Abstract
Adenosine receptors (A1, A2a, A2b and A3) have several physiological and pathological roles in cancer cell lines. The present study was carried out to evaluate the mRNA and protein expression profile and functional role of adenosine receptors in OVCAR-3, Caov-4 and SKOV-3 ovarian cancer cell lines. The levels of mRNA and protein expression of A1, A2a, A2b and A3 adenosine receptors in the ovarian cancer cell lines were measured by Real-time PCR and western blotting. The functional roles of adenosine receptors were investigated through measurement of cAMP levels after agonist treatment. The mRNA and protein of all adenosine receptors subtypes were expressed in the ovarian cancer cell lines. Our findings demonstrated that A2b and A3 had the most mRNA and protein expression. Moreover, cAMP assay confirmed the functional role of A2b and A3 adenosine receptors. This findings demonstrated that A2b and A3 subtypes are most important adenosine receptors in humn ovarian cancer cell lines. This information provide a strong possibility into the relationship of A2b and A3 adenosine receptor and ovarian cancer.
Keywords: Adenosine receptor, Expression, Ovarian cancer
INTRODUCTION
In gynecological tumors, ovarian cancer is the most cancer and is the seventh-leading cause of the cancer death in women (1). Human ovarian cancer is the 9th most frequent cancer and is the most cause of gynecological cancer death in women in Iran (2). The death incidences from ovarian cancer could be significantly decreased by developing novel methods for the early diagnosis and treatment of this fatal disease (3). Transmission of molecular signals from outside the cell into the cell occurs via receptor-ligand binding and by activation of a cascade of intracellular signaling pathways (4). Inhibition of intracellular signaling pathways could reducecancer cell proliferation (4).
Adenosine, a purine nucleoside acts via four receptors including A1, A2a, A2b, and A3 (5). Adenosine has some special biochemical, pharmacological and physiological effects that are conducted through its interaction with adenosine receptors (6). Adenosine receptors belong to a large family of G-protein-coupled receptors (5).
Classification of these receptors is based on pharmacological findings, molecular studies, tissue distribution, biochemical structure and intracellular messengers (5). These receptors exert their effects through adenylyl cyclase (7). A1 and A3 adenosine receptors are coupled to Gi and decrease cAMP level through inhibiting adenylyl cyclase activitiy. A2a and A2b receptors are coupled to Gs and increase cAMP concentrations by stimulating adenylyl cyclase activity (7). These receptors play a major role in the process of cell proliferation (8) and have been associated with higher levels in tumor cells such as breast (9), prostate (10), colorectal (11), human leukemia Jurkat (12) and human melanoma A375 cells (13).
Recently much attention has been given to the survey gene and protein expression profiles and functional role of adenosine receptors in different cancer cell lines for using therapeutic goals. Khoo and coworkers demonstrated that the expression of A1 adenosine receptor in human colon cancer tissues was significantly higher than those of normal tissues (14). Another study reported that mRNA expression of A1 adenosin receptor was upregulated in breast cancer cell lines (15). It was demonstrated that human umbilical vein endothelial cells (HUVECs) preferentially express A2a adenosine receptor and human microvascular endothelial cells (HMEC-1) express A2b receptor (16). High expression of A3 adenosine receptors reported in breast tumor and hepatocellular carcinoma (HCC) tissues (17). It was demonstrated that A2a, A2b, and A3 adenosine receptors were expressed in murine lung mast cells (18).
In a recent study, Qiang Wei and colleguease showed that mRNA and protein expression of A2b was significantly higher than those of other adenosine receptors in three prostate cancer cell lines (19). In another study, the mRNA expression of adenosine receptors was shown on peripheral blood lymphocytes (20). Our previous study demonstrated that A1, A2a and A2b receptors were expressed in human lung adenocarcinoma cell line, Calu-6 (21) and in human prostate cancer cell lines, DU-145, PC3 and LNcap-FGC10 (22). Since the adenosine receptor gene and protein expression profile has not been elucidated in ovarian cancer cells, the purpose of the present study was to explore the mRNA and protein expression profiles and the functional role of these receptors in OVCAR-3, Caov-4 and SKOV-3 ovarian cancer cell lines.
MATERIALS AND METHODS
Culture media, fetal bovine serum, penicillin and streptomycin were obtained from Gibco (Germany). Flasks and plates were purchased from Nunc Co. (Roskilde, Denmark). Electrophoresis reagents were procured from Bio-Rad (Hercules, CA, USA). Materials used for Real-time PCR (RT-PCR) obtained from Qiagen (Valencia, CA, USA). Rabbit polycolonal A1, A2a, A2b, and A3 adenosine receptor antibodies, and anti-rabbit IgG-horseradish peroxidase (IgG-HRP) antibody was purchased from Acris (Germany). Glycine, sodium dodecyl sulfate, Tris, acrylamide, N-methylene-bisacrylamide, mercaptoethanol were obtained from Merk Co (Germany). 5′-N-ethylcarboxamidoadenosine (NECA), 1-deoxy-1- [6-[[(3-iodophenyl) methyl] amino]-9H-purine-9-yl]- N- methyl-b-d-ribofuranuron-amide (IB-MECA), 2-[p-(2-carboxyethyl) phenylethylamino]-5′- N- ethyl-carboxamido-adenosine (CGS-21680), N6-(2- phenylisopropyl) adenosine (R-PIA) and direct cAMP enzyme immouno assay kit (CA-200) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Beta-actin (Quantum RNA™ β-Actin Internal Standards) Cat Num: AM1720. Enhanced chemiluminescence (ECL) kit was supplied from Amersham (Buckinghampshire, UK).
Cell culture
The human ovarian cancer cell lines, OVCAR-3 (NCBI code: C430), Caov-4 (NCBI code: C595) and SKOV-3 (NCBI code: C209) were obtained from the Pasteur Institute of Iran. Cells were cultured and maintained in Roswell Park Memorial Institute medium (RPMI-1640) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were incubated at 37 °C in the presence of 5% CO2 and 95% air.
Quantitative polymerase chain reaction
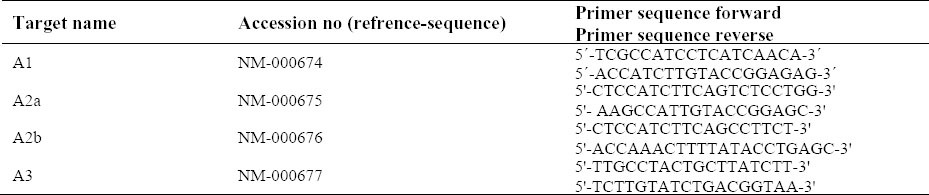
Total RNA was isolated from the cells using RNeasy Minikit (Qiagen, Inc) as previously described (23). Briefly, cells were lysed and homogenized in RLT buffer supplemented with 10 ml/ml mercaptoethanol (Sigma–Aldrich). The lysate was homogenized with a syringe and a 20-G needle. The sample was applied on a silica column followed by washing and eluting in RNase-free water according to the manufacturer's instructions. The RNA concentration was quantified by UV spectrophotometer at 260 nm and the purity and integrity was determined using the A260/A280 ratio. Total RNA was treated with DNase (Fermentas, Burlington, Ontario, Canada), and then reverse transcribed using Revert Aid M-MuLV Reverse Transcriptase (Fermentas) with Oligo dT primers (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Quantitative RT-PCR assays of A1, A2A, A2b, and A3 cDNA were carried out using the SYBR Green kit (Qiagen, Inc.) in an ABI 7500 Sequence Detection System (Applied Biosystems) in accordance with the manufacturer's recommendations. A dissociation curve was generated at the end of each PCR reaction to verify that a single product was amplified. Identical PCR conditions were performed using 1 ml of cDNA, and the relative expression levels of genes normalized to the endogenous housekeeping gene beta-actin (Quantum RNA™ β-Actin Internal Standards). The relative mRNA expression level was determined using the 2-ΔΔCt analysis method. The primers used for realtime RT-PCR are shown in Table 1.
Table 1.
Sequences of the primer pairs for human adenosine receptors and human housekeeping genes used for Real time-PCR
Western blotting
We used western blotting analysis to evaluate the protein levels of adenosine receptors in ovarian cancer cell lines. The cells were harvested in 6-well plate. After 24 h, cells were harvested at 4 °C in a lysis RIPA buffer (150 mmol/l NaCl, 50 mmol/l Tris-HCl, pH 8, 0.5% sodium deoxhycolate, 1% Nonidet P-40, 1 mmol/l phenylsulfonylfluoride, 10 μg/ml aprotinin, 100 μmol/l sodium orthovanadate). After 2 h cells were centrifuged at 10000 g for 10 min and the supernatant was utilized in the assay. The total amount of protein was determined using Bradford reagent, with bovine serum albumin (BSA) as standard. After determination of protein concentration the same amount of proteins was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were incubated with blocking buffer (5% non-fat dry milk in PBS containing 0.1% Tween 20 (PBST)) for 1 h at room temperature. Membranes were then incubated with Rabbit polycolonal A1, A2a, A2b and A3 primary antibody (1:1000 in PBS-T) overnight at 4 °C and washed three times (each for 5 min) with PBST. Membranes were incubated with corresponding secondary antibodies for 1 h at room temperature. After washing with PBST, proteins were detected with ECL detection reagent (Amersham Corp., Arlington Heights, IL, USA). The expression of beta actin was used as an internal control. Gene expression levels were quantified with Image-J software.
cAMP assay
To evaluate the cAMP levels in ovarian cancer cell lines, direct cAMP enzyme immouno assay kit (CA-200) was used. The cells (3 × 105 cells/ml) were transferred into 24-well plates over night prior to the assay. The cells were then washed twice with HHBS, Hank's 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) Buffer Solution, (20 mM, pH 7.4). After 15 min, the medium was replaced with fresh HHBS containing adenosine deaminase (ADA) 2 U/ml, Ro-20-1724 (100 μM) (as phosphodiestrase inhibitor) and incubated for 15 min at 37 °C after which different concentration (0.01–100) of adenosine receptor agonists including RPI-A (A1AR agonist), CGS21680 (A2aAR agonist), NECA (A2bAR agonist), IBMECA (A3AR agonist) were added to the mixture and incubated for 10 min. At the end of incubation, the solution was aspirated and replaced immediately with ice-cold HCl 0.1 M. The level of cAMP accumulation was determined using EIA kit (acetylated version). Dose response curves were calculated using Graphpad Prism.
Statistical analysis
The results were expressed as the mean ± S.D. and statistical analysis were performed by nonparametric analysis of variance between groups (ANOVA) followed by Dunnett's post hoc test. All experiments were repeated at least three times. Statistical analyses were done using the software package SPSS version 17. A difference was considered statistically significant at P<0.05.
RESULTS
Expression of mRNA of adenosine receptors in ovarian cancer cells
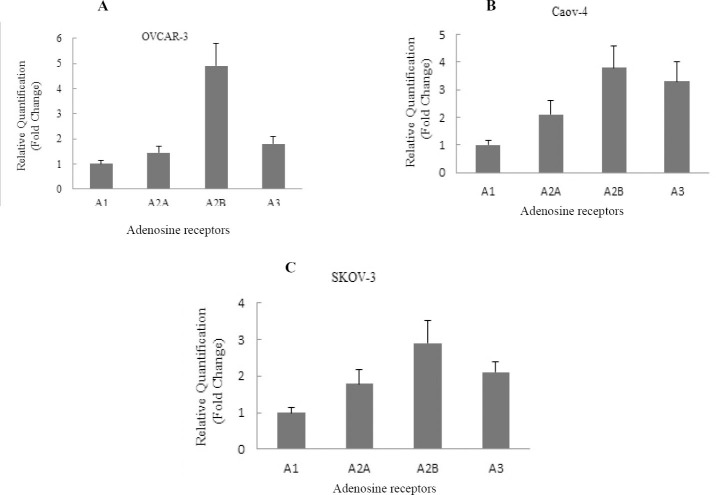
The gene expression of adenosine receptor subtypes, A1, A2a, A2b, and A3, were measured using RT-PCR system in three ovarian cancer cell lines, OVCAR-3, Caov-4, and SKOV-3. The results showed thatthe expression of mRNA in all four adenosine receptors in each ovarian cancer cell line took place (Fig. 1).
Fig. 1.
Expression of adenosine receptors in ovarian cancer cell lines. The relative gene expression of A1, A2a, A2b, and A3 adenosine receptors were detected by Real-time PCR in A; OVCAR-3, B; Caov-4 and C; SKOV-3 cell lines. The relative expression levels of A2b and A3 adenosine receptors in three cell lines were higher than A2a and A1 adenosine receptor. The data are representative of three independent experiments, and the relative expression values were calculated using the equation RQ = 2-ΔΔCt.
Data analysis showed that the pattern of gene expression levels of all four adenosine receptor subtypes was similar in all three cell lines (A2b>A3>A2a>A1) (Fig. 1). The mRNA expression of the A2bAR was higher than other adenosine receptors in studied ovarian cancer cell lines. The A1AR showed the lowest mRNA expression compared to another adenosine receptors in OVCAR-3, Caov-4, and SKOV-3 cell lines.
Expression of protein of adenosine receptors in ovarian cancer cells
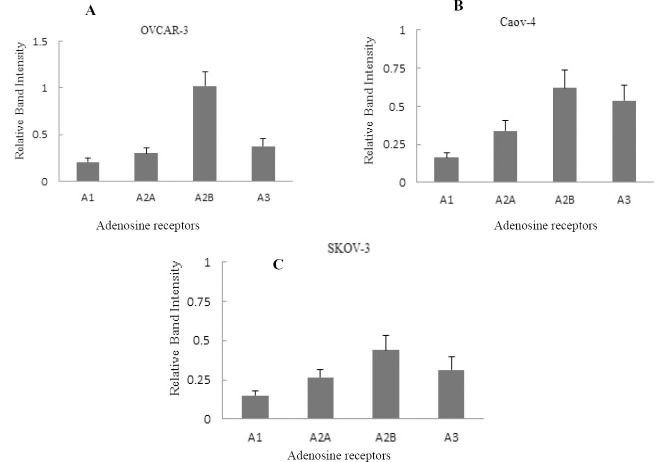
Western blotting was performed on OVCAR-3, Caov-4 and SKOV-3 ovarian cancer cell lines to evaluate the adenosine receptor protein expression. A single and strong band was detected in the western blot using the anti-A2b and anti-A3 receptor antibodies (Fig. 2). All findings analyzed with Image J software and quantified as bond intensity. Data analysis in all four adenosine receptor antibodies showed that A2b and A3 adenosine receptor were highly expressed in all three cell lines followed by low expression of A1 and A2aARs (Fig. 3).
Fig. 2.
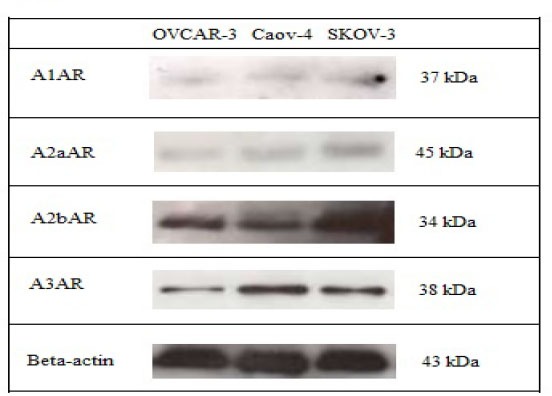
Western blotting detection of adenosine receptor protein expression in ovarian cancer cell lines. A single and strong band was detected in the western blot using the anti-A2B receptor antibody and anti-A3 receptor antibody.
Fig. 3.
Quantitative detection of adenosine receptor protein expression in ovarian cancer cell lines. The relative gene expression of A1, A2a, A2b, and A3 adenosine receptors were detected by western blotting in A; OVCAR-3, B; Caov-4 and C; SKOV-3 cell lines. The relative expression levels of A2b and A3 adenosine receptors in three cell lines were higher than A2a and A1 adenosine receptor. The data are representative of three independent experiments, and the relative expression values were calculated using the ImageJ software.
These results revealed a significant presence of A2bAR and A3AR in OVCAR-3 as well as Caov-4 and SKOV-3 cell lines. Another two subtypes, A1 and A2a also were detected by western blot assay but both of them showed weaker bands in all ovarian cancer cell lines examined (Fig. 2).
cAMP assay
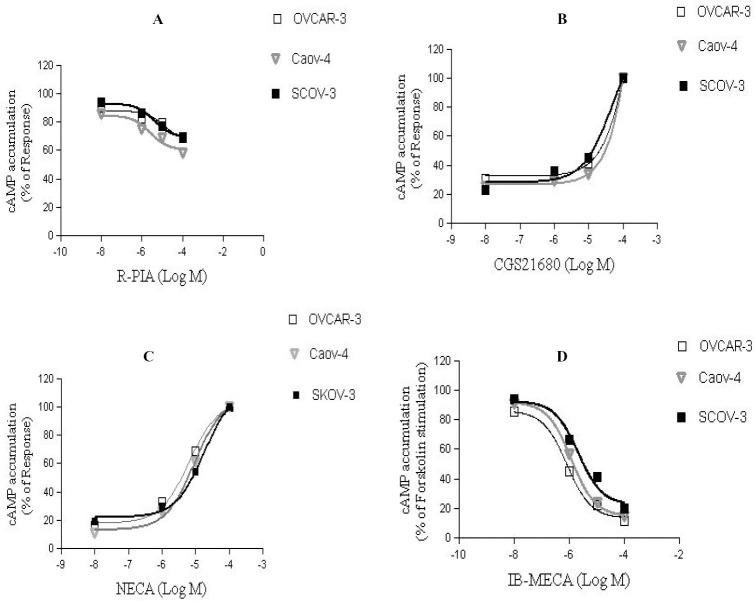
Adenosine receptors as G-protein coupled receptors act through affecting adenylyl cyclase. Therefore, the functional role of adenosine receptors was explored by measurement of the cAMP concentration changes. In this study, three ovarian cancer cell lines were treated with different concentrations (0.01-100 μm) of adenosine receptor agonists, and cAMP assay performed to determine the functional role of each receptor.
NECA (A2bAR agonist) increased cAMP levels in a concentration dependent manner corresponding to an EC50 value of 6.6 μM, 9.12 μM and 18 μM for OVCAR-3, Caov-4 and SKOV-3, respectively (Fig. 4). It confirmed a significant role of A2b adenosine receptor in ovarian cancer cell lines. The results showed that IB-MECA (A3AR agonist) was able to inhibit forskolin-stimulated cAMP levels with an EC50 value of 0.8 μM, 1.2 μM and 2.2 μM in OVCAR-3, Caov-4 and SKOV-3 cell lines, respectively (Fig. 4). The effect of R-PIA (A1AR agonist) and CGS 21680 (A2a agonist) also investigated on ovarian cancer cell lines. Our findings demonstrated that, A1 and A2a agonists had no significant effect on cAMP concentrations (Fig. 4).
Fig. 4.
Effect of adenosine receptor agonists on cyclic AMP accumulation. Cells were treated with increasing concentrations of A; R-PIA, B; CGS21680, C; NECA and D; IB-MECA and then cAMP accumulation was carried out using a calorimetric competitive ELISA kit. Data are given as a percentage. Each point is the mean ± S.D of three experiments. Each experiment was repeated three times.
DISCUSSION
One of the most effective ways to control the growth of cancer cells is to acquire sufficient knowledge about the gene and protein expression pattern of tumor cells. In our previous studies, we have identified the expression profile, signal transduction, molecular function and cell growth modulation of adenosine receptor subtypes in different cancer cell lines (21,22). In the present study, the mRNA and protein expression profile of adenosine receptors in three human ovarian cell lines, OVCAR-3, Caov-4 and SKOV-3 were evaluated. Different methods of determining gene expression have been utilized in various studies. Quantitative RT-PCR is one of the best methods for detecting gene expression. Xiang and coworkers reported that intensity of A2b mRNA expression in hepatocellular carcinoma was consistently higher than those of adjacent normal tissues (24). Qiang Wei and collegueas have investigated the profile expression of four adenosine receptors in three common prostate cancer cell lines (DU-145, PC3 and LNcap-FGC10) and their R-T PCR results showed that the expression level of A2B AR was the highest amongst four subtypes of ARs in all three cell lines (24). Feoktistov and coworkers showed that the mRNA of adenosine receptors were expressed in microvascular (HMEC-1) and umbilical vein (HUVEC) of human endothelial cells. Their results showed that HUVECs significantly expressed A2a adenosine receptors and HMEC-1 expressed A2b adenosine receptors (16). Zhong and collegueas demonstrated that A2a, A2b, and A3 adenosine receptors were expressed in murine lung mast cells (18).
In the present study, quantitative RT-PCR analysis was used to evaluate the mRNA expression of adenosine receptor subtypes in OVCAR-3, Caov-4 and SKOV-3. The results showed that A2b receptor was the most expressed receptor compared to other receptors in OVCAR-3, Caov-4 and SKOV-3; and the expression pattern of these receptors ranked as A2b>A3>A2a>A1. Quantitative differences in expression levels of each subtype were observed in OVCAR-3, Caov-4 and SKOV-3 but were not significant. Expression of A1AR was detectable but was lower than other subtypes. Moreover, results indicated that the expression of A2b was high in OVCAR-3 cells, moderate in Caov-4 and low in SKOV-3 cells, while the A3 adenosine receptor expression was high in Caov-4 cell, moderate in OVCAR-3 and low in SKOV-3 cells. There was no differences in the quantification of mRNA expression in other receptors, A1 and A2a.
In order to confirm mRNA expression, western blot assay have been used in some studies. Mirza and colleagues have investigated the expression of A1 adenosine receptor by quantitative RT-PCR method in seven breast tumor cell lines and demonstrated that A1R mRNA were expressed in all breast tumor cell lines. Also, to confirm the expression of A1 receptor protein in breast cancer tissues, western blot assay was performed and the results showed a significant higher A1AR protein expression in the breast tumor tissues (15).
Bar-Yehuda and coworkers showed that A3AR was highly expressed in human hepatocellular carcinoma tissues and in peripheral blood mononuclear cells (PBMCs) derived from patients with hepatocellular carcinoma (HCC) compared to healthy subjects (25). Qiang and colleagues demonstrated that the expression levels of A2a and A2b were higher than A1 and A3 in DU-145, LNcap-FGC-10 and PC3 prostate cancer cell lines (19). Xiang and coworkers evaluated the protein levels of A2b in HCC by western blot assay and their results showed that the protein levels of this receptor weresignificantly higher than in adjacent tissues (24). In this study protein expression of adenosine receptor subtypes were explored by western blot analysis. As shown in Fig. 2, A2b and A3 receptor were highly expressed with sharp bands, while other two adenosine receptors, A1 and A2a had poor expression in all three ovarian cancer cell lines.
As one of the parameters for functional adenosine mediated responses, the effects of adenosine receptor agonists on the activity of adenylyl cyclase were determined. In some studies signal transduction of adenosine receptors was explored in different cancer cell lines. In a previous study, functional role of A2a and A2b adenosine receptors were surveyed in microvascular (HMEC-1) and umbilical vein (HUVEC) of human endothelial cells with measurement of cAMP. The results showed a higher accumulation of cAMP levels in HUVEC after treating cells with CGS 21680 (A2aAR agonist) compared with NECA (A2bAR agonist).
Whereas on HMEC-1 cells, NECA increased cAMP levels considerably but CGS 21680 was ineffective (16). Qiang and coworkers showed a significant increase in cAMP accumulation after exposuring PC3 cells with NECA in a dose dependent manner (19). Another agonist of A2b adenosine receptor, BAY60-6583, induced accumulation of cAMP in PC3, DU-145 and LNcap-FGC-10 prostate cell lines (20). In the present study, to demonstrate the existence of functional A3 and A2b adenosine receptors in human ovarian cancer cells and to evaluate whether changes of mRNA and protein receptor expression were reflected at a functional level, we determined the potency of the most selective A3 and A2b agonist in the cAMP levels. A decresed level of cAMP observed after treating cells with IB-MECA and an increased level were occurred after affecting cells with NECA (Fig. 4). Another subtypes agonist, R-PIA and CGS 21680 caused no functional response.
CONCLUSION
In conclusion, the results of the present study showed the mRNA and protein expression and functionality of four adenosine receptors A1, A2a, A2b and A3 in three ovarian cancer cell lines OVCAR-3, Caov-4 and SKOV-3. Our findings revealed the role of adenosine receptors in ovarian cancer cell lines. Recently a lot of attention has been focused on the adenosine receptors because they may serve as drug targets for the treatment of cancers. A strong relationship between A2b and A3 adenosine receptor and ovarian cancer was observed.
ACKNOWLEDGMENTS
We would like to appreciate Dr. Mahdi Shabani from Department of Antibody and Antigen Engendering of Shahid Beheshti University, for his sincere help.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Arab M, Noghabaei G. Ovarian cancer incidence in Iran and the World. Report Radiother Oncol. 2013;1:69–72. [Google Scholar]
- 3.Chauhan SC, Kumar D, Jaggi M. Mucins in ovarian cancer diagnosis and therapy. J Ovarian Res. 2009:2–21. doi: 10.1186/1757-2215-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowinsky EK. Signal events: cell signal transduction and its inhibition in cancer. The Oncologist. 2003;8:5–17. doi: 10.1634/theoncologist.8-suppl_3-5. [DOI] [PubMed] [Google Scholar]
- 5.Fredholm BB, IJzerman AP, Jacobson KA, Klotz K-N, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman P, Bar-Yehuda S. Pharmacology and therapeutic applications of A3 receptor subtype. Curr Top Med Chem. 2003;3:463–469. doi: 10.2174/1568026033392147. [DOI] [PubMed] [Google Scholar]
- 7.St Hilaire C, Carroll SH, Chen H, Ravid K. Mechanisms of induction of adenosine receptor genes and its functional significance. J Cell Physiol. 2009;218:35–44. doi: 10.1002/jcp.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 9.Dhillon A, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 10.Jajoo S, Mukherjea D, Watabe K, Ramkumar V. Adenosine A3 receptor suppresses prostate cancer metastasis by inhibiting NADPH oxidase activity. Neoplasia. 2009;11:1132–1145. doi: 10.1593/neo.09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gessi S, Cattabriga E, Avitabile A, Lanza G, Cavazzini L, Bianchi N, et al. Elevated expression of A3 adenosine receptors in human colorectal cancer is reflected in peripheral blood cells. Clin Cancer Res. 2004;10:5895–5901. doi: 10.1158/1078-0432.CCR-1134-03. [DOI] [PubMed] [Google Scholar]
- 12.Gessi S, Varani K, Merighi S, Morelli A, Ferrari D, Leung E, et al. Pharmacological and biochemical characterization of A3 adenosine receptors in Jurkat T cells. Br J Pharmacol. 2001;134:116–126. doi: 10.1038/sj.bjp.0704254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merighi S, Varani K, Gessi S, Cattabriga E, Iannotta V, Ulouglu C, et al. Pharmacological and biochemical characterization of adenosine receptors in the human malignant melanoma A375 cell line. Br J Pharmacol. 2001;134:1215–1226. doi: 10.1038/sj.bjp.0704352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoo H-E, Ho C-L, Chhatwal VJ, Chan ST, Ngoi S-S, Moochhala SM. Differential expression of adenosine A1 receptors in colorectal cancer and related mucosa. Cancer Lett. 1996;106:17–21. doi: 10.1016/0304-3835(96)04289-9. [DOI] [PubMed] [Google Scholar]
- 15.Mirza A, Basso A, Black S, Malkowski M, Kwee L, Pachter JA, et al. Research paper RNA interference targeting of A1 receptor-overexpressing breast carcinoma cells leads to diminished rates of cell proliferation and induction of apoptosis. Cancer Biology & Therapy. 2005;4:1355–1360. doi: 10.4161/cbt.4.12.2196. [DOI] [PubMed] [Google Scholar]
- 16.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, et al. Differential expression of adenosine receptors in human endothelial cells role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 17.Madi L, Ochaion A, Rath-Wolfson L, Bar-Yehuda S, Erlanger A, Ohana G, et al. The A3 adenosine receptor is highly expressed in tumor versus normal cells potential target for tumor growth inhibition. Clin Cancer Res. 2004;10:4472–4479. doi: 10.1158/1078-0432.CCR-03-0651. [DOI] [PubMed] [Google Scholar]
- 18.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, et al. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003;171:338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 19.Wei Q, Costanzi S, Balasubramanian R, Gao Z-G, Jacobson KA. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal. 2013;9:271–280. doi: 10.1007/s11302-012-9350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiedel AC, Lacher SK, Linnemann C, Knolle PA, Muller CE. Antiproliferative effects of selective adenosine receptor agonists and antagonists on human lymphocytes: evidence for receptor-independent mechanisms. Purinergic Signal. 2013;9:351–365. doi: 10.1007/s11302-013-9354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panjehpour M, Movahedian A, Sadeghi H, Eghbali B, Yekdaneh A. Adenosine receptor expression in two different human cancer cell lines at molecular level. Iranian Journal of Cancer Prevention. 2012;3:111–116. [Google Scholar]
- 22.Aghaei M, Karami-Tehrani F, Panjehpour M, Salami S, Fallahian F. Adenosine induces cell-cycle arrest and apoptosis in androgen-dependent and-independent prostate cancer cell lines, LNcap-FGC-10, DU-145, and PC3. Prostate. 2012;72:361–375. doi: 10.1002/pros.21438. [DOI] [PubMed] [Google Scholar]
- 23.Nikhbakht Dastjerdi M, Babazadeh Z, Rabbani M, Gharagozloo M, Esmaeili A, Narimani M. Effects of disulfiram on apoptosis in PANC-1 human pancreatic cancer cell line. Res Pharm Sci. 2013;9:287–294. [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang H-J, Liu Z-C, Wang D-S, Chen Y, Yang Y-L, Dou K-F. Adenosine A2b receptor is highly expressed in human hepatocellular carcinoma. Hepatol Res. 2006;36:56–60. doi: 10.1016/j.hepres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, et al. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-êB signal transduction pathways. Int J Oncol. 2008;33:287–295. [PubMed] [Google Scholar]