Abstract
Background
Mother-to-child transmission of human immunodeficiency virus (HIV) infection was extremely common in southern Africa during the 1990s, and a substantial minority of infected infants have survived to reach adolescence undiagnosed. Studies have shown a high prevalence of HIV infection in hospitalized adolescents who have features associated with long-standing HIV infection, including stunting and frequent minor illnesses. We therefore investigated the epidemiology of HIV infection at the primary care level.
Methods
Adolescents (aged 10–18 years) attending two primary care clinics underwent HIV and Herpes simplex virus–2 (HSV-2) serological testing, clinical examination, and anthropometry. All were offered routine HIV counseling and testing. Patients attending for acute primary care (APC) who were HIV infected were asked about their risk factors.
Results
Five hundred ninety-four participants were systematically recruited (97% participation), of whom 88 (15%) were attending for antenatal care. HIV infection prevalence was higher among APC attendees than among antenatal care attendees (17% vs 6%; P < .007), but for the prevalence of HSV-2 infection, a marker of sexually acquired HIV, the converse was true (4% vs 14%; P < .002). Seventy (81%) of 86 HIV-positive APC attendees were previously undiagnosed. They had a broad range of presenting complaints, with a median CD4 cell count of 329 cells/μL (interquartile range, 176–485 cells/μL) and a high prevalence of stunting, compared with the corresponding prevalence among HIV-negative attendees (40% vs 12%; P < .001). Maternal transmission was considered to be likely by 69 (80%) of the 86 HIV-positive APC attendees, only one of whom was HSV-2 positive.
Conclusions
Unrecognized HIV infection was a common cause of primary care attendance. Routine HIV counseling and testing implemented at the primary care level may provide a simple and effective way of identifying older long-term survivors of mother-to-child transmission before the onset of severe immunosuppression and irreversible complications.
The global incidence of human immunodeficiency virus (HIV) infection remains high, with nearly 3 million new infections in 2008 [1]. Southern Africa is the most heavily affected region, with HIV infection prevalence rates >15% in most countries [1]. Adult HIV infection prevalence has been at these extremely high rates for the past 10 to 15 years, with even higher rates among pregnant women, resulting in large numbers of infants being exposed to HIV transmission.
Although untreated HIV infection in infants is characterized by rapidly progressing disease and by death occurring at a median age of 2 years [2], there is increasing evidence that up to one third of HIV-infected infants are intrinsically “slow progressors,” with a median survival of >10 years even in the absence of HIV diagnosis and care [3-5]. Given the high number of infants infected in this region during the 1990s (before the introduction of interventions for the prevention of mother-to-child transmission), it is anticipated that the number of adolescents in southern Africa who are HIV-infected survivors will continue to grow during the coming decade, peaking at ~1%–2% of all children aged 10 to 15 years [4].
Adolescents who present for the first time with features suggesting long-standing HIV infection are an increasingly prominent cause of adolescent morbidity in countries such as Zimbabwe, which has been severely affected since early in the HIV epidemic [6-8]. We recently reported that hospitalized adolescents in Harare had a high burden of HIV infection, with an adult spectrum of opportunistic infections plus severe complications of untreated pediatric HIV infection, such as chronic lung disease and growth failure [9]. Inpatient mortality was extremely high, and although most cases were known to be HIV infected by the time of admission, the diagnosis had often been made only a short time earlier, following presentation with severe immunosuppression or irreversible chronic complications [9]. These findings suggested the need to investigate routine HIV testing at the primary care level as a potential intervention for achieving earlier diagnosis and entry into care.
The aims of this current study were to investigate the burden of HIV infection, including previously undiagnosed infection, and to further explore the likely mode of HIV acquisition among adolescents attending primary care services in Harare, Zimbabwe.
METHODS
Study population
Participants were recruited from Epworth and Mabvuku clinics, both primary care polyclinics in the high-density suburbs of Harare, Zimbabwe, with catchment populations of about 120,000 and 60,000 people, respectively. Primary care clinics are run by nurses, and services offered by primary care polyclinics include acute primary care (APC) as well as antenatal care (ANC). ANC attendees, but not all APC attendees, are routinely offered provider-initiated testing and counseling. Single-dose nevirapine to pregnant HIV-infected mothers is offered for prevention of mother-to-child transmission.
Patients aged 10 through 18 years attending the primary care clinics for any reason were enrolled consecutively on weekdays during a 6-month period in 2009. ANC attendees were included in the study as a control group to investigate the association of factors suggestive of long-term survival following vertical transmission (such as stunting, pubertal delay, and maternal orphanhood) among APC attendees. The associations between these factors and HIV infection were compared in the ANC attendees (for whom HIV infection was acquired through sexual transmission) and among participants attending for APC.
Patients were excluded if they were too ill to take part (defined as needing immediate hospitalization), had been previously enrolled in the study, or were aged <16 years and not accompanied by a guardian.
Study procedures
All participants were asked to consent to providing blood for anonymized HIV testing for study purposes, and all were offered provider-initiated HIV testing and counseling following group pretesting counseling, either through study personnel (for the APC attendees) or through the program for the prevention of mother-to-child transmission. The HIV-infected APC attendees were given a self-administered questionnaire and were asked to choose the most likely source of their HIV infection from the following options: born with it, from injections or blood transfusion, from boyfriend or girlfriend or from husband or wife, or from an unwanted sexual encounter. Herpes simplex virus–2 (HSV-2) positivity was used as a biological marker of sexually acquired HIV [10, 11].
Pre-set diagnostic algorithms were adapted from the World Health Organization Integrated Management of Adult and Adolescent Illness to broadly classify the presenting complaints [12]. All participants had mid-upper arm circumference, height, and weight measured and received a Tanner pubertal staging to assess growth.
HIV testing was performed at the clinics in accordance with the national guidelines, by use of 2 rapid tests run in parallel (SD Bioline and Abbott Determine). Discordant HIV test results were resolved by repeating the rapid tests and by using an enzyme-linked immunosorbent assay (ELISA) (Vironostika Uniform II plus O). HSV-2 testing used an ELISA (Herpes-Select; Focus Technologies) with an optical density cut-off >1.1 for HSV-2 positivity.
HIV-positive participants were given cotrimoxazole and were referred for HIV infection care to adolescent clinics at one of the 2 central hospitals in Harare, where CD4 cell counts were determined by flow cytometry (CyFlow Counter; Partec).
Data analysis
Data were entered and analyzed using Stata 10 (StataCorp). The χ2 test or the Fisher exact test was used as a test for association between categorical variables, a Student t test for normally distributed variables, and a Mann Whitney U test for variables not normally distributed. P < .05 was considered to be statistically significant. We calculated z scores for height-for-age and weight-for-age by means of British 1990 Growth Reference Curves, which provide data for those aged >10 years [13], and z scores of less than −2 were considered to represent stunting and wasting, respectively.
Participants who were aged ≥16 years were presumed to be competent to give consent. Those aged <16 years were considered to be minors, and consent to participate in the study and to undergo HIV testing was sought from the guardian and assent was sought from the participants. However, emancipated minors (i.e., participants aged <16 years who were married or had children) could give consent independently. If there was disagreement between the guardian and adolescent about participating in the study or undergoing diagnostic HIV testing, both were counseled until consensus was reached. Written informed consent and assent was obtained from participants and from guardians. Participants were encouraged to have a guardian present when HIV test results were given, but the participant could refuse permission to have their test result disclosed to the guardian. In reality, refusal to have test results disclosed to a guardian did not occur. The study was approved by the Medical Research Council of Zimbabwe, the London School of Hygiene and Tropical Medicine Ethics Committee, and the Biomedical Research and Training Institute Ethics Committee.
RESULTS
Baseline participant characteristics
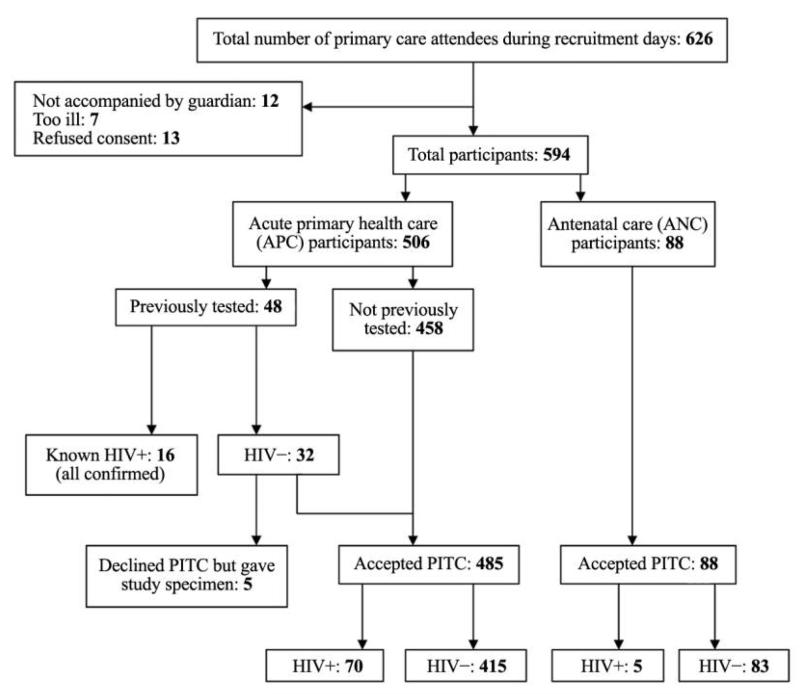
We recruited into the study 594 adolescent clinic attendees (97% of those eligible), of whom 88 (15%) were ANC attendees (Figure 1). Nearly half of all participants were orphaned, and 35% were not attending school (Table 1). ANC attendees were more likely than the APC participants to be married (85% vs 5%; P < .001) and were older (median age, 17 vs 14 years; P < .001).
Figure 1.
Study recruitment and results of human immunodeficiency virus (HIV) testing. PITC, provider-initiated testing and counseling.
Table 1. Baseline Characteristics of 594 Participants by Type of Clinic and Human Immunodeficiency Status (HIV) Status.
| Acute primary care (n = 506) |
Antenatal care (n = 88) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | HIV-positive (n = 86) |
HIV-negative (n = 420) |
P | HIV-positive (n = 5) |
HIV-negative (n = 83) |
P |
| Age, median (IQR), years | 14 (11–16) | 14 (11–16) | .63 | 16 (15–17) | 17 (16–18) | .09 |
|
| ||||||
| Female sex | 48 (56) | 211 (50) | .35 | 5 (100) | 83 (100) | |
|
| ||||||
| Clinic site | .69 | .44 | ||||
|
| ||||||
| Epworth | 35 (41) | 181 (43) | 5 (100) | 74 (89) | ||
|
| ||||||
| Mabvuku | 51 (59) | 239 (57) | 0 (0) | 9 (11) | ||
|
| ||||||
| Orphanhood status | ||||||
|
| ||||||
| Maternal orphan | 15 (17) | 36 (8) | .02 | 0 (0) | 6 (7) | .54 |
|
| ||||||
| Paternal orphan | 21 (24) | 84 (20) | .40 | 0 (0) | 18 (22) | .24 |
|
| ||||||
| Double orphan | 29 (34) | 56 (13) | .001 | 2 (40) | 13 (16) | .17 |
|
| ||||||
| Attending school | 62 (72) | 327 (78) | .23 | 0 (0) | 0 (0) | |
|
| ||||||
| Married | 7 (8) | 18 (4) | .13 | 3 (60) | 72 (87) | .10 |
|
| ||||||
| Biological parent as primary | 34 (40) | .001 | 0 (0) | 15 (18) | .30 | |
|
| ||||||
| MUAC, median (IQR), mm | 202 (177–225) | 219 (195–247) | .001 | 250 (242–255) | 243 (234–261) | .94 |
|
| ||||||
| Pubertal delaya | 15 (17) | 27 (6) | .001 | 0 (0) | 0 (0) | |
|
| ||||||
| Height-for-age z score, median (IQR) | −1.7 (−2.63 to −0.76) | −0.71 (−1.42 to −0.01) | .001 | −0.75 (−0.97 to −0.11) | −0.77 (−1.3 to −0.13) | .98 |
|
| ||||||
| Stuntingb | 34 (40) | 51 (12) | .001 | 1 (20) | 7 (8) | .38 |
|
| ||||||
| Weight-for-age z score, median (IQR) | −2.03 (−3.13 to −1.14) | −0.90 (−1.64 to −0.27) | .001 | −0.2 (−0.64 to 0.63) | 0.08 (−0.43 to 0.63) | .55 |
|
| ||||||
| Wastingc | 43 (50) | 63 (15) | .001 | 0 (0) | 0 (0) | |
NOTE. Data are no. (%) of participants, unless otherwise indicated. IQR, interquartile range; MUAC, mid-upper arm circumference.
Tanner puberty stage 1/2 in children aged ≥14 years.
Height-for-age z score less than −2.
Weight-for-age z score less than −2.
Burden of undiagnosed HIV infection in primary care
The overall HIV prevalence was 15%, being significantly higher among APC attendees (17%) than among ANC attendees (6%) (P < .007). Forty-eight APC participants (9%) had received an HIV test in the past. Only 5 participants (1%) declined diagnostic provider-initiated testing and counseling, all of whom were HIV negative on the study HIV test. Of the 86 HIV-infected APC participants, 70 (81%) were newly diagnosed by the study team. Four newly diagnosed HIV-infected participants died before registering for HIV care. The median CD4 cell count among patients with new diagnoses was 329 cells/μL (interquartile range [IQR], 176–485 cells/μL).
Presenting complaints
The most common presenting complaints in APC patients were diarrhea; ear, nose, or throat infection; and skin infection. HIV prevalence in patients presenting with these complaints ranged from 20% to 24% (Table 2) but was higher than the HIV prevalence in routine ANC attendees (5%) for all categories except malaria. Participants presenting with possible tuberculosis or a sexually transmitted infection had the highest HIV prevalence (50% and 40%, respectively), with those presenting with malaria (5%), chronic lung or heart conditions (7%), trauma (8%), nonspecific abdominal pain (8%), and urinary tract infection (8%) having the lowest risk of underlying HIV infection. HIV-infected participants were more likely to present with infectious conditions than were HIV-negative participants (82% vs 67%; P < .001).
Table 2. Syndromic Classification of the Presenting Complaints in 594 Adolescents in Zimbabwe.
| Cause of attendance (≤3 causes allowed) | No. of attendees with given presenting complaint (% of total) |
No. of HIV-infected attendees with given presenting complaint (% of attendees with given presenting complaint) |
|---|---|---|
| Acute primary care attendees (n = 506) | ||
| Diarrhea and/or dysentery | 106 (21) | 21 (20) |
| Ear, nose, or throat infection | 99 (20) | 22 (22) |
| Skin infection | 65 (13) | 15 (23) |
| Lower respiratory tract infection | 45 (9) | 11 (24) |
| Headache | 45 (9) | 7 (16) |
| Other noninfectious causea | 44 (9) | 7 (16) |
| Urinary tract infection | 29 (6) | 2 (7) |
| Possible tuberculosis | 26 (5) | 13 (50) |
| Traumab | 38 (8) | 3 (8) |
| Nonspecific abdominal pain | 24 (5) | 2 (8) |
| Malaria | 22 (4) | 1 (5) |
| Other infectionc | 22 (4) | 5 (23) |
| Surgical problem | 22 (4) | 2 (9) |
| Sexually transmitted infection | 20 (4) | 8 (40) |
| Chronic lung or heart problem, including asthma | 14 (3) | 1 (7) |
| ≥1 presenting complaint | 117 (23) | 35 (30) |
| Antenatal care attendees (n = 88) | ||
| Routine antenatal care visit | 79 (90) | 4 (5) |
| Sexually transmitted infection | 5 (6) | 1 (20) |
| Other infectiond | 4 (5) | 4 (100) |
NOTE. HIV, human immunodeficiency virus.
Among HIV-positive patients, the following other noninfectious presenting complaints were recorded: allergic conjunctivitis (n = 3), suspected pregnancy (n = 2), visual impairment (n = 1), and follow-up visit (n = 1). Among HIV-negative patients, the following other noninfectious presenting complaints were recorded: allergic conjunctivitis (n = 14), visual impairment (n = 3), suspected pregnancy (n = 3), gynecological problem (n = 3), request for HIV test (n = 2), epilepsy (n = 2), arthritis (n = 1), drug allergy (n = 1), diabetes mellitus (n = 1), epistaxis (n = 1), follow-up visit (n = 1), growth on eye (n = 1), and limb pain and general weakness with no cause identified (n = 4).
Includes wound infection following trauma in 3 participants.
Among HIV-positive patients, the following other infectious presenting complaints were recorded: oral candidiasis (n = 3), hepatitis (n = 1), and dental abscess (n = 1). Among HIV-negative patients, the following other infectious presenting complaints were recorded: hepatitis (n = 9), dental abscess (n = 2), gingivitis (n = 2), eye infection (n = 2), mastitis (n = 1), and worm infestation (n = 1).
The following other infections were recorded: skin infection (n = 2), possible tuberculosis (n = 1), and urinary tract infection (n = 1). All were among HIV-positive patients.
Mode of HIV acquisition
As shown in Table 1, age and sex did not differ by HIV status, but HIV-infected APC attendees were significantly more likely to be maternal or double orphans than were their HIV-negative counterparts and significantly more likely to be stunted. These associations were not observed for ANC participants, although numbers were small in this group. HIV-infected APC attendees were also significantly more likely to have pubertal delay than HIV-negative counterparts.
Of the 86 HIV-infected APC participants, 69 (80%) selected vertical transmission as the most likely source of their infection, 4 (5%) chose injections or blood transfusion, and 13 (15%) chose sexual transmission. HSV-2 prevalence among ANC attendees was significantly higher than the HSV-2 prevalence among APC attendees (14% vs 4%; P < .002). Being an ANC attendee, regardless of HIV status, was associated with increased odds of being HSV-2 positive (odds ratio [OR], 3.6; P < .001). Among APC participants, there was an association between HIV and HSV-2 for HIV-infected participants who considered themselves likely to have been sexually infected with HIV (OR, 24.8; P < .001) but not in those who considered themselves to have been vertically infected (OR, 0.4; P < .38) (Table 3). The median CD4 cell count was 305 cells/μL (IQR, 174–480 cells/μL) in the vertically infected group.
Table 3. Risk of Being Positive for Herpes Simplex Virus (HSV)–2 among Adolescents in Zimbabwe.
| Status of HIV infection, type of clinic, and self-reported most likely source of HIV infection |
Proportion (%) of participants who were HSV-2-positive |
OR (95% CI) | P |
|---|---|---|---|
| HIV-negative | |||
| Antenatal clinic | 10/83 (12) | 3.97 (1.7–9.3) | .001 |
| Acute primary care | 14/420 (3) | Reference | |
| HIV-positive | |||
| Antenatal clinic | 2/5 (40) | 19.33 (3.0–125) | .002 |
| Acute primary care | |||
| Sexually infected | 6/13 (46) | 24.85 (7.43–83.6) | .001 |
| Nonsexual mode of HIV infectiona | 1/73 (1) | 0.4 (0.05–3.1) | .38 |
NOTE. CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
Parenteral (n = 4) and vertical (n = 69).
DISCUSSION
The main finding of this study was the substantial burden of previously undiagnosed HIV infection across a wide range of presenting complaints among adolescents attending APC services in Zimbabwe. In contrast, there was a low prevalence of HIV infection among adolescents attending routine ANC services (6%), consistent with the decreasing HIV epidemic in Zimbabwe [1, 14, 15].
In common with hospitalized adolescents, HIV-infected primary care attendees had a high prevalence of features suggesting long-standing infection, such as pubertal delay and stunting [16, 17], and little to suggest sexual transmission as the predominant cause. There was an equal sex distribution of HIV infection; a strong association of HIV infection with maternal and double, but not paternal, orphanhood; and a low prevalence of HSV-2 infection. Although HSV-2 seropositivity does not establish an individual’s source of infection, HSV-2 infection is a highly prevalent sexually acquired infection in southern Africans that significantly increases the risk of HIV acquisition [11, 18, 19]. As such, it serves here as an independent marker of sexually acquired HIV that can be used to corroborate the self-reported data concerning likely mode of transmission that we collected from our HIV-infected participants. The high prevalence of HSV-2 infection in acutely unwell HIV-positive adolescents who selected sexual transmission as their most likely source of HIV infection, and the positive association between HIV and HSV-2 infections in ANC attendees, contrasts with the very low prevalence of HSV-2 infection (less than that of HIV-negative participants) in acutely unwell adolescents who selected vertical or parenteral transmission as their most likely source of HIV infection. Zimbabwe is one of the few African countries to have had strong policies to prevent parenteral transmission very early on in the course of the HIV epidemic, with good evidence of effective implementation [20, 21]. If maternal transmission is indeed the predominant source of HIV among the acutely unwell adolescents in this study, then the main implications are that there is still a very high burden of undiagnosed long-term survivors in Zimbabwe, and that routine testing of this age group at primary care level is strongly indicated, as discussed below.
Few studies have focused on the spectrum of morbidity related to undiagnosed HIV infection presenting at the primary care level and, to our knowledge, none have focused specifically on adolescents. As in other studies, HIV-infected individuals were significantly more likely to present with possible tuberculosis [22] or sexually transmitted infection [23] and were also more likely to have multiple complaints. However, HIV prevalence was high across the entire spectrum of common presenting complaints, suggesting that provider-initiated testing and counseling should be adopted universally, rather than targeted to specific clinical presentations [24].
Adolescents face barriers to accessing HIV testing, including the lack of availability of client-initiated HIV testing services and the need for guardian support and consent. At present, the majority of HIV-infected adolescent long-term survivors remain undiagnosed until they develop advanced disease [25], risking life-threatening illness and chronic complications that may not respond to antiretroviral therapy [26-28]. In contrast to hospitalized adolescents [9], most HIV-infected adolescents (81%) at this level of the health system were previously un-diagnosed, and their median CD4 cell count was relatively high (329 cells/μL). Studies have shown that HIV-infected adults commonly consult primary care with HIV-related symptoms before their eventual diagnosis, and children consult primary care services with greater frequency than do adults [29]. Thus, implementing provider-initiated testing and counseling at the primary care level is likely to have a much greater effect on reducing diagnostic delay and, if linked to prompt entry into HIV care, on improving long-term prognosis than would a similar intervention at hospital level. The acceptability of provider-initiated testing and counseling was very high (97%) in this study, with both adolescents and their guardians supporting routinely offered HIV testing, as has been shown for younger children in South Africa [30].
The study had several limitations. Our assessment of the likely mode of HIV infection was through a brief questionnaire asking participants to report their likely mode of HIV acquisition. Other studies have shown that adolescents, particularly girls, underreport sexual debut [31]. Participants may have been unwilling to disclose risk of infection through sexual transmission, and participants’ perception of personal risk of being HIV infected may have been influenced by the information obtained during pre-test counseling, particularly regarding vertical HIV infection. However, the very low prevalence of HSV-2 in participants selecting nonsexual transmission concurs with data obtained through self-report. We may have overestimated the proportion of HIV-infected adolescents who were newly diagnosed, because those with known HIV infection may preferentially present to their HIV care clinic with complaints.
Evidence from Zimbabwe suggests that there are increasing numbers of long-term survivors of mother-to-child transmission who are reaching adolescence, the majority of whom are not yet in HIV care [25]. This is likely to be generalizable to the region [6, 32]. The current study adds to the existing literature by demonstrating a high burden of undiagnosed HIV infection, with features suggesting mother-to-child transmission as the predominant source of infection in adolescents presenting to APC services. The relatively high CD4 cell counts in previously undiagnosed cases raises the possibility that HIV progression may be very slow in some older long-term survivors, as has been shown among HIV-infected adults who are “long-term nonprogressors” or “elite controllers” [33].
Our data strongly support routine implementation of diagnostic HIV testing for younger children as well as older children and adolescents attending primary care services in countries with long-standing generalized HIV epidemics, regardless of the presenting complaint, and possibly universal testing of infants at immunization clinics [34]. Missing these opportunities will delay identification of vertically infected children, exposing them to avoidable complications of HIV infection. Legal barriers to testing, such as the age of consent, need to be lowered to support routine testing of adolescents, especially when guardians are not available. There is also a need for frontline service providers to be made aware of the changing epidemiology of HIV infection in older children and adolescents and to be provided with appropriate information to give to affected adolescents and their guardians and siblings.
Acknowledgments
We thank Reggie Mutetwa for performing the HSV-2 tests.
Footnotes
Potential conflicts of interest. R.A.F. and E.L.C. are funded by the Wellcome Trust. All other authors: no conflicts.
References
- 1.AIDS epidemic update. Joint United Nations Programme on HIV and AIDS and World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- 2.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364(9441):1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 3.Marston M, Zaba B, Salomon JA, Brahmbhatt H, Bagenda D. Estimating the net effect of HIV on child mortality in African populations affected by generalized HIV epidemics. J Acquir Immune Defic Syndr. 2005;38(2):219–227. doi: 10.1097/00126334-200502010-00015. [DOI] [PubMed] [Google Scholar]
- 4.Ferrand RA, Corbett EL, Wood R, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23(15):2039–2046. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stover J, Walker N, Grassly NC, Marston M. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect. 2006;82(suppl 3):iii45–50. doi: 10.1136/sti.2006.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker AS, Mulenga V, Sinyinza F, et al. Determinants of survival without antiretroviral therapy after infancy in HIV-1–infected Zambian children in the CHAP Trial. J Acquir Immune Defic Syndr. 2006;42(5):637–645. doi: 10.1097/01.qai.0000226334.34717.dc. [DOI] [PubMed] [Google Scholar]
- 7.Zimbabwe National HIV and AIDS Estimates 2007. Ministry of Health and Child Welfare; Harare, Zimbabwe: 2007. [Google Scholar]
- 8.Ferrand RA, Luethy R, Bwakura F, Mujuru H, Miller RF, Corbett EL. HIV infection presenting in older children and adolescents: a case series from Harare, Zimbabwe. Clin Infect Dis. 2007;44(6):874–878. doi: 10.1086/511873. [DOI] [PubMed] [Google Scholar]
- 9.Ferrand RA, Bandason T, Musvaire P, et al. Causes of acute hospitalization in adolescence: burden and spectrum of HIV-related morbidity in a country with an early-onset and severe HIV epidemic: a prospective survey. PLoS Med. 2010;7(2):e1000178. doi: 10.1371/journal.pmed.1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss HA, Buve A, Robinson NJ, et al. The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS. 2001;15(suppl 4):S97–S108. doi: 10.1097/00002030-200108004-00011. [DOI] [PubMed] [Google Scholar]
- 11.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . Acute care: integrated management of adolescent and adult illness. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 13.Cole TJ. Growth monitoring with the British 1990 growth reference. Arch Dis Child. 1997;76(1):47–49. doi: 10.1136/adc.76.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregson S. Evidence for HIV decline in Zimbabwe: a comprehensive review of epidemiological data. Joint United Nations Programme on HIV and AIDS; Geneva, Switzerland: 2005. [Google Scholar]
- 15.The 2009 ANC sentinel surveillance report. Ministry of Health and Child Welfare; Harare, Zimbabwe: 2009. [Google Scholar]
- 16.Arpadi SM. Growth failure in children with HIV infection. J Acquir Immune Defic Syndr. 2000;25(suppl 1):S37–S42. doi: 10.1097/00042560-200010001-00006. [DOI] [PubMed] [Google Scholar]
- 17.Buchacz K, Rogol AD, Lindsey JC, et al. Delayed onset of pubertal development in children and adolescents with perinatally acquired HIV infection. J Acquir Immune Defic Syndr. 2003;33(1):56–65. doi: 10.1097/00126334-200305010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Glynn JR, Carael M, Auvert B, et al. Why do young women have a much higher prevalence of HIV than young men? a study in Kisumu, Kenya and Ndola, Zambia. AIDS. 2001;15(suppl 4):S51–S60. doi: 10.1097/00002030-200108004-00006. [DOI] [PubMed] [Google Scholar]
- 19.Amornkul PN, Vandenhoudt H, Nasokho P, et al. HIV prevalence and associated risk factors among individuals aged 13–34 years in rural western Kenya. PLoS ONE. 2009;4(7):e6470. doi: 10.1371/journal.pone.0006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopman BA, French KM, Baggaley R, Gregson S, Garnett GP. HIV-contaminated syringes are not evidence of transmission. AIDS. 2006;20(14):1905. doi: 10.1097/01.aids.0000244215.00704.73. [DOI] [PubMed] [Google Scholar]
- 21.Lopman BA, Garnett GP, Mason PR, Gregson S. Individual level injection history: a lack of association with HIV incidence in rural Zimbabwe. PLoS Med. 2005;2(2):e37. doi: 10.1371/journal.pmed.0020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munyati SS, Dhoba T, Makanza ED, et al. Chronic cough in primary health care attendees, Harare, Zimbabwe: diagnosis and impact of HIV infection. Clin Infect Dis. 2005;40(12):1818–1827. doi: 10.1086/429912. [DOI] [PubMed] [Google Scholar]
- 23.Arrington-Sanders R, Ellen J, Trent M. HIV testing in adolescents and young adults receiving STI testing in an urban primary care setting. Sex Transm Dis. 2008;35(7):686–688. doi: 10.1097/OLQ.0b013e31816b1f9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mwachari CW, Shepherd BE, Cleopa O, Odhiambo JA, Cohen CR. Mortality and burden of disease in a cohort of HIV-seropositive adults in Nairobi, Kenya. Int J STD AIDS. 2004;15(2):120–126. doi: 10.1258/095646204322764325. [DOI] [PubMed] [Google Scholar]
- 25.Ferrand RA, Lowe S, Whande B, et al. Survey of children accessing HIV services in a high HIV prevalence setting: time for HIV-infected adolescents to count? Bull World Health Organ. 2010;88(6):428–434. doi: 10.2471/BLT.09.066126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newell ML, Patel D, Goetghebuer T, Thorne C. CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: is it associated with age at initiation? J Infect Dis. 2006;193(7):954–962. doi: 10.1086/500842. [DOI] [PubMed] [Google Scholar]
- 27.Walker AS, Doerholt K, Sharland M, Gibb DM, Collaborative HIV Paediatric Study (CHIPS) Steering Committee Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS. 2004;18(14):1915–1924. doi: 10.1097/00002030-200409240-00007. [DOI] [PubMed] [Google Scholar]
- 28.Kekitiinwa A, Lee KJ, Walker AS, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49(4):384–392. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan AK, Curtis H, Sabin CA, Johnson MA. Newly diagnosed HIV infections: review in UK and Ireland. BMJ. 2005;330(7503):1301–1302. doi: 10.1136/bmj.38398.590602.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwood C, Voce A, Vermaak K, Rollins N, Qazi S. Routine checks for HIV in children attending primary health care facilities in South Africa: attitudes of nurses and child caregivers. Soc Sci Med. 2010;70(2):313–320. doi: 10.1016/j.socscimed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Cowan FM, Pascoe SJ, Langhaug LF, et al. The Regai Dzive Shiri Project: a cluster randomised controlled trial to determine the effectiveness of a multicomponent community-based HIV prevention intervention for rural youth in Zimbabwe—study design and baseline results. Trop Med Int Health. 2008;13(10):1235–1244. doi: 10.1111/j.1365-3156.2008.02137.x. [DOI] [PubMed] [Google Scholar]
- 32.Shisana O, Methtar S, Mosala T, et al. HIV risk exposure among young children: a study of 2- to 9-year-olds served by the public health fa cilities in the Free State. Human Social Research Council; South Africa. Cape Town, South Africa: 2005. [Google Scholar]
- 33.Saksena NK, Rodes B, Wang B, Soriano V. Elite HIV controllers: myth or reality? AIDS Rev. 2007;9(4):195–207. [PubMed] [Google Scholar]
- 34.Rollins N, Mzolo S, Moodley T, Esterhuizen T, van Rooyen H. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23(14):1851–1857. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]