Abstract
Purpose.
Although the impact of homonymous visual field defects (HFDs) on mobility has been investigated previously, the emphasis has been on obstacle detection. Relatively little is known about HFD patients' ability to judge collisions once an obstacle is detected. We investigated this using a walking simulator.
Methods.
Patients with HFDs (n = 29) and subjects with normal vision (NV; n = 21) were seated in front of a large screen on which a visual simulation of walking was displayed. They made collision judgments for a human figure that appeared for 1 second at lateral offsets from the virtual walking path. A perceived-collision threshold was calculated for right and left sides.
Results.
Symmetrical collision thresholds (same on left and right sides) were measured for participants with NV (n = 21), and right (n = 9) and left (n = 7) HFD without hemispatial neglect. Participants with left neglect (n = 10) showed significant asymmetry with thresholds smaller (compared to the NV group and other HFD groups) on the blind (P < 0.001) and larger on the seeing (P = 0.05) sides. Despite the asymmetry, the overall width of the zone of perceived collision risk was not different, suggesting a relatively uniform rightward deviation in judgments of the left neglect group.
Conclusions.
Left neglect was associated with rightward asymmetry in collision judgments, which may cause collisions on the left side even when an obstacle is detected. These behaviors may represent the spatial misperceptions in body midline described previously in patients with left neglect.
Keywords: hemianopia, mobility, stroke, brain injury, visual midline shift, egocentric spatial localization
Brain-injured patients with left hemineglect exhibited rightward deviated collision judgments unlike other brain-injured patients and normal participants whose judgements were not different on their right and left sides.
After stroke or other brain injury, colliding with obstacles, or drifting left or right while walking or wheelchair driving are common problems1 that have been attributed to motor impairments2 and homonymous visual field defects (HFDs, loss of vision on the same side in both eyes resulting from damage to the postchiasmal visual pathways) impairing blind side detection.3,4 However, misjudgment of obstacle location related to dysfunctional visuospatial or spatiomotor processing from damage to higher visual-cortical areas also may contribute. While the impact of the HFDs on detection has been documented in walking5 and driving tasks,3,4 relatively little is known about the ability of patients with HFDs to judge the likelihood of a collision once an obstacle has been detected. Using a collision judgment task in a walking simulator, we started to address this gap in the literature.
When making a collision judgment, people might use an internal representation of the volume of space they occupy6–8 (sometimes referred to as the collision envelope (Woods RL, et al. IOVS 2003;44:ARVO E-Abstract 4321) to determine when an approaching object poses a collision hazard. Presumably, this collision envelope includes the body volume and a safety margin. The outer edges of this space can be defined by the individual's collision thresholds on the right and left sides, which represent the perceived safe passing distances (Fig. 1). These thresholds are the person's own perception and are related to their perception of collision risk, which presumably would be used to decide whether an avoidance maneuver is required. A biased or inaccurate perception of collision risk may lead to real collisions or unnecessary actions in real-world mobility (i.e., to avoid a collision that would not occur). In people with normal vision, the collision envelope was approximately symmetrical; was not correlated with various measures of the subjects' physical size, but did show some relationship to physical size (wearing a “sandwich board” increased the envelope width; Woods RL, et al. IOVS 2003;44:ARVO E-Abstract 4321). Patients with tunnel vision were found to have larger (but still symmetrical) collision thresholds than people with normal vision, possibly as a compensatory strategy for their visual field loss.8
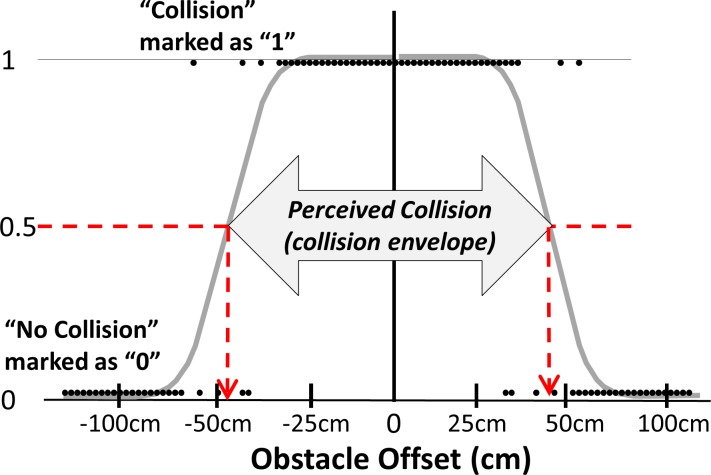
Figure 1.
Illustration of the raw data of a normal vision subject fitted with two probit functions which can be used to determine the collision threshold for right and left sides at 50% (dashed arrows pointing to the x-axes). “Collision” responses are assigned a 1 and “no collision” a 0. Obstacle offset refers to the distance of the closest edge of the obstacle from the center of the virtual walking path.
The collision envelope of patients with HFDs has not been investigated previously to our knowledge. In a driving simulator, Bowers et al.9 found that, while drivers with normal vision maintained a central lane position on straight road segments, patients with HFDs adopted an average lane position away from the side of field loss (i.e., a larger safety margin on the blind side). In an on-road study, a similar behavior was documented by Wood et al.10 It is not clear whether that asymmetry in behavior is due to strategic compensation or biased perception. There is indirect evidence from performance on line bisection11 that patients with HFDs have small, but statistically significant spatial misjudgments erring towards the side of the visual field loss (i.e., less line length on the blind side),12 which might suggest the lateral lane offset9,10 away from the field loss was a symptom of visuospatial dysfunction rather than a strategy to increase safety. Thus, these prior studies lead us to predict that the collision threshold of patients with HFDs would be larger on the blind side (as illustrated in Fig. 2) either from a spatial misjudgment or as a compensatory strategy.
Figure 2.
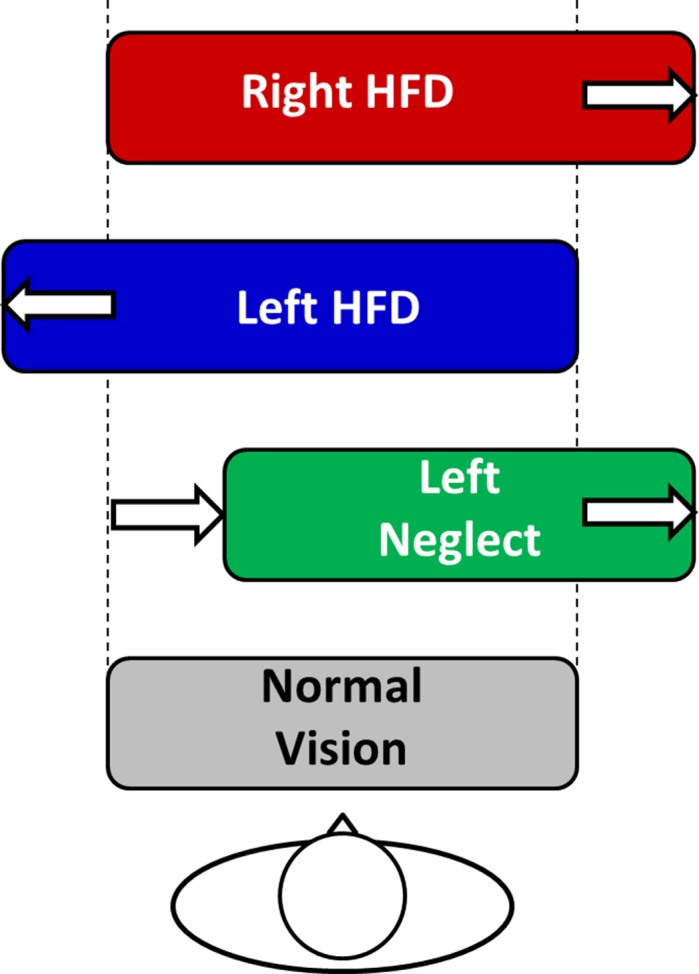
Rounded rectangles represent the hypothesized collision envelopes showing an increase on the blind side in patients with HFD (without left neglect) and a rightward shift in patients with left neglect. Patients with right neglect might show behaviors similar to left neglect, but in the opposite direction.
When studying stroke and brain-injured patients with HFDs, hemispatial neglect also must be considered as they often occur together. This is because the optic radiations are anatomically juxtaposed and share a blood supply with cortical spatial networks in the overlying temporal and parietal cortices.13 Injury to these structures (and other frontal and subcortical structures) are correlated with neglect behaviors.14 Left neglect, often with left HFD, occurs after approximately half of all right hemisphere strokes,15 characterized by a rightward gaze preference,16 reduced scanning to the left hemifield,17 decreased response to one or more types of sensory stimuli (visual, auditory, tactile) on the left side,18 deviated judgments of straight ahead to the right,11,18,19 and rightward spatial aiming errors.20 Right neglect can occur with right HFD, manifesting opposite behaviors to left neglect; however, it is less common, less severe, and less persistent.21–23 Neglect diagnosis is not straightforward and may require as few as six and as many as 10 tests to sufficiently detect various subtypes.24 Even the most sensitive paper and pencil tests (Bells, line bisection, Behavioral Inattention Test Battery) were all negative in 28% of right brain stroke patients who tested positive in other modalities; perceptual, personal, or motor (see Table 2 in the report of Buxbaum et al.14). Therefore, negative paper and pencil testing does not definitively confirm an absence of neglect. A by-proxy history dependent on extended semiquantitative rating of functional performance may capture more disability-related symptoms,25 and can be combined with paper and pencil tests.26,27
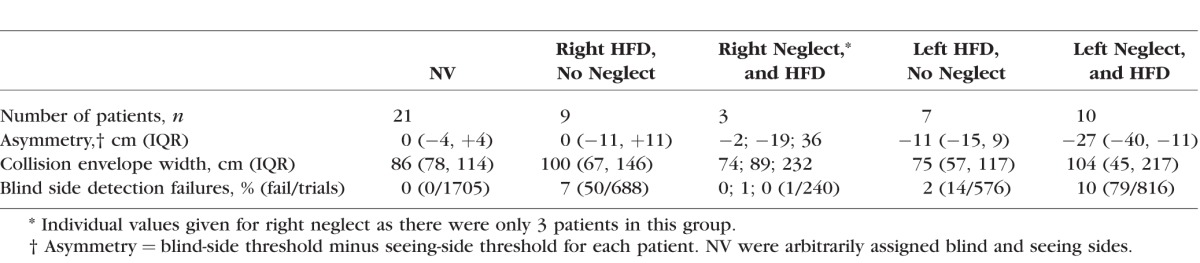
Table 2.
Median (IQR) for Collision Judgment Measures Grouped by Visual Status
Abnormal formation, access, or implementation of spatial representations is widely recognized to occur in neglect,19,28–31 and typically respects the midline of the trunk.32,33 A possible mechanism is distortion of body-centered representations such that the body midline is shifted (i.e., body perceived as smaller on the left and larger on the right, or rotated); the egocentric shift hypothesis.19,28–31 Alternatively, there may be a distortion of the extra-personal space surrounding the body such that a greater spatial extent is represented to the right of the body than to the left.34–39 Either theoretical model would predict that patients with left neglect would judge collision risk closer to the virtual walking path on the left and farther on the right (Fig. 2). This might be the case for right neglect patients as well, but in the opposite direction.
In this study, we used the collision envelope paradigm to characterize the collision judgments of patients with HFDs, without or with left neglect. Our primary hypotheses were that, compared to people with normal vision, patients with HFDs without neglect would have collision thresholds that were larger on their blind side and not different on the seeing side, while patients with left neglect would have collision thresholds that were smaller on the left (blind-neglected) side and larger on the right (seeing) side, as illustrated in Figure 2, and that this effect would be independent of detection rates.
Methods
Participants
The study was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from the participants after explanation of the nature and possible consequences of the study. The protocol was approved by the institutional review board at Massachusetts Eye and Ear. Patients with HFDs were recruited from area hospitals and eye care practices. Inclusion criteria were the presence of HFD (complete or incomplete hemianopia) on Goldmann perimetry with V4e stimulus, ≥3 months since onset, binocular visual acuity of at least 20/40, and a normal visual field on the seeing side. Of 51 HFD patients screened, 31 met the study criteria and were enrolled. Reasons for ineligibility were subject declined to participate (n = 10), no visual field loss (n = 3), vision loss in both hemifields (n = 2), cognitive or physical impairments (n = 2), onset < 3 months (n = 1), age < 14 years (n = 1), and active eye disease (n = 1).
For neglect assessment, we reviewed medical records for documentation of neglect, and patients performed the Schenkenberg Line Bisection Test (LBT)11 and Bells Test,40 which are regarded as being among the most sensitive paper and pencil tests for neglect.24,27 The LBT requires a spatial judgment that is similar to collision judgment (although it requires a motor response in peri-personal space unlike collision judgment). Prior studies on neglect have described challenges with diagnosis, requiring as many as 10 tests to achieve adequate sensitivity.15,24 Therefore, we also documented if a patient had a history of neglect and used a questionnaire to identify neglect behaviors. With a neglect history, the patient was at risk for persistent neglect behaviors. A patient was classified as having a history of neglect if they provided a positive report of neglect history with at least one confirmatory source, or without self-reported history, but with definitive documentation in a medical record or behavioral report. Confirmatory sources included a positive caregiver report of neglect history on an intake questionnaire that included two questions adopted from the Catherine Bergego Scale,27 which were selected to capture neglect behaviors for which paper and pencil testing is insensitive (i.e., difficulty adjusting the left sleeve or slipper and difficulty finding way to the left), or positive documentation in the physical medicine, neurology, therapy, or vision rehabilitation records. If a patient reported neglect history, but it could not be confirmed, they were excluded from the analysis. On that basis, we excluded one patient reporting right neglect history and one patient reporting left neglect history. Preliminary analyses of the collision judgment data supported grouping of patients with measured neglect and neglect history together, since they were not significantly different from each other, but were significantly different from the other groups (see Supplementary Material).
Therefore, 29 HFD patients were included in the study; of the 12 patients with right HFD, 9 did not have neglect (right HFD group) and 3 had right neglect or neglect history (right neglect group), and of the 17 patients with left HFD, 7 did not have neglect (left HFD group) and 10 had neglect or neglect history (left neglect group, Table 1). Most (25/29) patients had complete HFD,41 as this is our typical referral population; the remaining 4 had incomplete HFD. We did not exclude patients based on their degree of HFD, since we intended to measure their judgments once the obstacle was detected (rather than if they would detect it).
Table 1.
Characteristics of the Patients With HFD Without Neglect (Left and Right HFD) and With Neglect (Left and Right Neglect)
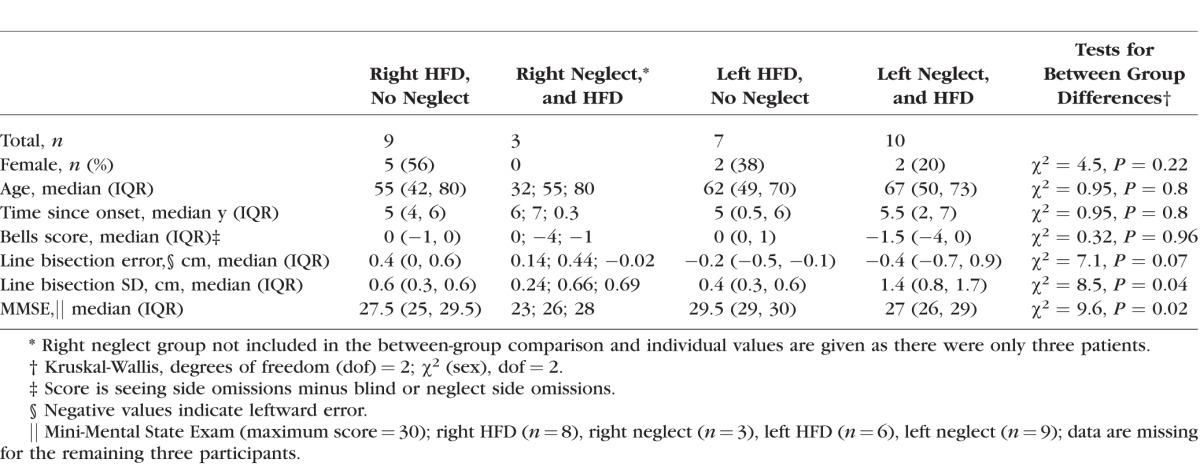
In addition, 21 naïve volunteers with normal vision (NV; 31 ± 9 years, 48% female) were recruited as a control group. They provided data on the collision judgment symmetry for our experimental set up (i.e., seated with head restrained, see below). In the prior study, symmetry of the collision envelope was reported for NV participants; however, they walked on a treadmill without any head restraint (Woods RL, et al. IOVS 2003;44:ARVO E-Abstract 4321). A non–age-matched NV group was expected to be adequate, because age was not a significant factor affecting collision judgments in the prior study (Spearman ρ = −0.22, P = 0.31).
Collision Judgment Measurements
To limit motor impairment as a confounding variable, participants performed the collision judgment task when seated (i.e., with the motor component of walking eliminated), enabling a purely visual judgment to be measured. Only the perceived collision width of the participant was measured (the actual width of the participants was not measured). As shown in Figure 3, their head was stabilized in a head-chinrest mounted on a dark table top 100 cm from a wide (170 × 125 cm) rear-projection screen displaying a virtual model of a shopping mall corridor. The headrest kept the head aligned with the center of the virtual path and limited postural shifts. The tabletop restricted visual reference of body position and any objects below the screen were covered to prevent them from being used as a landmark to aid collision judgments. The animated mall corridor generated an optic flow background of 1.5 m/s along a straight path similar to that experienced during actual locomotion.
Figure 3.

Virtual mall walking simulator setup and collision judgment task. Photograph from above and behind a participant performing the experimental task showing the mall with life-sized human obstacle.
Participants performed the collision judgment task with unconstrained gaze (i.e., free to scan), wearing spectacle correction when appropriate. Each trial consisted of “walking” one straight segment of the path when suddenly a single life-sized human obstacle (the same obstacle on all trials) appeared either directly in the path of virtual movement, or at different offsets to the left or right of the path (Fig. 3). The obstacle was stationary within the optic flow field of the corridor, initially appearing at a virtual distance of 5 m from the participant, was visible for 1 second as the participant continued to “walk,” and disappeared at a virtual distance of 3.5 m. The virtual walking (optic flow) ceased 0.75 seconds later, signaling the end of the trial. Participants were instructed to “imagine walking down an actual mall corridor” and to report verbally whether they would make any contact with the human figure if they continued on the same path, assuming they could not adjust their body or change direction to avoid the collision. A forced choice paradigm required a verbal response of “collision,” “no collision,” or “nothing” (i.e., no obstacle was seen). The scene then rotated to the next trajectory and the next trial began.
Each session consisted of 80 trials (40 obstacles per side) interleaved with eight trials in which no obstacle was presented (9% [8/88]). There were 40 obstacle offsets on each side (measured from the inside edge of the obstacle to the virtual path) evenly spaced from −0.2 m out to 1.2 m (virtual world distances), presented in a random order. Some of the offsets were clearly collisions; for example, when the obstacle was directly in the virtual walking path or only at a small offset. The trajectory of walking also was randomly changed for each trial so that it simulated either walking directly down the center of the mall corridor, or angled to the left or right. A practice session comprising 20 trials was used to familiarize participants with the task. Data collection took approximately 45 minutes.
Statistical Analysis
Collision responses were scored as 1 for collision and 0 for no collision. Detection failures were scored as “no collision,” since subsequent behavior would have been the same as a judgment that there was no collision. Collision judgment summary statistics were obtained for each patient and NV participant by probit functions to derive the collision threshold (at 50%) for the left and right sides (Fig. 1). One left neglect patient responded “collision” on every trial except one, and another patient with left neglect responded “collision” for all trials on the right (seeing) side. Both showed this behavior on two or more sessions, despite repeating the instructions and asking the patient to report where on their body they believed they would have bumped the obstacle. As far as could be determined, their responses were valid (i.e., they perceived that they would have collided). As a threshold could not be calculated in these cases, for the descriptive statistics they were instead assigned an arbitrary value of 200 cm (maximum tested eccentricity was 120 cm) for the threshold.
The collision judgment asymmetry (blind-side threshold minus seeing-side threshold) and the overall collision envelope width (the sum of the absolute thresholds for the two sides) were calculated for each patient, and then median and interquartile range (IQR) calculated for each group. Adaptive behavior would have a higher safety margin on the blind side (i.e., asymmetry > 0). Collision behavior was maladaptive when the asymmetry was negative (threshold smaller on the blind side than the seeing side). Normal vision subjects were arbitrarily assigned a blind side, which is relevant for the calculation of the descriptive statistics, but not for the regression analysis described below.
Mixed-effects logistic regression analyses were performed for dependent variables of (1) detection failures and (2) collision judgment responses, to evaluate the asymmetry of responses and collision envelope width. Diagnosis (HFD, neglect) and other factors that might affect collision judgments or detection were included as independent variables (described below). We hypothesized that side of the obstacle (patient's blind or seeing) would be predictive of the collision response (Fig. 2). Duration of vision loss was selected as a factor with the rationale that spatial biases may recover over an extended time period, or strategies might develop gradually. Scores for the LBT were selected (where Bells test was not) because LBT requires spatial judgments not unlike collision judgment (although with some potentially substantial differences including, but not limited to requiring a motor response in peripersonal space). Performance factors for the LBT included the mean and standard deviation of the errors in bisecting the 18 lines on the test. Detection failures were included as a factor when analyzing collision responses, since reduced attention related to not seeing the obstacle might affect behavior. Age was included as it could be a negative predictor for the success of rehabilitation.42 On 6% (144/2320) of trials, patients failed to detect the obstacle. Analyses of collision responses reported below were conducted, including those trials scored as a “no collision” response. Analyses conducted without those detection-failure trials provided similar outcomes and did not alter the interpretation.
All statistical analyses were performed with STATA/IC 13.1 (College Station, TX, USA); α ≤ 0.05 was taken to indicate statistical significance. Since the sample sizes in each group were relatively small, we also noted marginal significances, where 0.05 < α ≤ 0.10.
Results
Detection Failures
No NV participant had a detection failure, as expected. Most HFD patients had low rates of failing to detect the obstacle (Table 2), almost all of which (140/144) were on the blind side, with the majority of detection failures being for obstacles with offsets outside 60 cm eccentricity (75%, 108/144). Among the HFD patients, left neglect was associated with a higher proportion of detection failures (P = 0.015), while there was a tendency for the patients with right neglect to have fewer detection failures (P = 0.08). Significant predictors of a higher proportion of detection failures were older age (P = 0.008) and had greater LBT error (P = 0.04). Duration of vision loss was borderline (P = 0.09), longer durations being associated with a higher proportion of detection failures, even when adjusted for age. When two patients with left neglect and unusually high LBT errors (3.9 and 4.5 cm, greater than 1.5 times the IQR) were excluded, left neglect (P = 0.03), age (P = 0.01), and duration (P = 0.04) remained significantly related to a higher likelihood of detection failures, but LBT error was no longer a significant predictor of detections (P = 0.40).
Collision Judgment Asymmetry
The left neglect group (median −27 cm) had a negative (maladaptive) asymmetry compared to the NV group (Wilcoxon-Mann-Whitney, P < 0.001) with eight of the 10 left neglect patients having a negative asymmetry of more than 10 cm (Fig. 4). The left HFD group (median −11 cm) was not significantly different from the NV (median 0 cm, P = 0.19) or right HFD (median 0 cm, P = 0.56) groups. A mixed-effects logistic regression model with an interaction term for side of the obstacle found no significant effect for the left HFD group (P > 0.14), while left neglect patients were significantly more likely to judge left side obstacles “no collision” (P < 0.001) and right side obstacles “collision” (P = 0.05, Fig. 5). This asymmetric response in left neglect represents a significant maladaptive asymmetry, consistent with our hypothesis for this group (Fig. 2). For the other groups, side of obstacle was not a significant predictor of collision judgment response and so there was no asymmetry (Table 2; Figs. 4, 5). Therefore, contrary to our hypothesis (Fig. 2), HFD patients without neglect did not have a larger safety margin on their blind side. Right neglect patients did not show maladaptive asymmetry like their left neglect counterparts (seeing side, P = 0.46; blind side, P = 0.61). Looking at the right neglect cases individually, one of the three patients showed substantial negative asymmetry (−19 cm), while another showed large positive asymmetry (+36 cm, Table 2).
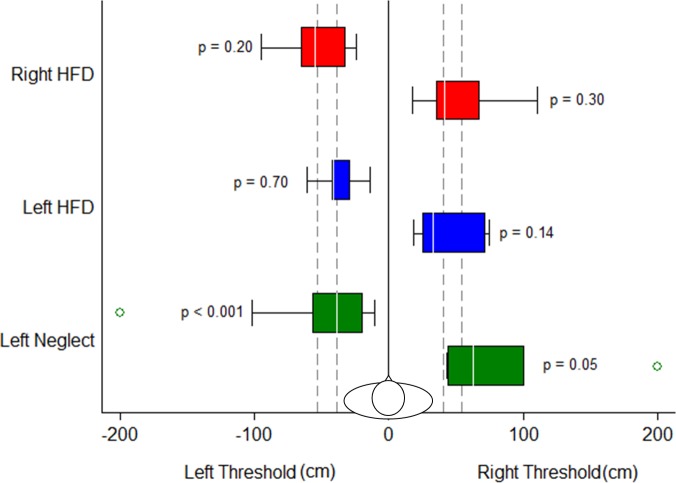
Figure 4.
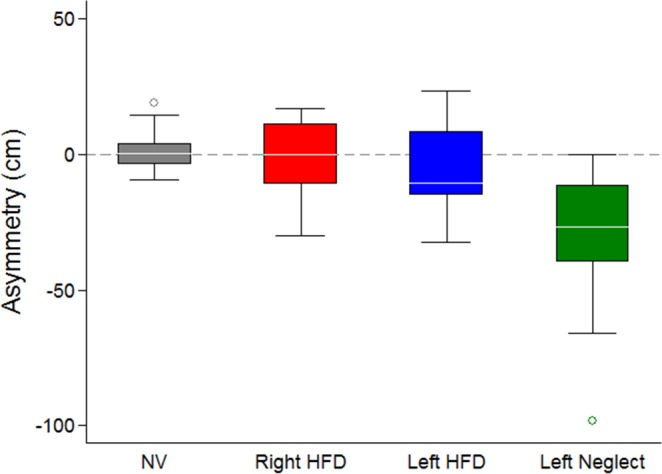
There was a significant collision threshold asymmetry in the left neglect group but not in the left HFD group or other groups. The white lines within the boxes are the median thresholds, box length represents IQR, whiskers show the range of the data within 1.5 × IQR, and open circles are outliers (> 1.5 × IQR). The right neglect group is not plotted as there were only three patients.
Figure 5.
Collision thresholds on the left and right sides. Side of the obstacle was only a significant predictor for the left neglect group (P values are for the interaction terms between each group and side of the obstacle from the logistic regression analysis). Format of bars and whiskers is the same as for Figure 4. Outliers > 1.5 × IQR are shown as open circles. The NV group is represented by the vertical dashed lines (25th and 75th quartiles of the thresholds). The right neglect group is not shown as there were only three patients.
There was a marginal effect of detection failures on the collision response (P = 0.07). Higher detection failure rates in the left neglect group could conceivably cause a smaller threshold on the blind side since they were scored “no collision” (see methods). To investigate this, the analysis was repeated excluding detection failures, and the blind side effect in the left-neglect group was still highly significant (P < 0.001).
To evaluate any predictive value of the LBT and duration of vision loss, the logistic regression analysis was repeated with HFD patients only (NV participants did not have these measures). There was a marginal effect of duration of vision loss (P = 0.07), such that longer duration was associated with more “collision” responses and so more cautious mobility. There was no association with LBT mean error (P = 0.12) or standard deviation (P = 0.73). In addition, when the analysis was repeated including only those HFD patients who had MMSE data (n = 26), there was no significant association (P = 0.48) with MMSE score.
Extreme outliers with very large thresholds were present in the left neglect group representing two patients with left neglect who responded “collision” on most trials, as described above in the statistical analysis section. Excluding these patients did not affect significance on the blind side (P < 0.005), but removed significance on the seeing side (P = 0.11).
Width of the Collision Envelope
A similar mixed-effects logistic regression model for the width of the perceived collision envelope found that patients were not more likely to have wider or narrower collision envelopes than the NV subjects (right HFD, P = 0.31; left HFD, P = 0.15; right neglect, P = 0.71; left neglect, P = 0.30). Excluding the two left neglect patients who responded collision on most trials or excluding detection-failure trials had no impact on the interpretation. Thus, patients with left neglect had an asymmetry, but not an abnormality in their overall perceived “width” (or safe passing zone).
Discussion
Consistent with our hypothesis (Fig. 2), patients with left neglect allowed obstacles to be closer to their virtual path of movement on their left (blind) side and farther away on their right (seeing) side compared to other HFD patients and NV participants (Fig. 5). This could cause incorrect positioning during mobility tasks and collisions on the blind side even when obstacles are detected. This effect was strong, with every left neglect patient, but one, exhibiting a negative asymmetry (the one who did not show asymmetry responded “collision” on every trial). The larger seeing-side threshold was borderline for significance, and so should be interpreted with caution. Unlike many left neglect studies that enroll patients with moderate-to-severe left neglect in rehabilitation facilities, our sample was fairly high functioning with chronic impairments, more similar to patients who might be encountered in typical ophthalmology or vision rehabilitation practices with primary goals of independent mobility and return to driving. The overall width of the collision envelope of the left neglect group was not different to that of the other groups, consistent with a deviation of body or extrapersonal space representation to the right rather than a general expansion or narrowing of the perceived safe passing distance on one side. The direction of the asymmetry is consistent with previously reported rightward deviations in the LBT11 and judgments of straight ahead18,29,43 by people with left neglect, and with the egocentric shift and size distortion theories of representational neglect.30,31
While our findings seem to support an egocentric shift or spatial distortion, we caution that there are other possible explanations that this study was not designed to distinguish. For example, while we controlled for motor impairments, we did not control for attentional or cognitive biases which also could have produced our findings. One example is anosognosia, a hallmark cognitive characteristic of left neglect, where patients deny or de-emphasize the severity of their impairments (most notably hemiparesis). Anosognosia might manifest during collision judgments as a devaluation of risk for obstacles on the left side and over valuation of risk on the right side, resulting in the observed fairly uniform shift in the collision envelope. It also is possible, since the effect on the seeing side was marginal and dependent on the two outliers with large collision envelopes, that the effect of neglect is only a smaller threshold on the blind side (i.e., seeing side is normal).
Detection failures on the blind or neglected side might have explained the measured asymmetry; however, this was not the case. While having left neglect was associated with higher detection failures, conducting the analysis when the detection failures were omitted rather than assigning them “no collision” did not change the outcome, with the asymmetry being just as strong. Most detection failures were for obstacles with an offset from the path of 60 cm or more on the blind or neglected side, where most patients would have responded “no collision” had they seen the obstacle. Cueing the patient to look or repeating the trial until the patient detected the obstacle was considered, but may have affected the results by unnaturally modifying visuospatial attention.
Given that the stimuli had an abrupt onset and offset, attentional capture44 could have played a role in the asymmetry, with more attention being drawn to the seeing side. The prediction might then be for a negative asymmetry. Attentional capture should have occurred in all HFD patients, but asymmetry was not seen in patients without neglect. From our study, it is not possible to distinguish between attentional capture and detection failure. However, as noted above, the asymmetry of the left neglect group was found even when removing the detection failure trials from the analysis.
An interesting and clinically relevant finding is that patients who passed the LBT and Bells test still showed evidence of left neglect in their collision judgment behavior. This suggests that spatial biases are overcome, perhaps strategically, for common clinical tests, but may still present problems in mobility. Persistent neglect behaviors in seemingly recovered patients have been reported in other studies as well.15,45 Our results provide further evidence that left neglect rarely fully recovers.
While we did not measure body size in this study, prior reports (Woods RL, et al. IOVS 2003;44:ARVO E-Abstract 4321) found that physical width at the shoulders was not correlated with the collision envelope. In that study, the only variable that was correlated was body mass index, and the correlation was negative, such that smaller people tended to have a larger collision envelope. The collision envelope is a perception of collision likelihood, perhaps related to perceived risk. We presume that, if a person reports that they perceive a likelihood of a collision, that they are highly likely to make some avoidance maneuver. Conversely, if they perceive that a collision is unlikely, they will not change their path. Thus, from a behavioral perspective, the perception of a collision risk may be as important and informative as whether a real collision would occur.
If a patient with left neglect would allow an obstacle closer on the blind side before considering it a collision risk, then obstacles on the blind side would need to be closer to the path before the patient would make a collision avoidance maneuver, elevating the risk of a collision. Similarly, that patient would consider as a risk an obstacle on the seeing (right) side that was farther away and, thus, be expected to make an unnecessary avoidance maneuver. In a similar manner, they may misjudge stationary obstacles (such as door frames) on the blind (left) side as being farther from the path of movement than they truly are, and bump them with their left arm or shoulder. Some prior studies looking at veering behavior in people with left neglect did find leftward drifting and collisions, particularly when driving a power wheelchair,1 whereas other studies found the opposite behavior (for a review, see the report of Turton et al.1). It is important to remember that visual perceptual judgment is not the only factor influencing actual mobility. For example, a cognitive strategy of staying far to the right (after multiple collisions on the left) may mask or cause misinterpretation of biases in purely visual spatial judgments. Conversely, residual motor intentional neglect may result in failure to make leftward movements of sufficient amplitude46 causing collisions on the seeing side in certain situations.
Patients with HFD but without neglect had symmetrical collision thresholds (Fig. 4), which was contrary to our hypothesis of a larger threshold on the blind side as a compensatory strategy (Fig. 2). It appears that, at least in a virtual walking situation, HFD patients without neglect do not strategically increase their blind-side safety margin, as seems to have occurred during driving.9,10 One possible reason for this discrepancy is that the perceived level of risk from oncoming traffic in those driving and driving simulator studies is much higher than our simulated walking environment, resulting in the patient strategically increasing their blind side safety margin. It is possible that left neglect patients would exhibit the same rightward offset to avoid the dangerous oncoming traffic despite their spatial misjudgments (which would predict a leftward lane offset).
Determining how asymmetric collision judgments manifest during actual mobility is an important next step in this line of research. Studies which isolate the visual perceptual and motor components of mobility and compare to actual mobility in the same patients would be useful to understand the relative contributions. Since neglect patients have more falls47 and are frequently denied motorized wheelchairs for safety reasons,48 further research in this area is critical. Mobility-related issues in subclinical neglect (i.e., patients with a history of neglect) are no less important, since patients with maladaptive collision judgment behavior may be at a higher risk for falls and other collision-related events. Most of the patients in our study reported a return to driving as one of their primary goals, making an understanding of how behaviors are modified by subclinical neglect an important public health issue. Future studies of collision avoidance in real world situations are needed to investigate the effects of the collision judgment asymmetry that we have found among people with measured neglect and with a history of neglect. If those studies do find an effect on collision avoidance, rehabilitation efforts to normalize collision judgments would be justified.
Supplementary Material
Supplement 1
Acknowledgments
We thank A.M. Barrett for discussions about spatial neglect; Qu Tang for making modifications to the collision judgment software; Jeffrey Churchill, Jean-Paul Wiegand, Azma Rehman, Rui Liu, and Sarah Sheldon for their assistance with data collection and processing; and Doris Apfelbaum, Jackie Doherty, and Amy Doherty for their assistance with scheduling and coordination.
Supported in part by Department of Defense Grant DM090420 and National Institutes of Health (Bethesda, MD, USA) Grants K12EY016335 and P30EY003790.
Disclosure: K.E. Houston, None; R.L. Woods, None; R.B. Goldstein, None; E. Peli, None; G. Luo, None; A.R. Bowers, None
References
- 1. Turton AJ,, Dewar SJ,, Lievesly A,, O'Leary K,, Gabb J,, Gilchrist ID. Walking and wheelchair navigation in patients with left visual neglect. Neuropsychol Rehabil. 2009; 19: 274–290. [DOI] [PubMed] [Google Scholar]
- 2. Boyadjian A,, Marin L,, Danion F. Veering in human locomotion: the role of the effectors. Neurosci Lett. 1999; 265: 21–24. [DOI] [PubMed] [Google Scholar]
- 3. Bowers AR,, Mandel AJ,, Goldstein RB,, Peli E. Driving with hemianopia: I. Detection performance in a driving simulator. Invest Ophthalmol Vis Sci. 2009; 50: 5137–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papageorgiou E,, Hardiess G,, Ackermann H,, et al. Collision avoidance in persons with homonymous visual field defects under virtual reality conditions. Vision Res. 2012; 52: 20–30. [DOI] [PubMed] [Google Scholar]
- 5. Iorizzo DB,, Riley ME,, Hayhoe M,, Huxlin KR. Differential impact of partial cortical blindness on gaze strategies when sitting and walking - an immersive virtual reality study. Vision Res. 2011; 51: 1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warren WH,, Whang S. Visual guidance of walking through apertures: body-scaled information for affordances. J Exp Psychol Hum Percept Perform. 1987; 13: 371–383. [DOI] [PubMed] [Google Scholar]
- 7. Gerin-Lajoie M,, Richards CL,, Fung J,, McFadyen BJ. Characteristics of personal space during obstacle circumvention in physical and virtual environments. Gait Posture. 2008; 27: 239–247. [DOI] [PubMed] [Google Scholar]
- 8. Luo G,, Woods RL,, Peli E. Collision judgment when using an augmented-vision head-mounted display device. Invest Ophthalmol Vis Sci. 2009; 50: 4509–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowers AR,, Mandel AJ,, Goldstein RB,, Peli E. Driving with hemianopia: II. Lane position and steering in a driving simulator. Invest Ophthalmol Vis Sci. 2010; 51: 6605–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wood JM,, McGwin G,, Elgin J,, et al. Hemianopic and quadrantanopic field loss, eye and head movements, and driving. Invest Ophthalmol Vis Sci. 2011; 52: 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schenkenberg T,, Bradforn DC,, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980; 30: 509–517. [DOI] [PubMed] [Google Scholar]
- 12. Barton JJ,, Black SE. Line bisection in hemianopia. J Neurol Neurosurg Psychiatry. 1998; 64: 660–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mort DJ,, Malhotra P,, Mannan SK,, et al. The anatomy of visual neglect. Brain. 2003; 126: 1986–1997. [DOI] [PubMed] [Google Scholar]
- 14. Heilman KM,, Watson RT,, Valenstein E. Neglect and related disorders. : Clinical Neuropsychology. 3 ed. New York, NY: Oxford University Press; 1993: 279–336. [Google Scholar]
- 15. Buxbaum LJ,, Ferraro MK,, Veramonti T,, et al. Hemispatial neglect: subtypes, neuroanatomy, and disability. Neurology. 2004; 62: 749–756. [DOI] [PubMed] [Google Scholar]
- 16. Berger MF,, Johannsen L,, Karnath HO. Time course of eye and head deviation in spatial neglect. Neuropsychology. 2008; 22: 697–702. [DOI] [PubMed] [Google Scholar]
- 17. Behrmann M,, Watt S,, Black SE,, Barton JJ. Impaired visual search in patients with unilateral neglect: An oculographic analysis. Neuropsychologia. 1997; 35: 1445–1458. [DOI] [PubMed] [Google Scholar]
- 18. Heilman KM,, Bowers D,, Watson RT. Performance on hemispatial pointing task by patients with neglect syndrome. Neurology. 1983; 33: 661–664. [DOI] [PubMed] [Google Scholar]
- 19. Brain R. Visual disorientation with special reference to lesions of the right cerebral hemisphere. Brain. 1941; LXIV: 244–272. [Google Scholar]
- 20. Barrett AM,, Muzaffar T. Spatial cognitive rehabilitation and motor recovery after stroke. Curr Opin Neurol. 2014; 27: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wee JYM,, Hopman WM. Comparing consequences of right and left unilateral neglect in a stroke rehabilitation population. Am J Phys Med Rehabil. 2008; 87: 910–920. [DOI] [PubMed] [Google Scholar]
- 22. Chen P,, Chen CC,, Hreha K,, Goedert KM,, Barrett AM. Kessler foundation neglect assessment process uniquely measures spatial neglect during activities of daily living. Arch Phys Med Rehabil. 2015; 96: 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen P,, Goedert KM,, Shah P,, Foundas AL,, Barrett AM. Integrity of medial temporal structures may predict better improvement of spatial neglect with prism adaptation treatment. Brain Imaging Behav. 2014; 8: 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindell AB,, Jalas MJ,, Tenovuo O,, Brunila T,, Voeten MJ,, Hamalainen H. Clinical assessment of hemispatial neglect: evaluation of different measures and dimensions. Clin Neuropsychol. 2007; 21: 479–497. [DOI] [PubMed] [Google Scholar]
- 25. Goedert KM,, Chen P,, Botticello A,, Masmela JR,, Adler U,, Barrett AM. Psychometric evaluation of neglect assessment reveals motor-exploratory predictor of functional disability in acute-stage spatial neglect. Arch Phys Med Rehabil. 2012; 93: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azouvi P,, Olivier S,, de Montety G,, Samuel C,, Louis-Dreyfus A,, Tesio L. Behavioral assessment of unilateral neglect: study of the psychometric properties of the Catherine Bergego Scale. Arch Phys Med Rehabil. 2003; 84: 51–57. [DOI] [PubMed] [Google Scholar]
- 27. Azouvi P,, Bartolomeo P,, Beis JM,, Perennou D. A battery of tests for the quantitative assessment of unilateral neglect. Restor Neurol Neurosci. 2006; 24: 273–285. [PubMed] [Google Scholar]
- 28. Ventre J,, Flandrin M,, Jeannerod M. In search for the egocentric reference. A neurophysiological hypothesis. Neuropsychologia. 1984; 22: 797–806. [DOI] [PubMed] [Google Scholar]
- 29. Vallar G. Spatial hemineglect in humans. Trends Cogn Sci. 1998; 2: 87–97. [DOI] [PubMed] [Google Scholar]
- 30. Chokron S. Right parietal lesions unilateral spatial neglect, and the egocentric frame of reference. Neuroimage. 2003; 20 (suppl 1): S75–S81. [DOI] [PubMed] [Google Scholar]
- 31. Karnath HO. Disturbed coordinate transfomation in the neural representation of space as the crucial mechanism leading to neglect. Neuropsychol Rehabil. 1994; 4: 147–150. [Google Scholar]
- 32. Karnath HO,, Schenkel P,, Fischer B. Trunk orientation as the determining factor of the 'contralateral' deficit in the neglect syndrome and as the physical anchor of the internal representation of body orientation in space. Brain. 1991; 114: 1997–2014. [DOI] [PubMed] [Google Scholar]
- 33. Karnath HO,, Fetter M,, Niemeier M. Disentangling gravitational environmental, and egocentric reference frames in spatial neglect. J Cogn Neurosci. 1998; 10: 680–690. [DOI] [PubMed] [Google Scholar]
- 34. Milner AD,, Harvey M. Distortion of size perception in visuospatial neglect. Curr Biol. 1995; 5: 85–89. [DOI] [PubMed] [Google Scholar]
- 35. Milner AD,, Harvey M,, Roberts RC,, Forster SV. Line bisection errors in visual neglect: Misguided action or size distortion? Neuropsychologia. 1993; 31: 39–49. [DOI] [PubMed] [Google Scholar]
- 36. Keller I,, Ditterich J,, Eggert T,, Straube A. Size distortion in spatial neglect. Neuroreport. 2000; 11: 1655–1660. [DOI] [PubMed] [Google Scholar]
- 37. Pritchard CL,, Dijkerman HC,, McIntosh RD,, Milner AD. Visual and tactile size distortion in a patient with right neglect. Neurocase. 2001; 7: 391–396. [DOI] [PubMed] [Google Scholar]
- 38. Dijkerman HC,, McIntosh RD,, Milner AD,, Rossetti Y,, Tilikete C,, Roberts RC. Ocular scanning and perceptual size distortion in hemispatial neglect: effects of prism adaptation and sequential stimulus presentation. Exp Brain Res. 2003; 153: 220–230. [DOI] [PubMed] [Google Scholar]
- 39. Harvey M,, Gilchrist ID,, Olk B,, Muir K. Eye-movement patterns do not mediate size distortion effects in hemispatial neglect: Looking without seeing. Neuropsychologia. 2003; 41: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 40. Vanier M,, Gauthier L,, Lambert J,, et al. Evaluation of left visuospatial neglect: norms and discrimination power of two tests. Neurology. 1990; 4: 87–96. [Google Scholar]
- 41. Giorgi RG,, Woods RL,, Peli E. Clinical and laboratory evaluation of peripheral prism glasses for hemianopia. Optom Vis Sci. 2009; 86: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bagg S,, Pombo AP,, Hopman W. Effect of age on functional outcomes after stroke rehabilitation. Stroke. 2002; 33: 179–185. [DOI] [PubMed] [Google Scholar]
- 43. Farne A,, Ponti F,, Ladavas E. In search for biased egocentric reference frames in neglect. Neuropsychologia. 1998; 36: 611–623. [DOI] [PubMed] [Google Scholar]
- 44. Yantis S,, Jonides J. Abrupt visual onsets and selective attention: evidence from visual search. J Exp Psychol Hum Percept Perform. 1984; 10: 601–621. [DOI] [PubMed] [Google Scholar]
- 45. Goodale MA,, Milner AD,, Jakobson LS,, Carey DP. Kinematic analysis of limb movements in neuropsychological research: subtle deficits and recovery of function. Can J Psychol. 1990; 44: 180–195. [DOI] [PubMed] [Google Scholar]
- 46. Goedert KM,, Chen P,, Boston RC,, Foundas AL,, Barrett A. Presence of motor-intentional aiming deficit predicts functional improvement of spatial neglect with prism adaptation. Neurorehabil Neural Repair. 2013; 28: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Campbell GB,, Matthews JT. An integrative review of factors associated with falls during post-stroke rehabilitation. J Nurs Scholarsh. 2010; 42: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dawson J,, Thornton H. Can patients with unilateral neglect following stroke drive electrically powered wheelchairs? Br J Occup Ther. 2003; 66: 496–504. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1