constructed by plotting the paw withdrawal latency as a function of time. A two-way analysis of variance (ANOVA) with repeated measures followed by Bonferroni's post-hoc analysis was used to examine the time-courses of behavioral changes after various treatments.
The overall antinociceptive effects of drugs during the 14-day observation period were expressed as the area under the curve (AUC) calculated by trapezoidal method (26). The results then converted to the MPE%. If the observed MPE% of combination therapy exceeded the theoretical sum of MPE% of individual drugs, it was considered as a potentiating effect and if they were similar, it was considered as additive effect and for the case of theoretical sum less than observed effect, it was considered as a sub additive interaction (26). Data of MPE% were analyzed by ANOVA followed by Tukey post-hoc comparison test and presented as mean ± SEM for 6-8 rats per group. Statistical significance between two groups (the theoretical response and the experimentally derived values) was evaluated using the Student's t-test. For analysis of data, SPSS software was used. Differences at P<0.05 were considered as significant.
RESULTS
Anti-hyperalgesia effects of single administration of drugs
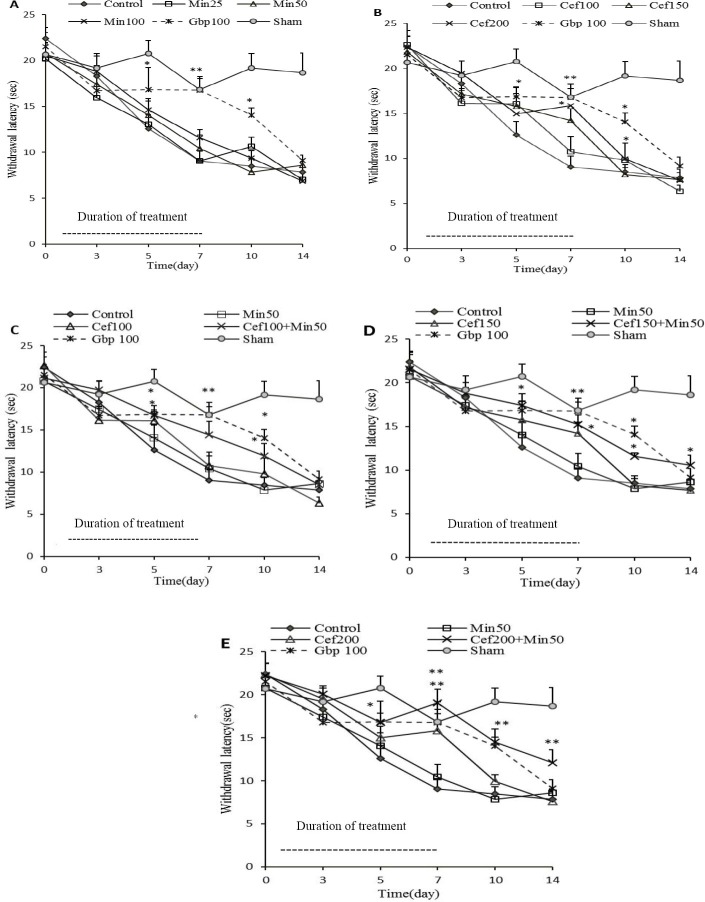
When paw withdrawal latency to painful (constant intensity) radiant heat stimulus decreases, it means that thermal hyperalgesia has been developed. After five days of operation, vehicle-treated CCI group (control) showed significant hyperalgesia to radiant heat beam compared to pre-surgery day (day 0). This reduction in latency time to heat stimulus was from 22.4 ± 1.5 s on day 0 to 12.7 ± 1.5 s on day 5 following surgery (P<0.01 by paired t-test). On the contrary, sham operated group behaved normally and showed no significant variation to radiant heat stimulation (20.7 ± 1.7 s and 20.6 ± 1.9 s on day 0 and 5, respectively) which was stable throughout the study. High doses of ceftriaxone and minocycline administered to sham animals showed no anti-hyperalgesic effect per se (data not shown). Thermal hyperalgesia was not attenuated in CCI animals receiving three different doses of minocycline as compared to control group. Ceftriaxone at low doses of 100 and 150 mg/kg also was not superior to vehicle in increasing the latency to thermal stimulus. However, latency to thermal stimulus was significantly increased by ceftriaxone at the dose of 200 mg/kg on days 5, 7, and 10 post-surgery. Repeated administration of gabapentin attenuated thermal hyperalgesia in CCI rats, although this improvement was reversible and declined following discontinuation of treatment. The time course of anti-hyperalgesic effects of drugs is depicted in Fig. 1 (A and B).
Fig. 1.
Time course of the antihyperalgesic effects produced by repeated administration of minocycline (Min 25, 50 and 100 mg/kg, A) and ceftriaxone (Cef 100,150, and 200 mg/kg, B), as well as the combination of a fixed dose of minocycline (50 mg/kg) with three different doses of ceftriaxone (100,150, and 200 mg/kg) in chronic constriction injury (CCI)animals (C, D and E, respectively). Administration of drugs was performed once daily from the day of surgery, for 7 consecutive days. Dotted horizontal bars indicate the duration of drug administration. Withdrawal threshold to radiant heat stimulus was assessed at days 3, 5,7,10, and 14 post-surgery. Data were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc analysis to examine the time-courses of behavioral changes after various treatments. Data were expressed as mean ± SEM for n=6-8/group. *P<0.05, **P<0.01 indicate significant difference in comparison to control (CCI-vehicle) group. Gabapentin (Gbp 100 mg/kg) is the reference drug.
Anti-hyperalgesia effects of drugs in simultaneous administration
No visible adverse effects were observed with the doses of ceftriaxone tested in this study whereas minocycline treated animals with dose of 100 mg/kg daily showed diarrhea during the study. Due to ineffectiveness of minocycline at any of the administered doses, the interaction of both drugs could not be evaluated by the isobolographic method. Thereby, we selected a fixed dose of minocycline (50 mg/kg) with three different doses of ceftriaxone.
The AUC of combination therapies was calculated based on the time course of anti-hyperalgesic activity of drugs in combination and then anti-hyperalgesic effect was expressed as the percentage of MPE% and compared to the theoretical sum of the effects when administered individually (expected value).
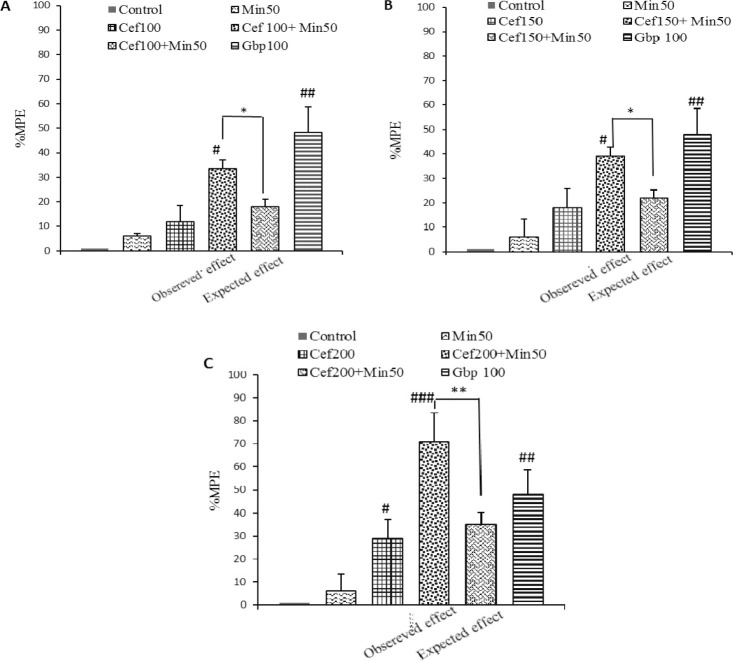
Comparison of the MPE% on thermal hyperalgesia with combination of minocycline (50 mg/kg/day), and ceftriaxone 100 mg/kg which had no anti-hyperalgesic effect in CCI animals, resulted in a significantly higher effect than the one corresponding to theoretical sum of individual drugs (33 ± 13 vs. 18.5 ± 3, P<0.05 by student's t-test; Fig. 2, panel A). Giving minocycline (50 mg/kg) to animals receiving ceftriaxone 150 mg/kg produced even a higher latency time to thermal stimulus with a MPE about 39 ± 2. 5 which was significantly different from that was expected from alone administration of drugs (21 ± 3.1, P<0.05 by student's t-test; Fig. 2, panel B), indicating a potentiation of effect.
Fig. 2.
Antihyperalgesia effect produced by repeated i.p. co-administration of minocycline (Min 50 mg/kg) with A; ceftriaxone 100 mg/kg (Cef 100), B; ceftriaxone 150 mg/kg (Cef 150) and C; ceftriaxone 200 mg/kg (Cef 200) daily to animals subjected to chronic constriction injury(CCI) surgery. Data are expressed as the percentage of maximal possible effect (%MPE). Withdrawal threshold was assessed at days 3, 5, 7, 10, and 14 after surgery. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey post-hoc test. Statistical significance between two groups (the theoretical response and the experimentally derived values) was evaluated using the Student's t-test and expressed as mean ± SEM for n=6-8 group.*P<0.05, **P<0.01 comparison is made between observed and expected effects of drug combination with paired t test, #P<0.05, ##P<0.01, ###P<0.001 indicate significant change in comparison to control (CCI-vehicle) group.
When comparing percentages of maximum experimental and theoretical effects of 50 mg/kg minocycline combined with 200 mg/kg ceftriaxone for seven days, combination again yielded a potentiating effect (64 ± 12.6 vs. 33 ± 5.1, P<0.01 by student's t-test; Fig. 2, panel C).
Gabapentin exhibited an MPE about 48.2 ± 10.5. Time course of antihyperalgesic effects of the combination treatments revealed that antihyperalgesic effect remained even up to the 14th day after surgery (seven days after the discontinuation of drugs) with ceftriaxone 200 plus minocycline 50 (P<0.01) as well as ceftriaxone 150 plus minocycline 50 (P<0.05), but not the low dose of ceftriaxone (100 mg/kg) + minocycline and reference drug, gabapentin (Fig. 1, panel C, D and E).
DISCUSSION
Glutamate neurotoxicity and increase in the pro-inflammatory cytokines have been contributed to the central sensitization of neuropathic pain. Minocycline, a second-generation tetracycline which is a selective inhibitor of microglia activation (7,8), and ceftriaxone, a member of beta lactam family, which is selectively activates and increases the expression of GLT1 (19), have been suggested to possess therapeutic potential for the treatment of a variety of neurological disorders, distinct from their antimicrobial properties.
This study aimed to evaluate anti-hyperalgesic activity of repeated admin-istration of minocycline and ceftriaxone against painful thermal stimulus alone and in combination, in rats underwent to CCI model of peripheral neuropathic pain. An advantage of CCI is inducing a profound thermal hyperalgesia compared to what is seen in other kinds of neuropathic pain models (27).
Vehicle-treated animals subjected to CCI developed hypersensitivity to radiant thermal stimulus is in line with previous studies (27). We observed that administration of ceftriaxone, alone, was effective in reducing CCI-induced thermal hyperalgesia only at the highest dose (200 mg/kg, i.p.).
Hu and colleagues also evaluated thermal antihyperalgesic activity of ceftriaxone in CCI model of neuropathic pain in Sprague–Dawley rats. In Hu and co-workers study, thermal hyperalgesia was evoked 3 days post-surgery with a mean latency about 14 s before surgery, whereas in the present study, it was observed on the 5th day after the surgery with a baseline threshold about 22 s. The observed discrepancies could be due to using different animal species, suggesting the importance of genetic impact on the hypersensitivity to pain, and different applied methods in experiments (i.e. different intensity of applied thermal stimuli). In contrast, thermal hyperalgesia was unaffected by 7-days administration of minocycline at any of doses tested for, in our laboratory.
Our data are in agreement with the results obtained by i.p. minocycline on mechanical allodynia and cold hyperalgesia in Mika and co-workers and Hua co-workers studies (14,15) but are in contrast to the results by intrathecal administration of minocycline (9,28) which suggests the important role of administration route. However, minocycline crossing from blood brain barrier has completely been established (29). Therefore it might be hypothesized that i.p. administration of minocycline is not able to reach enough high concentration in the CNS or after systemic administration some interfering peripheral or central mechanisms oppose the possible spinal anti-nociception effect of minocycline.
Since anti-allodynic effect of minocycline has been shown in many studies (12,21), another plausible explanation would be participation of different mechanisms in the induction of thermal hyperalgesia and mechanical allodynia/hyperalgesia. It has previously been shown that myelinated A fibers (Aδ and Aβ) convey mechanical noxious and the non-noxious signals along with cold noxious signals, while the non-myelinated C fibers mediate mainly heat nociception (30,31).
Therefore, it might be speculated that microglia activation is somewhat different in the stated pathways and have more important role in the allodynia induced by myelinated A fibers rather than to thermal hyperalgesia induced by non-myelinated C fibers. The lack of efficacy was observed with minocycline in our study might be due to different treatment procedures as in most of studies, one or two preemptive doses of minocycline is administered before induction of injury (11). In our study however, treatment was started following surgery suggesting the limited role of microglia after induction of injuries.
However, analysis of MPE% based on the AUC of combination treatments at all of applied doses of ceftriaxone showed potentiation against thermal hypersensitivity in CCI animals. Interestingly more antihyperalgesic effect against thermal stimulus was observed with the high dose of ceftriaxone plus minocycline compared to reference drug gabapentin. Although the attenuation of thermal hyperalgesia with combination therapies decreased after discontinuation of treatment (on the 7th day post-surgery) but remained significantly different through 14th day after the surgery with doses 200 + 50 and 150 + 50 mg/kg of ceftriaxone+minocycline respectively, compared to ceftriaxone alone. As a result, a sustained antinociceptive effect would be hypothesized with the combination therapy.
It should be mentioned that although enhanced concentration of glutamate leads to excitotoxicity and pathological events; however, it is a crucial neurotransmitter in CNS which takes part in many physiologic processes including excitatory synaptic transition (32).
As a result, complete clearance of glutamate might not be a good strategy. Furthermore, reversal of microglia activity is not a desirable goal because these cells are also responsible for producing trophic factors and immune surveillance (33,34). Selective ablation of microglia led to exacerbation of ischemia injury in brain (35). Hence, combination therapy with lower doses of applied drugs could prevent such undesirable adverse effects. Generally, since minocycline had no effect on thermal hypersensitivity of its own, it seems that spinal neuroimmune dysregulation has less powerful effect in the development of thermal hyperalgesia. Colburn and co-workers. reported less correlation of microglia activation compared to astrocytes in inducing peripheral neuropathic pain behavioral changes (36).
In summary, the exact mechanisms involved in the thermal antihyperalgesic effects of minocycline and ceftriaxone in combined was not evaluated in our study. However, considering the beneficial effects of combination pharmacotherapy for neuropathic pain and based on our results, it seems that application of minocycline together with ceftriaxone could have more antinociceptive effect. Designing new antibiotic derivatives that are devoid of antibacterial activity with interfering on the stated pathways would be a promising neuroprotective strategy in neurodegenerative diseases such as neuropathic pain.
CONCLUSION
In summary, the exact mechanisms involved in the thermal antihyperalgesic effects of combination of minocycline and ceftriaxone were not evaluated in our study. However, considering the beneficial effects of combination pharmacotherapy for neuropathic pain and based on our results, it seems that application of minocycline together with ceftriaxone could have more antinociceptive effect. Designing new derivatives of these antibiotics that are devoid of antibacterial activity with effect on the stated pathways would be a promising neuroprotective strategy in neurodegenerative diseases such as neuropathic pain.
ACKNOWLEDGMENTS
The content of this paper is extracted from Ph.D thesis NO. 389239 submitted by B. Amin which was financially supported by Vice Chancellor for Research, Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73:123–139. doi: 10.1016/S0304-3959(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ, Mannion RJ. Neuropathic pain: Etiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 3.Sah DWY, Ossipo MH, Porreca F. Neurotrophic factors as novel therapeutics for neuropathic pain. Nat Rev Drug Discov. 2003;2:460–472. doi: 10.1038/nrd1107. [DOI] [PubMed] [Google Scholar]
- 4.Vranken JH. Mechanisms and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem. 2009;9:71–78. doi: 10.2174/187152409787601932. [DOI] [PubMed] [Google Scholar]
- 5.Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996;73:625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 6.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 7.Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. ProcNatAcad Sci. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- 9.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 12.Zanjani TM, Sabetkasaei M, Mosaffa N, Manaheji H, Labibi F, Farokhi B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. Eur J pharmacol. 2006;538:66–72. doi: 10.1016/j.ejphar.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 13.Padi SSV, Kulkarni SK. Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol. 2008;601:79–87. doi: 10.1016/j.ejphar.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 15.Mika J, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, Przewlocka B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun. 2009;23:75–84. doi: 10.1016/j.bbi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirzaei V, Manaheji H, Maghsoudi N, Zaringhalam J. Comparison of changes in mRNA expression of spinal glutamate transporters following induction of two neuropathic pain models. Spinal Cord. 2010;48:791–797. doi: 10.1038/sc.2010.21. [DOI] [PubMed] [Google Scholar]
- 19.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. â-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Li W, Lu L, Cai J, Xian X, Zhang M, et al. An anti-nociceptive role for ceftriaxone in chronic neuropathic pain in rats. Pain. 2010;148:284–301. doi: 10.1016/j.pain.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Amin B, Hajhashemi V, Hosseinzadeh H, AbnousKh Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat. Neuroscience. 2012;224:15–25. doi: 10.1016/j.neuroscience.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 23.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 24.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 25.Ortega-Varela LF, Herrera JE, Caram-Salas NL, Rocha-Gonzalez HI, Torres-López JE, Granados-Soto V. Synergistic antiallodynic interaction of the metamizol-gabapentin combination. Drug Develop Res. 2009;70:386–394. [Google Scholar]
- 26.Rowland M, Tozer TN. 3rd ed. Philadelphia, London: Lea &Febigger; 1989. Clinical pharmacokinetics: concepts and applications; pp. 115–119. [Google Scholar]
- 27.Baliki M, Calvo O, Chialvo DR, Apkarian AV. Spared nerve injury rats exhibit thermal hyperalgesia on an automated operant dynamic thermal escape task. Mol Pain. 2005;1:1–18. doi: 10.1186/1744-8069-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, et al. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Aronson A. Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc. 1980;176:1061–1068. [PubMed] [Google Scholar]
- 30.Shir Y, Seltzer Ze. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. NeuroscieLett. 1990;115:62–67. doi: 10.1016/0304-3940(90)90518-e. [DOI] [PubMed] [Google Scholar]
- 31.Simone DA, Kajander KC. Responses of cutaneous A-fiber nociceptors to noxious cold. J Neurophysiol. 1997;77:2049–2060. doi: 10.1152/jn.1997.77.4.2049. [DOI] [PubMed] [Google Scholar]
- 32.Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. ProgNeurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi Y, Tomimatsu Y, Suzuki H, Yamada J, Wu Z, Yao H, et al. The intra-arterial injection of microglia protects hippocampal CA1 neurons against global ischemia-induced functional deficits in rats. Neuroscience. 2006;142:87–96. doi: 10.1016/j.neuroscience.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 35.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]