Abstract
BACKGROUND
Recent large clinical trials show lower rates of late cardiovascular events by extending clopidogrel >12 months after percutaneous coronary revascularization (PCI). However, concerns of increased bleeding have elicited support for limiting prolonged treatment to high-risk patients.
OBJECTIVES
The aim of this analysis was to determine the effect of prolonging clopidogrel therapy >12 months versus ≤12 months after PCI on very late outcomes in patients with diabetes mellitus (DM).
METHODS
Using the Veterans Health Administration, 28,849 patients undergoing PCI between 2002 and 2006 were categorized into 3 groups: 1) 16,332 without DM; 2) 9,905 with DM treated with oral medications or diet; and 3) 2,612 with DM treated with insulin. Clinical outcomes, stratified by stent type, ≤4 years after PCI were determined from the Veterans Health Administration and Medicare databases and risk was assessed by multivariable and propensity score analyses using a landmark analysis starting 1 year after the index PCI. The primary endpoint of the study was the risk of all-cause death or myocardial infarction (MI).
RESULTS
In patients with DM treated with insulin who received drug-eluting stents (DES), prolonged clopidogrel treatment was associated with a decreased risk of death (hazard ratio [HR]: 0.59; 95% confidence interval [CI]: 0.42 to 0.82) and death or MI (HR: 0.67; 95% CI: 0.49 to 0.92). Similarly, in patients with noninsulin-treated DM receiving DES, prolonged clopidogrel treatment was associated with less death (HR: 0.61; 95% CI: 0.48 to 0.77) and death or MI (HR: 0.61; 95% CI: 0.5 to 0.75). Prolonged clopidogrel treatment was not associated with a lower risk in patients without DM or in any group receiving bare-metal stents.
CONCLUSIONS
Extending the duration of clopidogrel treatment >12 months may decrease very late death or MI only in patients with DM receiving first-generation DES. Future studies should address this question in patients receiving second-generation.
Keywords: clopidogrel, diabetes mellitus, long-term outcomes, percutaneous coronary interventions
The duration of clopidogrel therapy after percutaneous coronary intervention (PCI) remains a vexing issue. Older studies showed that discontinuing dual antiplatelet therapy (DAPT) in the first 12 months after PCI increased the risk of myocardial infarction (MI), stent thrombosis, and death and led to the current recommendations of DAPT for ≥1 year (1). More recently, the DAPT study showed that in patients free of ischemic and major bleeding events in the first year after PCI, prolonging DAPT a further 18 months reduced recurrent ischemic events (2,3). However, concerns of harm from increased bleeding and the results of several smaller studies supporting shorter term DAPT (4–10) have generated uncertainty, with suggestions of individualizing DAPT duration according to clinical judgment of perceived risks and benefits (11). Exactly which patient characteristics should be used to determine prolonged DAPT remains unclear.
Diabetes mellitus (DM) is consistently identified as a risk factor for poorer outcomes after PCI (12–14). DM could be an important determinant of DAPT duration. The aims of this analysis were to assess whether DM could serve as a clinical indicator of benefit of prolonging DAPT >12 months after PCI.
METHODS
PATIENT POPULATION
We identified all patients who received coronary stents at any Veterans Affairs (VA) facility in the United States between April 2002 and September 2006 and who were alive 12 months after their index PCI (15). We used the International Classification of Diseases-Ninth Revision (ICD-9) procedure codes for coronary artery stent placement (36.06 for bare-metal stents [BMS] and 36.07 for drug-eluting stents [DES]) to identify patients. During this time frame, only first-generation DES were available. The index procedure was defined as the first coronary artery stent procedure between 2002 and 2006. Of 42,254 patients receiving coronary stents, 2,930 (7%) died within 12 months of their index PCI (Figure 1). We excluded another 10,475 patients, as outlined in Figure 1. Subjects free of the clinical outcomes at 1 year were followed to September 1, 2007, with a maximum follow-up of 4 years after their index PCI.
FIGURE 1. Study Population.
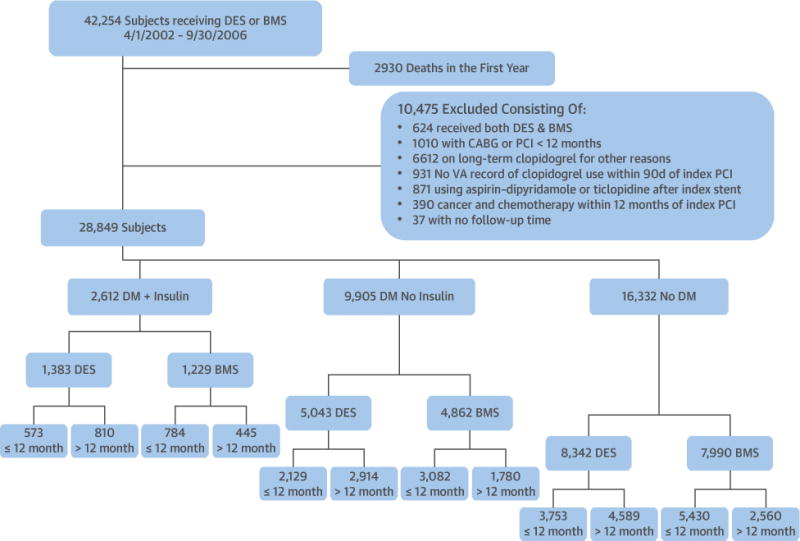
Study population with exclusion criteria. BMS = bare-metal stent(s); CABG = coronary artery bypass grafting; DES = drug-eluting stent(s); DM = diabetes mellitus; PCI = percutaneous coronary intervention; VA = Veterans Affairs.
DEMOGRAPHIC DATA
Data from the time of the index procedure included the patient age, sex, race, and presentation with an acute coronary syndrome. Comorbid conditions were defined by ICD-9 codes as those from 5 years before 12 months after the index PCI and included previous angioplasty (ICD-9: 36.01-2, 36.05-7,00.66), coronary bypass surgery (ICD-9: 36.1), smoking (ICD-9: 305.1), hypertension (ICD-9: 401), congestive heart failure (ICD-9: 428), previous stroke (ICD-9: 433.01, 433.11, 434.91, 436, V1254), peripheral arterial disease (ICD-9: 443), chronic obstructive lung disease (ICD-9: 496), anemia (ICD-9: 281–285), and chronic kidney disease (ICD-9: 585).
CLASSIFICATION OF DM
Patients were classified into 3 groups based on the ICD-9 codes for DM (250.x) and use of outpatient hypoglycemic agents in the first 12 months after the index PCI, including insulin (VA drug classification: HS501) or oral hypoglycemic agents (VA drug classification: HS502). These data were used to stratify patients into the following 3 groups: 1) no ICD-9 code for DM (no DM group); 2) ICD-9 code for DM and treated with oral medications or no medications (DM, no insulin group); and 3) ICD-9 code for DM and treated with insulin (DM and insulin).
MEDICATION USE
The VA National Pharmacy Database provided medications used at the index PCI and clopidogrel during the follow-up period. Because VA prescriptions are usually written for 90-day periods, we defined baseline cardiovascular medications as prescriptions filled within 90 days before the index procedure to up to 7 days after the index procedure. Aspirin use is a VA quality-control measure, and other studies show very high rates of outpatient aspirin use in VA patients with coronary artery disease (16). The database tracks the dates of prescription and the amount and delivery of clopidogrel up to 4 years after their index PCI. If a clopidogrel prescription lapsed >30 days from the last day of the supply, the patient was considered to be not taking clopidogrel. We defined clopidogrel use as either prolonged (>12 months of use after the index PCI) or ≤12 months of use after the index PCI.
OUTCOMES
Clinical outcomes after the index PCI were identified from the Department of Veterans Affairs National Patient Care, VA Death Database, and the Centers for Medicaid and Medicare database using ICD-9 codes until October 2007. These included all-cause death, the combined outcome of death or MI (ICD-9: 410), admissions with a new discharge diagnosis of revascularization by PCI (ICD-9: 36.01, 36.02, 36.05, 00.66) or coronary artery bypass grafting (CABG) (ICD-9:36.1), ischemic stroke (ICD-9: 436, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91), and hospitalization for severe bleeding (ICD-9: rectal bleeding, 569.3; esophageal hemorrhage, 530.82; hematemesis, 578; gastroduodenitis with bleeding, 535.01–535.61; intracranial hemorrhage, 430–432; intraocular, 379.23; hemorrhage not specified, 459).
STATISTICAL ANALYSIS
The primary endpoint was the combined endpoint of death or MI in the 1 to 4 years after PCI, with secondary outcomes of death alone, repeat surgical or percutaneous coronary revascularization, ischemic stroke, or serious or life-threatening bleeding. Because previous studies indicate different outcomes in patients with DES versus BMS, the analysis was prospectively stratified by stent type. We used event curves and landmark analyses to analyze outcomes in subjects who were free of the outcome under analysis 12 months after the index PCI. Patients were followed until they died or had the outcome of interest. Follow-up was censored after September 2007, 12 months after the last recorded VA visit, or 4 years after the index procedure. We calculated the hazard ratio (HR) and 95% confidence interval (CI) for prolonged clopidogrel use versus clopidogrel ≤12 months for each outcome within each stent and DM subgroup using Cox proportional hazards regression. Multivariable models were developed from more than 50 variables and included whether they were related to the outcome of death or MI. The HR from the final multivariable models for each outcome were adjusted for the baseline characteristics of age, hypertension, smoking, acute coronary syndrome on admission, peripheral vascular disease, chronic kidney disease, congestive heart failure, warfarin use within 7 days of the index procedure, and the year of stent placement. The multivariable models excluded patients with the outcome of interest in the first year and also adjusted for the other major outcomes in the first year (e.g., the HR for long-term death or MI excluded patients with death or MI in the first year and adjusted for stroke, revascularization, or bleeding in the first year). We also used Cox models to assess stent type by prolonged clopidogrel interactions within each DM group. We used a second method of adjustment for confounders by constructing propensity models for prolonged clopidogrel from statistically significant baseline characteristics using multivariable logistic regression (15) (Online Table 1). We estimated HRs for each outcome using inverse probability weighting of the propensity score in the Cox proportional hazards models. Sensitivity analyses included the following: 1) repeating the analyses after excluding subjects with any primary or secondary endpoint in the first year to simulate the patient selection in the DAPT trial (3); and 2) varying the definition of stopping clopidogrel from a gap in treatment >30 days to a gap in treatment of >15 days and >60 days. To account for the multiple comparisons, statistical significance was p <0.01.
TABLE 1.
Baseline Data for Patients Receiving DES for PCI by DM Group and Duration of Clopidogrel Treatment
| Insulin-Treated DM (n = 1,383) | DM, No Insulin (n = 5,043) | No DM (n = 8,342) | ||||
|---|---|---|---|---|---|---|
| Clopidogrel duration, months | ≤12 (n = 573) | >12 (n = 810) | ≤12 (n = 2,129) | >12 (n = 2,914) | ≤12 (n = 3,753) | <12 (n = 4,589) |
|
| ||||||
| Age, yrs | 63.9 ± 9.0 | 64.7 ± 9.9 | 64.6 ± 9.2 | 63.9 ± 9.0 | 64.7 ± 9.9 | 63.7 ± 10.4 |
|
| ||||||
| Men | 799 (98.6) | 2,092 (98.3) | 2,866 (98.4) | 799 (98.6) | 2,092 (98.3) | 2,515 (98.2) |
|
| ||||||
| Comorbidities 5 yrs before to 1 yr after index procedure | ||||||
| Smoking | 181 (31.6) | 253 (31.2) | 808 (38.0) | 1,030 (35.4) | 1,879 (50.1) | 2,166 (47.2) |
| Previous MI | 195 (34.0) | 284 (35.1) | 581 (27.3) | 917 (31.5) | 1,032 (27.5) | 1,459 (31.8) |
| Angina | 239 (41.7) | 375 (46.3) | 865 (40.6) | 1,228 (42.1) | 1,363 (36.3) | 1,707 (37.2) |
| Hypertension | 564 (98.4) | 798 (98.5) | 2,039 (95.8) | 2,826 (97.0) | 3,274 (87.2) | 4,119 (89.8) |
| COPD | 173 (30.2) | 267 (33.0) | 636 (29.9) | 884 (30.3) | 993 (26.5) | 1,280 (27.9) |
| CKD | 132 (23.0) | 190 (23.5) | 200 (9.4) | 322 (11.1) | 201 (5.4) | 289 (6.3) |
| PVD | 189 (33.0) | 252 (31.1) | 428 (20.1) | 602 (20.7) | 495 (13.2) | 639 (13.9) |
| Anemia, Hb <12 mg/dl | 146 (27.4) | 178 (22.0) | 350 (17.7) | 457 (15.7) | 520 (14.9) | 513 (11.2) |
| Stroke | 52 (9.1) | 61 (7.5) | 128 (6.0) | 181 (6.2) | 174 (4.6) | 188 (4.1) |
| Heart failure | 268 (46.8) | 390 (48.2) | 635 (29.8) | 855 (29.3) | 739 (19.7) | 899 (19.6) |
| Previous CABG | 22 (3.8) | 21 (2.6) | 55 (2.6) | 50 (1.7) | 58 (1.6) | 38 (0.8) |
| Previous PCI | 19 (3.3) | 18 (2.2) | 50 (2.4) | 42 (1.4) | 66 (1.8) | 56 (1.2) |
| ACS on index admission | 322 (56.2) | 479 (59.1) | 1,264 (59.4) | 1,776 (61.0) | 2,500 (66.6) | 3,101 (67.6) |
|
| ||||||
| Baseline medication | ||||||
| Statin or lipid-lowering agent | 492 (85.9) | 698 (86.2) | 1,746 (82.0) | 2,438 (83.7) | 2,976 (79.3) | 3,699 (80.6) |
| Beta-blocker | 538 (93.9) | 769 (94.9) | 1,950 (91.6) | 2,710 (93.0) | 3,329 (88.7) | 4,157 (90.6) |
| Calcium-channel blocker | 529 (92.3) | 745 (92.0) | 1,884 (88.5) | 2,589 (88.9) | 3,155 (84.1) | 4,001 (87.2) |
| ACEI or ARB | 193 (33.7) | 286 (35.3) | 585 (27.5) | 917 (31.5) | 751 (20.0) | 1,049 (22.9) |
| PPI | 444 (77.5) | 634 (78.3) | 1,597 (75.0) | 2,254 (77.4) | 2,444 (65.1) | 3,149 (68.6) |
| Warfarin | 306 (53.4) | 453 (55.9) | 972 (45.7) | 1,507 (51.7) | 1,679 (44.7) | 2,290 (49.9) |
Values are mean ± SD or n (%).
ACEI = angiotensin-converting enzyme inhibitor; ACS = acute coronary syndrome(s); ARB = angiotensin receptor blocker; CABG = coronary artery bypass grafting; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DES = drug-eluting stent(s); DM = diabetes mellitus; Hb = hemoglobin; MI = myocardial infarction; PCI = percutaneous coronary intervention; PPI = proton pump inhibitor; PVD = peripheral vascular disease.
RESULTS
After exclusions (Figure 1), the study population consisted of 28,849 patients who were alive 12 months after their index PCI. Tables 1 and 2 show baseline characteristics for all patients who received PCI with DES (Table 1) or BMS (Table 2), by DM group and duration of clopidogrel treatment.
TABLE 2.
Baseline Data for Patients Receiving BMS for PCI by DM Group and Duration of Clopidogrel Treatment
| Insulin-Treated DM (n = 1,229) | DM, No Insulin (n = 4,862) | No DM (n = 7,990) | ||||
|---|---|---|---|---|---|---|
| Clopidogrel duration, months | ≤12 (n = 784) | >12 (n = 445) | ≤12 (n = 3,082) | >12 (n = 1,780) | ≤12 (n = 5,430) | >12 (n = 2,560) |
|
| ||||||
| Age, yrs | 63.9 ± 9.9 | 64.4 ± 9.5 | 64.9 ± 9.8 | 65.3 ± 9.5 | 63.4 ± 10.6 | 63.7 ± 10.4 |
|
| ||||||
| Men | 764 (97.5) | 439 (98.7) | 3,032 (98.4) | 1,753 (98.5) | 5,349 (98.5) | 2,515 (98.2) |
|
| ||||||
| Comorbidities 5 yrs before to 1 yr after index procedure | ||||||
| Smoking | 262 (33.4) | 135 (30.3) | 1125 (36.5) | 651 (36.6) | 2,479 (45.7) | 1,125 (44.0) |
| Previous MI | 279 (35.6) | 170 (38.2) | 995 (32.3) | 646 (36.3) | 1,719 (31.7) | 867 (33.9) |
| Angina | 403 (51.4) | 223 (50.1) | 1,522 (49.4) | 879 (49.4) | 2,402 (44.2) | 1,132 (44.2) |
| Hypertension | 772 (98.5) | 437 (98.2) | 2,959 (96.0) | 1,741 (97.8) | 4,797 (88.3) | 2,312 (90.3) |
| COPD | 251 (32.0) | 140 (31.5) | 906 (29.4) | 555 (31.2) | 1,532 (28.2) | 727 (28.4) |
| CKD | 179 (22.8) | 112 (25.2) | 297 (9.6) | 168 (9.4) | 271 (5.0) | 136 (5.3) |
| PVD | 243 (31.0) | 157 (35.3) | 722 (23.4) | 411 (23.1) | 842 (15.5) | 401 (15.7) |
| Anemia, Hb <12 mg/dl | 163 (27.1) | 126 (28.3) | 418 (18.2) | 306 (17.2) | 628 (15.7) | 394 (15.4) |
| Stroke | 90 (11.5) | 54 (12.1) | 263 (8.5) | 130 (7.3) | 283 (5.2) | 131 (5.1) |
| Heart failure | 385 (49.1) | 238 (53.5) | 983 (31.9) | 589 (33.1) | 1,222 (22.5) | 572 (22.3) |
| Previous CABG | 27 (3.4) | 16 (3.6) | 84 (2.7) | 47 (2.6) | 80 (1.5) | 36 (1.4) |
| Previous PCI | 60 (7.7) | 24 (5.4) | 180 (5.8) | 87 (4.9) | 213 (3.9) | 64 (2.5) |
| ACS on index admission | 465 (59.3) | 276 (62.0) | 1,861 (60.48) | 1,145 (64.3) | 3,546 (65.3) | 1,749 (68.3) |
|
| ||||||
| Baseline medication | ||||||
| Statin or lipid-lowering agent | 716 (91.3) | 407 (91.5) | 2,832 (91.9) | 1,663 (93.4) | 4,902 (90.3) | 2,349 (91.8) |
| Beta-blocker | 730 (93.1) | 412 (92.6) | 2,829 (91.8) | 1,649 (92.6) | 4,891 (90.1) | 2,294 (89.6) |
| Calcium-channel blocker | 315 (40.2) | 195 (43.8) | 989 (32.1) | 597 (33.5) | 1,341 (24.7) | 669 (26.1) |
| ACEI or ARB | 640 (81.6) | 354 (79.6) | 2,498 (81.1) | 1,453 (81.6) | 3,891 (71.7) | 1,824 (71.3) |
| PPI | 404 (51.5) | 248 (55.7) | 1,395 (45.3) | 927 (52.1) | 2,370 (43.7) | 1,281 (50.0) |
| Warfarin | 86 (11.0) | 46 (10.3) | 355 (11.5) | 126 (7.1) | 452 (8.3) | 180 (7.0) |
Values are mean ± SD or n (%).
BMS = bare-metal stent(s); other abbreviations as in Table 1.
Events for insulin-treated DM between the index PCI and 1 year included MI in 7.6%, repeat revascularization with PCI or CABG in 17.3%, stroke in 1.0%, and major bleeding in 1.8%. Events in the DM and no insulin group between the index PCI and 1 year included MI in 5.4%, repeat revascularization with PCI or CABG in 14.8%, stroke in 0.4%, and severe bleeding in 1.2%. Events in the no DM group between the index PCI and 1 year included MI in 4.4%, repeat revascularization with PCI or CABG in 11.7%, stroke in 0.3%, and major bleeding in 1.2%. Among patients receiving DES, the mean durations of clopidogrel use for patients in the >12 versus ≤12 months clopidogrel groups were 18.5 versus 6.1 months for insulin-treated DM, 17.9 versus 6.2 months for DM and no insulin, and 17.0 versus 6.2 months for no DM. Among patients receiving BMS, the corresponding durations were 23.1 versus 3.0 months for insulin-treated DM, 22.3 versus 2.6 months for DM and no insulin, and 20.0 versus 2.8 months for no DM.
RISK OF DEATH OR MI WITH DM VERSUS NO DM
Among patients alive at 12 months, both DM groups had higher rates of death or MI 1 to 4 years after PCI compared with patients without DM. Among patients receiving DES, death or MI occurred in 150 (22%) of patients with insulin-treated DM (HR: 1.97; p < 0.0001 compared with no DM), 393 (16%) with DM not requiring insulin (HR: 1.31; p < 0.0001 compared with no DM), and 129 (12%) with no DM. Among patients treated with BMS, this combined endpoint occurred in 300 patients (31%) with insulin-treated DM (HR: 2.28; p < 0.0001 compared with no DM), 726 (19%) patients with DM not requiring insulin (HR: 1.27; p < 0.0001 compared with no DM), and 233 (15%) of patients without DM.
Compared with 2006, index procedures in earlier years were associated with a slightly higher risk of death or MI (HRs ranging from 1.23 to 1.33) with statistically significant differences in death or MI for years 2002 through 2004 versus 2006 (p = 0.01 to 0.03). For this reason, stent year was included in our multivariable models.
RISK OF EVENTS ASSOCIATED WITH PROLONGED CLOPIDOGREL USE
Figures 2 and 3 display the cumulative event curves for death, and death or MI for the 3 DM groups stratified by both duration of clopidogrel therapy after the index PCI and stent type. Tables 3 and 4 show the HRs for death, death or MI, repeat coronary revascularization, stroke, and severe bleeding from univariate, multivariable, and propensity-score models. In the multivariable and propensity-score models, prolonged clopidogrel treatment remained associated with a lower risk of death and death or MI only in patients with DM receiving DES (Table 3), but not BMS (Table 4). Prolonged clopidogrel treatment was not related to repeat revascularization, stroke, or severe bleeding. However, the number of stroke and severe bleeding events >12 months after the index PCI was relatively low.
FIGURE 2. Landmark Analyses of Death or Myocardial Infarction.
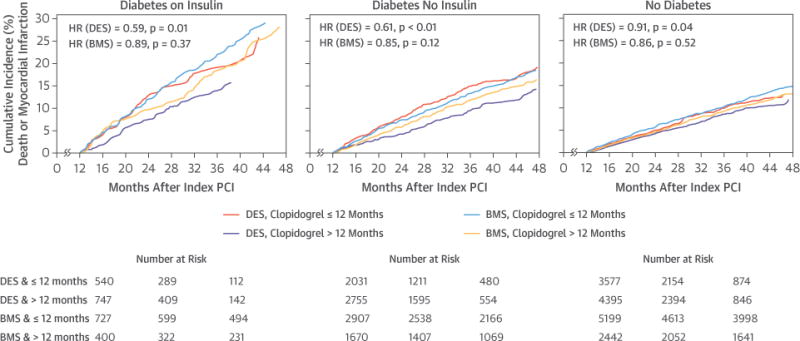
Landmark analyses starting 12 months after the index PCI showing death or myocardial infarction by diabetes mellitus group, stent type, and clopidogrel duration >12 months versus ≤12 months. BMS = bare-metal stent(s); DES = drug-eluting stent(s); HR = hazard ratio; other abbreviations as in Figure 1.
FIGURE 3. Landmark Analysis for Death.
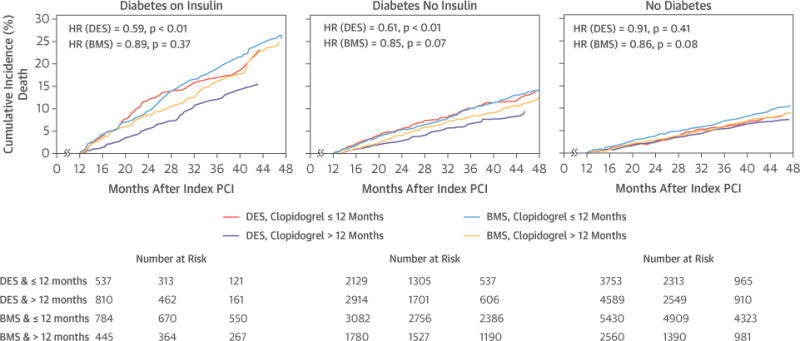
Landmark analyses starting 12 months after the index PCI showing death by diabetes mellitus group, stent type, and clopidogrel duration >12 months versus ≤12 months. Abbreviations as in Figures 1 and 2.
TABLE 3.
Landmark Analysis of the Risk of Clinical Endpoints for Patients With and Without DM Treated With >12 Months Versus ≤12 Months of Clopidogrel After Index Percutaneous Coronary Revascularization With DES*
| Events: Clopidogrel Duration
|
Univariate
|
Multivariable Adjusted†
|
Propensity Adjusted‡
|
|||||
|---|---|---|---|---|---|---|---|---|
| ≤12 Months | >12 Months | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| DM treated with insulin | ||||||||
| Death | 75 (23) | 61 (16) | 0.59§ | 0.42–0.82 | 0.53§ | 0.38–0.75 | 0.60‡ | 0.43–0.85 |
| Death or MI | 79 (26) | 71 (19) | 0.67‖ | 0.49–0.92 | 0.67 | 0.48–0.93 | 0.71 | 0.61–0.98 |
| CABG or PCI | 32 (14) | 42 (11) | 1.00 | 0.63–1.59 | 0.97 | 0.61–1.55 | 1.03 | 0.65–1.65 |
| Stroke | 3 (1) | 3 (1) | 0.71 | 0.14–3.53 | 0.76 | 0.15–3.85 | 0.76 | 0.15–3.83 |
| Severe bleed | 20 (8) | 9 (2) | 0.32§ | 0.14–0.70 | 0.28§ | 0.13–0.63 | 0.43 | 0.20–0.96 |
|
| ||||||||
| DM, no insulin | ||||||||
| Death | 163 (15) | 127 (9) | 0.61§ | 0.48–0.77 | 0.60§ | 0.48–0.76 | 0.65§ | 0.51–0.82 |
| Death or MI | 220 (19) | 173 (13) | 0.61§ | 0.50–0.75 | 0.60§ | 0.49–0.74 | 0.63§ | 0.52–0.77 |
| CABG or PCI | 148 (14) | 149 (12) | 0.81 | 0.64–1.01 | 0.81 | 0.64–1.02 | 0.83 | 0.66–1.05 |
| Stroke | 16 (1) | 14 (1) | 0.70 | 0.34–1.43 | 0.76 | 0.37–1.58 | 0.85 | 0.41–1.75 |
| Severe bleed | 31 (3) | 29 (3) | 0.73 | 0.44–1.22 | 0.73 | 0.44–1.22 | 0.99 | 0.59–1.65 |
|
| ||||||||
| No DM | ||||||||
| Death | 161 (9) | 162 (7) | 0.91 | 0.73–1.14 | 0.92 | 0.73–1.14 | 0.94 | 0.76–1.18 |
| Death or MI | 254 (13) | 236 (11) | 0.83 | 0.69–0.99 | 0.83 | 0.69–0.99 | 0.86 | 0.72–1.03 |
| CABG or PCI | 180 (11) | 190 (10) | 1.00 | 0.81–1.22 | 1.02 | 0.83–1.25 | 1.04 | 0.85–1.28 |
| Stroke | 15 (1) | 13 (0.5) | 0.80 | 0.38–1.68 | 0.81 | 0.38–1.71 | 0.86 | 0.40–1.82 |
| Severe bleed | 29 (2) | 30 (1) | 0.94 | 0.56–1.57 | 0.94 | 0.56–1.58 | 1.35 | 0.80–2.27 |
Values are n (%) unless otherwise indicated.
Subjects free of the endpoint at 12 months were assessed for risk of the endpoint from 12 months to end of follow-up.
Adjusted for age, year of stenting, smoking, acute coronary syndrome during index admission, hypertension, peripheral vascular disease, heart failure, chronic kidney disease, warfarin at discharge, and myocardial infarction, stroke, revascularization, and bleeding during the 1 year after the index procedure.
Adjusted for the propensity to use clopidogrel for >12 months.
p < 0.001.
p < 0.01.
CI = confidence interval; HR = hazard ratio; other abbreviations as in Table 1.
TABLE 4.
Landmark Analysis of the Risk of Clinical Endpoints for Patients With and Without DM Treated With >12 Months Versus ≤12 Months of Clopidogrel After an Index Percutaneous Coronary Revascularization With BMS*
| Events Clopidogrel Duration
|
Univariate
|
Multivariable Adjusted†
|
Propensity Adjusted‡
|
|||||
|---|---|---|---|---|---|---|---|---|
| ≤12 Months | >12 Months | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| DM treated with insulin | ||||||||
| Death | 190 (27) | 87 (25) | 0.89 | 0.69–1.15 | 0.78 | 0.60–1.02 | 0.90 | 0.69–1.16 |
| Death or MI | 208 (32) | 92 (30) | 0.88 | 0.69–1.13 | 0.79 | 0.61–1.02 | 0.87 | 0.67–1.12 |
| CABG or PCI | 88 (17) | 44 (18) | 0.97 | 0.67–1.39 | 0.95 | 0.66–1.39 | 0.87 | 0.60–1.27 |
| Stroke | 16 (3) | 6 (2) | 0.73 | 0.29–1.87 | 0.82 | 0.31–2.18 | 0.81 | 0.31–2.11 |
| Severe bleed | 27 (4) | 13 (4) | 0.90 | 0.46–1.74 | 0.77 | 0.39–1.54 | 0.88 | 0.45–1.73 |
|
| ||||||||
| DM, no insulin | ||||||||
| Death | 405 (15) | 179 (13) | 0.85 | 0.71–1.01 | 0.83 | 0.70–1.00 | 0.89 | 0.74–1.07 |
| Death or MI | 498 (19) | 228 (17) | 0.88 | 0.75–1.03 | 0.87 | 0.74–1.02 | 0.88 | 0.75–1.04 |
| CABG or PCI | 324 (15) | 171 (15) | 1.03 | 0.86–1.24 | 1.08 | 0.89–1.31 | 1.00 | 0.82–1.21 |
| Stroke | 53 (2) | 18 (1) | 0.65 | 0.38–1.11 | 0.68 | 0.39–1.19 | 0.69 | 0.40–1.19 |
| Severe bleed | 71 (3) | 31 (2) | 0.83 | 0.54–1.27 | 0.87 | 0.56–1.34 | 0.99 | 0.64–1.54 |
|
| ||||||||
| No DM | ||||||||
| Death | 526 (11) | 193 (10) | 0.86 | 0.73–1.02 | 0.84 | 0.71–1.00 | 0.89 | 0.75–1.06 |
| Death or MI | 711 (15) | 268 (14) | 0.88 | 0.77–1.02 | 0.86 | 0.75–1.00 | 0.88 | 0.76–1.02 |
| CABG or PCI | 474 (12) | 200 (12) | 1.03 | 0.87–1.21 | 1.08 | 0.91–1.28 | 0.97 | 0.81–1.15 |
| Stroke | 51 (1) | 18 (1) | 0.84 | 0.49–1.44 | 0.87 | 0.50–1.52 | 0.90 | 0.52–1.58 |
| Severe bleed | 100 (2) | 37 (2) | 0.86 | 0.59–1.25 | 0.90 | 0.61–1.33 | 0.92 | 0.62–1.37 |
Values are n (%) unless otherwise indicated.
Subjects free of the endpoint at 12 months were assessed for risk of the endpoint from 12 months to end of follow-up.
Adjusted for age, year of stenting, smoking, acute coronary syndrome during index admission, hypertension, peripheral vascular disease, heart failure, chronic kidney disease, warfarin at discharge, and myocardial infarction, stroke, revascularization, and bleeding during the 1 year after the index procedure.
Adjusted for the propensity to use clopidogrel for >12 months.
MODELS WITH INTERACTION EFFECTS
Interaction terms for prolonged clopidogrel treatment and stent type within each of the 3 DM cohorts were assessed. Among DM patients not requiring insulin, there was a significant interaction for prolonged clopidogrel treatment and stent type, with a significantly greater reduction in the risk of death for DES versus BMS patients (interaction p = 0.03) and death or MI in DES versus BMS patients (interaction p = 0.005). There were similar trends in patients with insulin-treated DM, with prolonged clopidogrel treatment having a greater effect on risk of death (interaction p = 0.055) and death or MI (interaction p = 0.19) in patients receiving DES versus BMS.
SENSITIVITY ANALYSES
The HRs for prolonged clopidogrel treatment, statistical significance, and the conclusions were unchanged in analyses from which patients with any endpoint in the first 12 months were excluded simulating the DAPT trial (Tables 5 and 6), and for analyses with varying definitions of stopping clopidogrel treatment (Online Tables 2 and 3).
TABLE 5.
Landmark Analysis of the Risk of Clinical Endpoints for Patients With and Without DM Treated With >12 Months Versus ≤12 Months of Clopidogrel After Percutaneous Coronary Revascularization With DES*
| Univariate
|
Multivariable Adjusted†
|
Propensity Adjusted‡
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| DM treated with insulin | ||||||
| Death | 0.51§ | 0.33–0.77 | 0.50§ | 0.32–0.76 | 0.53§ | 0.35–0.81 |
| Death or MI | 0.60‖ | 0.42–0.86 | 0.61‖ | 0.43–0.88 | 0.63‖ | 0.44–0.91 |
| CABG or PCI | 0.92 | 0.57–1.50 | 0.93 | 0.57–1.51 | 0.96 | 0.58–1.56 |
| Stroke | 0.75 | 0.15–3.74 | 0.76 | 0.15–3.85 | 0.77 | 0.15–3.87 |
| Severe bleed | 0.31‖ | 0.13–0.76 | 0.29‖ | 0.12–0.70 | 0.42 | 0.17–1.03 |
|
| ||||||
| DM, no insulin | ||||||
| Death | 0.57§ | 0.43–0.75 | 0.56§ | 0.42–0.74 | 0.62§ | 0.46–0.82 |
| Death or MI | 0.62§ | 0.50–0.77 | 0.60§ | 0.48–0.75 | 0.64§ | 0.52–0.80 |
| CABG or PCI | 0.81 | 0.64–1.03 | 0.82 | 0.65–1.04 | 0.84 | 0.66–1.06 |
| Stroke | 0.72 | 0.33–1.55 | 0.78 | 0.36–1.69 | 0.85 | 0.39–1.86 |
| Severe bleed | 1.24 | 0.63–2.44 | 1.26 | 0.64–2.49 | 1.63 | 0.82–3.22 |
|
| ||||||
| No diabetes mellitus | ||||||
| Death | 0.98 | 0.76–1.25 | 0.98 | 0.77–1.26 | 1.02 | 0.80–1.31 |
| Death or MI | 0.85 | 0.70–1.03 | 0.85 | 0.70–1.03 | 0.89 | 0.73–1.08 |
| CABG or PCI | 1.00 | 0.81–1.23 | 1.02 | 0.83–1.26 | 1.05 | 0.85–1.30 |
| Stroke | 0.82 | 0.38–1.77 | 0.83 | 0.38–1.81 | 0.87 | 0.40–1.89 |
| Severe bleed | 0.97 | 0.56–1.69 | 0.96 | 0.55–1.66 | 1.41 | 0.81–2.47 |
Subjects with any adverse events in the first year were excluded.
Adjusted for age, year of stenting, smoking, acute coronary syndrome during index admission, hypertension, peripheral vascular disease, heart failure, chronic kidney disease, and warfarin at discharge.
Adjusted for the propensity to use clopidogrel for >12 months.
p < 0.001.
p < 0.01.
TABLE 6.
Landmark Analysis of the Risk of Clinical Endpoints for Patients With and Without DM Treated With >12 Months Versus ≤12 Months of Clopidogrel After Index Percutaneous Coronary Revascularization With BMS*
| Univariate
|
Multivariable Adjusted†
|
Propensity Adjusted‡
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| DM treated with insulin | ||||||
| Death | 0.83 | 0.61–1.12 | 0.70 | 0.51–0.97 | 0.85 | 0.62–1.16 |
| Death or MI | 0.94 | 0.71–1.23 | 0.82 | 0.62–1.08 | 0.93 | 0.70–1.23 |
| CABG or PCI | 0.96 | 0.66–1.39 | 0.95 | 0.65–1.39 | 0.86 | 0.59–1.27 |
| Stroke | 0.68 | 0.24–1.89 | 0.73 | 0.26–2.08 | 0.72 | 0.26–2.03 |
| Severe bleed | 0.67 | 0.26–1.70 | 0.58 | 0.22–1.54 | 0.70 | 0.28–1.80 |
|
| ||||||
| DM, no insulin | ||||||
| Death | 0.78§ | 0.64–0.96 | 0.77 | 0.62–0.95 | 0.82 | 0.66–1.02 |
| Death or MI | 0.84 | 0.71–1.00 | 0.83 | 0.69–0.99 | 0.85 | 0.71–1.01 |
| CABG or PCI | 0.98 | 0.81–1.19 | 1.03 | 0.84–1.26 | 0.94 | 0.77–1.15 |
| Stroke | 0.55 | 0.29–1.04 | 0.58 | 0.30–1.13 | 0.56 | 0.29–1.08 |
| Severe bleed | 0.72 | 0.44–1.19 | 0.77 | 0.46–1.30 | 0.89 | 0.52–1.50 |
|
| ||||||
| No DM | ||||||
| Death | 0.87 | 0.73, 1.05 | 0.86 | 0.71, 1.04 | 0.91 | 0.75–1.10 |
| Death or MI | 0.89 | 0.76–1.03 | 0.86 | 0.73–1.00 | 0.89 | 0.76–1.04 |
| CABG or PCI | 1.02 | 0.86–1.21 | 1.08 | 0.91–1.29 | 0.96 | 0.80–1.15 |
| Stroke | 0.93 | 0.53–1.63 | 0.97 | 0.55–1.73 | 0.99 | 0.55–1.76 |
| Severe bleed | 0.72 | 0.47–1.11 | 0.76 | 0.49–1.18 | 1.79 | 0.51–1.24 |
Subjects with any adverse events in the first year were excluded.
Adjusted for age, year of stenting, smoking, acute coronary syndrome during index admission, hypertension, peripheral vascular disease, heart failure, chronic kidney disease, and warfarin at discharge.
Adjusted for the propensity to use clopidogrel for >12 months.
DISCUSSION
Patients with DM are at heightened risk of poor clinical outcomes after PCI (12–14), and our analysis showed that this risk persisted even in those who were free of events during first year (Central Illustration). Importantly, this analysis identified the interaction of DM and stent type as factors that may guide long-term DAPT duration. Only patients with DM receiving a DES had lower risk of death or MI with prolonged clopidogrel use. Patients without DM receiving DES or any patient receiving BMS did not benefit from prolonged clopidogrel use.
CENTRAL ILLUSTRATION. Long-Term Outcomes in Patients With Diabetes Mellitus Related to Prolonging Clopidogrel After Coronary Stenting.
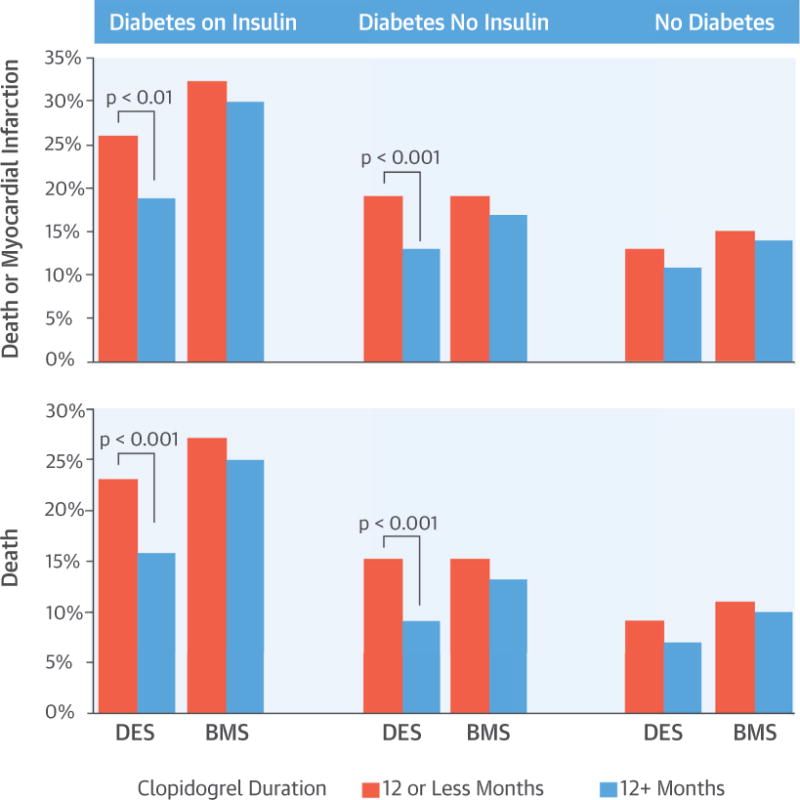
Thukkani, A.K. et al. J Am Coll Cardiol. 2015; 66(10):1091–101.
COMPARISON WITH THE DAPT RANDOMIZED TRIAL
These results need to be considered in the context of several important randomized trials of DAPT duration after PCI. Compared with the DAPT trial, the largest randomized trial to date (2,3,15), subjects in our study had higher rates of a number of risk factors including hypertension (95% to 98% vs. 76%), smoking (31% to 47% vs. 25%), previous cerebrovascular accident (4% to 7% vs. 3%), and peripheral vascular disease (13% to 33% vs. 6%). Not surprisingly, the absolute rates of major cardiovascular events were also higher in our 3 groups compared with DAPT (∼12% to 20% vs. 5% to 6% over 3 years). Thus, our population overall, and the DM subgroups in particular, were at higher risk than participants in the DAPT study.
Overall, our observational results compare favorably with those of the randomized DAPT study (2,3), with a 10% to 30% lower risk of major cardiovascular events by prolonging DAPT treatment >12 months after PCI (2,3,15). This supports the validity of the conclusions of our current analysis examining subgroups of DM.
In contrast to the DAPT study, we found a lower risk of all-cause mortality with prolonged clopidogrel treatment in our overall results (15) and in patients with DM receiving DES. However, our observational study had greater statistical power as it included 50% more patients receiving DES than the DAPT study and the risk of death was >5 times higher than in the DAPT study. The higher risk likely reflects a more inclusive “real-world” experience in our cohort, the longer follow-up in our study, and a higher cardiovascular risk of veterans compared with nonveteran populations. A recent meta-analysis found no difference in mortality with prolonged clopidogrel use, but included trials with a diverse range of indications including medical therapy for cardiovascular disease and atrial fibrillation and post-PCI patients with much shorter durations of clopidogrel therapy (17).
In recent months, at least 6 meta-analyses of randomized trials of prolonged versus short term DAPT were published (18–23). Four analyses pooled 10 studies including the DAPT trial. The different meta-analyses include frequentist, Bayesian, and individual data techniques, as well as intention-to-treat (risk from the time of the index PCI) and landmark (risk after a standard treatment time) approaches. The meta-analyses do not assess high-risk subgroups such as DM, but overall suggest a lower risk of MI and a higher risk of all-cause death with prolonged DAPT. However, the smaller meta-analysis did not find any statistically significant differences in these endpoints, and all analyses found no effect on cardiac mortality when this was assessed. Overall, our study has 10% to 300% more deaths in our DES subgroup and 240% to 610% more deaths overall than the total number of deaths in these meta-analyses. This enables us to assess the value of prolonged clopidogrel use in the important subgroup of DM with sufficient statistical power. Identifying subgroups of patients where the risk benefit ratio of prolonged DAPT is more favorable may help to rationalize post-PCI therapy. In another example of this approach, patients in the DAPT trial with an acute coronary syndrome at their index PCI had a greater reduction in major adverse cardiovascular events from prolonged DAPT than those with stable syndromes (24). However, this analysis lacked the power to assess individual endpoints in these subgroups.
Newer second-generation DES may modify the benefit of prolonged clopidogrel. First-generation DES were the only DES in our study and the TL-PAS (TAXUS Liberte Post Approval Study) (2) and accounted for 30% of DES in the DAPT study. In the DAPT study, the reduction in risk of MI with prolonged clopidogrel use tended to be lower in second- versus first-generation DES. Other randomized trials concluded that there is little difference between 6 months of DAPT and 12 or 24 months of DAPT (4–10). However, many of these studies are much smaller than DAPT with lower statistical power. Nevertheless, they have led to uncertainty and concern in prolonging DAPT for several years after PCI in every patient. Our study provides evidence that prolonged DAPT can be tailored to specific patient and stent characteristics.
OTHER ENDPOINTS
Prolonged clopidogrel treatment did not alter the rate of repeat coronary revascularization for either the stent type or DM group, a result similar to the TL-PAS for revascularization not related to MI (2). Although the number of strokes and serious bleeding events was small, there was no suggestion of an increased risk in these other important clinical endpoints with prolonged clopidogrel.
The lack of a consistent effect on serious bleeding with prolonged clopidogrel treatment may reflect our definition and the exclusion of patients with this event in the first year after PCI, which likely selected patients with a lower risk of bleeding in the 1- to 4-year time frame of this study. This emulates the clinical situation, as most physicians would not consider prolonging clopidogrel treatment in patients with serious bleeding events in the first year after PCI. Our definition of bleeding based on major bleeding defined by ICD-9 codes included intracranial and intraocular hemorrhage and is less likely to identify mild or moderate bleeding events. These events are similar to the more severe bleeding categories used in the DAPT study that were also uncommon and not increased with prolonged clopidogrel use over a similar time frame (3). Other studies suggest that patients with DM may benefit from more intensive antiplatelet therapy without an increase in bleeding. In the TRITON-TIMI 38 (Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction) study of prasugrel versus clopidogrel in acute coronary syndromes, an increase in bleeding occurred with prasugrel in patients without DM but not those with DM (25). However, in our analysis, we cannot exclude an increased risk of minor or moderate bleeding with prolonged clopidogrel treatment.
STUDY LIMITATIONS
The results of this analysis are derived from several administrative databases and could be affected by unknown confounders. Patients in this large analysis were not randomized to prolonged clopidogrel or placebo treatment, and therefore we may not have captured unknown factors influencing extended clopidogrel use beyond 12 months and the clinical outcomes. However, both multivariable and propensity-adjusted analyses had HRs consistent with the univariate analyses, suggesting no major effects from known confounders. This study did not include the use of second-generation DES and the more potent antiplatelet agents as these were introduced after the time frame of this study.
CONCLUSIONS
The present study demonstrates that for DM patients who have not experienced an adverse event within the first 12 months after the PCI, extending clopidogrel treatment >12 months after PCI is associated with a reduced risk of very late death or MI only in those receiving DES. There was no such benefit for prolonged clopidogrel treatment in DM patients receiving BMS or patients without DM treated with either stent type. Large randomized trials of first- and second-generation DES should examine outcomes by subgroups of DM to further assess whether this could be a useful indication for prolonging clopidogrel therapy after DES.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS
Continuation of clopidogrel therapy beyond 12 months should be considered for patients with DM undergoing PCI with DES.
TRANSLATIONAL OUTLOOK
Future randomized trials should include sufficiently powered, pre-specified subgroup analyses to determine specifically whether patients with DM gain greater benefit from extended adjunctive antiplatelet therapy after PCI than nondiabetic patients and explore the potential pathophysiological mechanisms underlying this difference.
Acknowledgments
This research was supported by Award Number I01CX000440 from the Clinical Science Research and Development Service of the VA Office of Research and Development. Dr. Kinlay has received research grants from Medtronic and The Medicines Company.
ABBREVIAT IONS AND ACRONYMS
- BMS
bare-metal stent(s)
- CABG
coronary artery bypass grafting
- CI
confidence interval
- DAPT
dual antiplatelet therapy
- DES
drug-eluting stent(s)
- DM
diabetes mellitus
- HR
hazard ratio
- ICD-9
International Classification of Diseases-Ninth Revision
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- VA
Veterans Affairs
APPENDIX
For supplemental tables, please see the online version of this article.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–41. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Garratt KN, Weaver WD, Jenkins RD, et al. Prasugrel plus aspirin beyond 12 months is associated with improved outcomes after Taxus Liberte paclitaxel-eluting coronary stent placement. Circulation. 2015;131:62–73. doi: 10.1161/CIRCULATIONAHA.114.013570. [DOI] [PubMed] [Google Scholar]
- 3.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–66. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feres F, Costa RA, Bhatt DL. Dual antiplatelet therapy after stent implantation–reply. JAMA. 2014;311:1446–7. doi: 10.1001/jama.2014.2107. [DOI] [PubMed] [Google Scholar]
- 5.Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss after Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505–13. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 6.Kim BK, Hong MK, Shin DH, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation) J Am Coll Cardiol. 2012;60:1340–8. doi: 10.1016/j.jacc.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362:1374–82. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 8.Valgimigli M, Campo G, Monti M, et al. Short-versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–26. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 9.Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: The SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64:2086–97. doi: 10.1016/j.jacc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Gilard M, Barragan P, Noryani AA, et al. Six-month versus 24-month dual antiplatelet therapy after implantation of drug eluting stents in patients non-resistant to aspirin: ITALIC, a randomized multicenter trial. J Am Coll Cardiol. 2015;65:777–86. doi: 10.1016/j.jacc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Colombo A, Chieffo A. Dual antiplatelet therapy after drug-eluting stents—how long to treat? N Engl J Med. 2014;371:2225–6. doi: 10.1056/NEJMe1413297. [DOI] [PubMed] [Google Scholar]
- 12.Abizaid A, Kornowski R, Mintz GS, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998;32:584–9. doi: 10.1016/s0735-1097(98)00286-1. [DOI] [PubMed] [Google Scholar]
- 13.Elezi S, Kastrati A, Pache J, et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998;32:1866–73. doi: 10.1016/s0735-1097(98)00467-7. [DOI] [PubMed] [Google Scholar]
- 14.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 15.Faxon DP, Lawler E, Young M, et al. Prolonged clopidogrel use after bare metal and drug-eluting stent placement: the Veterans Administration drug-eluting stent study. Circ Cardiovasc Interv. 2012;5:372–80. doi: 10.1161/CIRCINTERVENTIONS.111.967257. [DOI] [PubMed] [Google Scholar]
- 16.Jha AK, Perlin JB, Steinman MA, et al. Quality of ambulatory care for women and men in the Veterans Affairs Health Care System. J Gen Intern Med. 2005;20:762–5. doi: 10.1111/j.1525-1497.2005.0160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmariah S, Mauri L, Doros G, et al. Extended duration dual antiplatelet therapy and mortality: a systematic review and meta-analysis. Lancet. 2015;385:792–8. doi: 10.1016/S0140-6736(14)62052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulluck H, Kwok CS, Ryding AD, Loke YK. Safety of short-term dual antiplatelet therapy after drug-eluting stents: An updated meta-analysis with direct and adjusted indirect comparison of randomized control trials. Int J Cardiol. 2015;181:331–9. doi: 10.1016/j.ijcard.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Cassese S, Byrne RA, Ndrepepa G, et al. Prolonged dual antiplatelet therapy after drug-eluting stenting: meta-analysis of randomized trials. Clin Res Cardiol. 2015 Apr 23; doi: 10.1007/s00392-015-0860-1. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Giustino G, Baber U, Sartori S, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015;65:1298–310. doi: 10.1016/j.jacc.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Navarese EP, Andreotti F, Schulze V, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ. 2015;350:h1618. doi: 10.1136/bmj.h1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385:2371–82. doi: 10.1016/S0140-6736(15)60263-X. [DOI] [PubMed] [Google Scholar]
- 23.Palmerini T, Sangiorgi D, Valgimigli M, et al. Short- versus long-term dual antiplatelet therapy after drug-eluting stent implantation: an individual patient data pairwise and network meta-analysis. J Am Coll Cardiol. 2015;65:1092–102. doi: 10.1016/j.jacc.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 24.Yeh RW, Kereiakes DJ, Steg PG, et al. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol. 2015;65:2211–21. doi: 10.1016/j.jacc.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626–36. doi: 10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


