Abstract
We review the substantial progress recently made in understanding the underlying mechanisms controlling breathing and the applicability of these findings to selected human diseases. Emphasis is placed on the sites of central respiratory rhythm and pattern generation as well as newly described functions of the carotid chemoreceptors and the integrative nature of the central chemoreceptors and the interaction between peripheral and central chemoreception. Recent findings supporting critical contributions from cortical central command and muscle afferent feedback to exercise hyperpnea are also reviewed. These basic principles and the evidence supporting chemoreceptor and ventilatory control system plasticity during and following constant and intermittent hypoxemia and stagnant hypoxia are applied: a) to the pathogenesis, consequences and treatment of obstructive sleep apnea; and b) to exercise hyperpnea and its control and limitations with aging, COPD and CHF.
In healthy humans ventilation is tightly controlled by a system which is concerned with both the precise constancy of alveolar and arterial blood gases and acid-base status as well as with minimizing the work and metabolic cost of a breath. Breathing must remain largely an involuntary act of which we are not made aware. To this end a three component system is required consisting of a central medullary rhythm/pattern generator and integrator, extensive sensory inputs to the central integrator and finally the precise synchronous distribution of motor output to respiratory musculature of the upper airway as well as the chest and abdominal walls. Our understanding of how this is all accomplished with such high precision and efficiency in the healthy human has made significant strides over the past two decades. In this brief review we summarize a few of these accomplishments in the basic science of ventilatory control and how these findings have impacted our understanding and treatment of selected clinical problems.
Central rhythm/integration
The advent of the in vitro neonatal rodent brainstem preparation has allowed for precise identification of specific medullary sites for separate but coupled rhythm generation or “oscillators”. These neurons reside in the pre-Bötzinger complex for inspiration and in the parafacial respiratory group (pFRG) for (active) expiration [41]. Of the several models proposed for producing respiratory rhythm the most promising appears to be a hybrid model which combines emergent properties of networks of synaptic connections and intrinsic membrane properties of individual neurons together with independent pacemaker– type neurons [118, 119]. In order for the underlying respiratory rhythm to generate a physiologic breathing pattern requires a highly complex coordinated process wherein the premotor and motor respiratory neuronal activities influence the timing and amplitude of a broad array of respiratory muscles including those controlling upper airway resistance as well as the respiratory pump. Research into how abnormalities or mutations of the medullary neuronal networks responsible for rhythm and pattern generation may impact human disease is in its infancy, although abnormal breathing patterns often with CO2 retention in waking and especially in sleep have been documented in neurodegenerative diseases such as Parkinson’s, ALS post-polio syndrome with bulbar involvement and multiple system atrophy and linked to deficits in neurons in the pre-Bötzinger complex, pontine raphe and adjacent areas [2, 99, 114]. Furthermore the pre-Bötzinger complex has been identified as a major site of action mediating the markedly depressive effects of opiate agonists on respiratory rhythm and the reversal of this depressive affect via μ–opioid antagonists [75, 85].
Chemoreception
This past decade has provided the most significant advances in understanding peripheral and central chemoreceptor function since Nobel laureate Heymans discovery of carotid chemoreceptor function in the 1930s [57] and Mitchell and Loeschke’s identification of medullary chemosensitive areas in the 1960s [84]. A summary of key developments most relevant to human pathophysiology are as follows.
Carotid body chemoreception
(see Fig 1). We now know that hypoxia triggers sensory input from the carotid body by inhibiting O2 sensitive potassium channels in the glomus cells of the carotid body acting through several mechanisms, including release of gaseous transmitters (NO, CO, H2S), AMP activated protein kinases and/or reactive oxygen species [102, 108]. Basic knowledge of these mechanisms will prove invaluable in the pursuit of pharmacological approaches to inhibiting or stimulating carotid chemoreceptor function in some chronic diseases (also see “Plasticity” section below). It is also now clear that carotid bodies are polymodal receptors responsive to several circulating stimuli beyond just O2, CO2 and H+ such as K+, norepinephrine, temperature, osmolarity as well as glucose and insulin. Further, reductions in carotid body blood flow (in addition to changes in PaO2) also provide powerful carotid body stimulation and remodeling over time [30, 74]. On the effector end, in addition to ventilation, the carotid bodies are now well established as key mediators of sympathetic vasoconstrictor outflow and this mediation occurs through medullary pathways that operate independently of the respiratory rhythm generating network [51].
Figure 1.
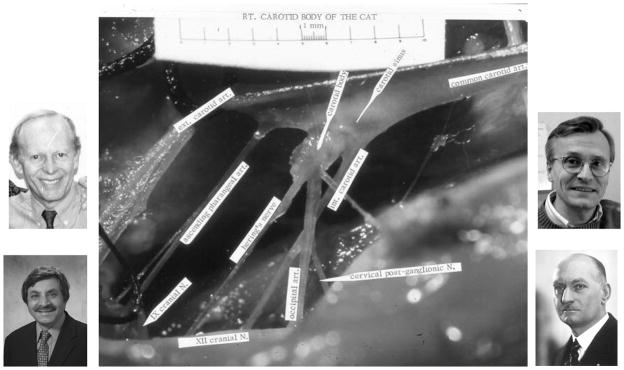
Carotid chemoreceptor in the cat. Heart is to the right and brain to the left, with the carotid chemoreceptor located at the bifurcation of the common carotid artery. Note the sensory nerve from the chemoreceptor, designated here as Hering’s nerve. Photos here are of Nobel laureate Corneille Heymans on the right who first described the function of the carotid chemoreceptor in the 1930s and on the left Gerald Bisgard (top) and Nanduri Prabhakar (bottom) who contributed importantly to our understanding of carotid chemoreceptor O2 sensing mechanisms and its plasticity over the past two decades.
Peripheral/central chemoreceptor interdependence and central chemoreceptors as a site of convergence
Several hydrogen ion sensitive sites in the medulla and midbrain have been identified that stimulate breathing [52, 88]. However the parafacial retrotrapezoid nucleus (pFRG/RTN) characterized by glutamatergic interneurons that strongly express Phox2b are likely to be the major site of central CO2 chemoresponsiveness. Phox2b has also been identified as a key gene participating in early embryonic development of the autonomic nervous system [122]. Moreover, a Phox2b mutation has been identified as a predisposing genotype underlying congenital central hypoventilation syndrome (CCHS), wherein severe hypoventilation and apnea routinely attend administration of sedatives and anesthetics or with sleep onset [136]. The RTN chemo sensors also appear to serve as important sites of integration of several stimuli, as these neurons are significantly modulated by inputs from vagally mediated pulmonary stretch receptors and from the hypothalamus[122]. Recent evidence (using c–FOS immunoreactivity) also shows these RTN Phox2b neurons to be activated by acute exercise in the rodent [9] – indirectly suggesting potential participation of the RTN chemosensitive neurons in the “central command” stimulus to exercise hyperpnea (also see Exercise Hyperpnea section below).
The Phox2b neurons are part of an uninterrupted chain of neurons in a circuit that includes the carotid bodies and their afferents as well as the nucleus of the tractus solitarius projections to the RTN [122]. The functional consequences of this linkage are that stimulation of the peripheral chemoreceptors enhances the slope of the central CO2 ventilatory response; and conversely, inhibition of the carotid bodies reduces the slope of the central CO2 response [13]1 (see Fig 2:A). Accordingly, when carotid bodies are bilaterally denervated (CBX) in several species – including humans – not only is the hypoxic ventilatory response eliminated (as expected) but in addition the central hyperoxic CO2 response is also markedly depressed [19, 113]. These findings are clearly inconsistent with the common presumption that hyperoxic CO2 rebreathe tests selectively test central chemoresponsiveness, per se. [31, 109]. Even during normoxic i.e. air breathing conditions, denervation of the carotid bodies or inhibition of the isolated, perfused intact carotid bodies [13, 43] results in substantial hypoventilation and CO2 retention on the order of +5 to 13 mmHg PaCO2 which persists for days and often weeks following CBX. We believe this substantial contribution of the carotid body to eupneic breathing represents not only a contribution of tonic activity from the carotid bodies to medullary rhythm generating neurons but also the powerful interactive effects of chemoreceptor input on RTN CO2 responsive neurons. The central projections of the carotid chemoreceptors to the hypothalamus and specifically to the paraventricular nucleus (PVN) may also be of significance as shown by the depressed sympathetic and phrenic nerve responses to acute carotid body stimulation achieved by blocking or lesioning neurons in these regions [110].
Figure 2.
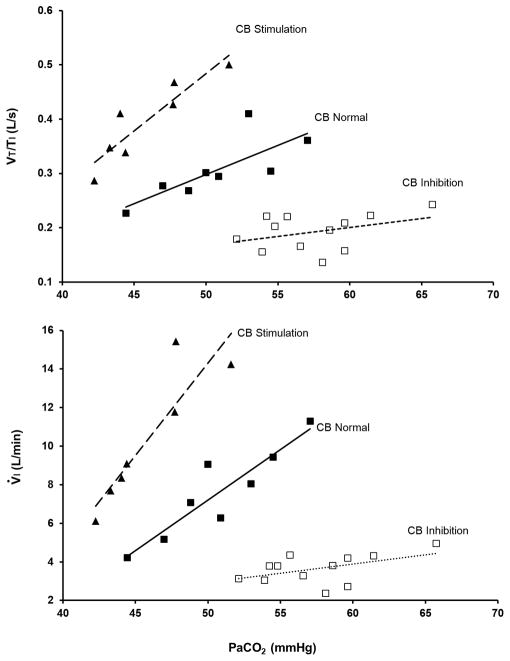
A. Hyperadditive effects of carotid chemoreceptor input on central CO2 response. In a canine, the carotid chemoreceptor is denervated on one side. The remaining carotid chemoreceptor is vascularly isolated from the systemic and cerebral circulation and perfused extracorporeally. The central chemoreceptor response to CO2, by itself, is determined by steady-state inhalation of CO2-enriched air. Animals were studied during quiet wakefulness. Note that, when the isolated CB is inhibited (CB PCO2 = 20 Torr and CB PO2 = 500 Torr), the central CO2 response was reduced to about one-fifth of normal, and when the isolated CB was stimulated (CB PO2 = 40 Torr, CB PCO2 = 40 Torr), the central CO2 response increased an average of twofold. The effects on VT/Ti indicate changes in the “drive” to breathe. Effects on V̇E reflect changes in both fb and VT. (From Blain et al. [13]).
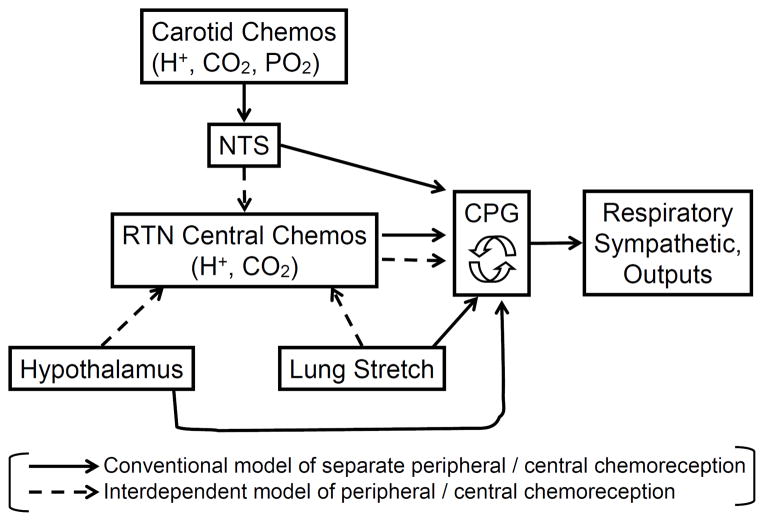
Figure 2. B. Schematic of central-peripheral chemoreceptor interdependence. Shown is the traditional concept supporting only separate chemoreceptor functions (solid lines) and the newer concept of interdependent chemoreceptor function (dashed lines). NTS, nucleus tractus solitarius; RTN, retrotrapezoid nucleus; CPG, central pattern generator. See Fig. 2: A and text for explanation and references to original research.
So we can no longer view either the peripheral or central carotid chemoreceptors as “stand-alone” receptors responding only to changes in their immediate environment (see Fig. 2:B). Further, the carotid bodies are not just reflex O2 sensors – rather they appear to provide a nonspecific tonic afferent input that sensitizes – through multiple CNS pathways – respiratory pattern generating medullary neurons.
Plasticity/after effects of sustained chemoreceptor activation
Substantial evidence has accumulated to demonstrate that the ventilatory control system is highly plastic in response to chemoreceptor stimuli. For example, with hypoxia-induced chemoreceptor stimulation three types of post stimulus after-effects and plasticity have been observed. First, an acute short-term potentiation (STP) occurs as manifested in a slowly declining hyperpnea persistent over several seconds following withdrawal of carotid body simulation [38] – a centrally mediated output that provides a stabilizing effect to breathing pattern following transient ventilatory overshoots – especially in sleep [8]. Secondly, a time–dependent hyperventilation and increased sympathetic nerve activity occurs over hours and days in the face of sustained hypoxic exposure which is mediated primarily by increasing carotid sinus nerve output from the carotid body– a chemosensitization which begins within a few hours of hypoxic exposure [90] and which coincides with increased protein expression and multiplication of O2 sensory glomus cells of the carotid body [133]. Thirdly, upon reversal of the sustained hypoxic stimulus via acute normoxia or even hyperoxia, hyperventilation and the increased sympathetic nerve activity continue, declining only very slowly over several days [27, 53].
In order to explain the persistent hyperventilation and excessive sympathoexcitation following removal of the hypoxic stimulus requires some type of ongoing “central” stimulating effect resulting from the prolonged chemoreceptor input. Accordingly, central sensitization of phrenic nerve activity in response to augmented carotid sinus nerve input has also been shown to occur during prolonged hypoxic exposure [33] and this might be explained, at least in part, by sensitization of central chemoreceptors by heightened carotid body input (see Fig. 2). In addition, acute CNS hypoxia, per se, especially in the presence of normal tonic input from the carotid chemoreceptors causes a tachypneic hyperventilation and increased sympathetic nerve activity in unanesthetized canines and goats [18, 29, 40] – an effect which likely reflects the balance struck between hypoxic-induced inhibition vs. excitation of different groups of medullary and hypothalamic neurons [89]. Sensitivity of the CNS hypoxic sensitive neurons appears to be enhanced after a few days of hypoxic exposure [92].
Plasticity/after-effects of intermittent hypoxia (IH)
Following even very brief periods of intermittent hypoxia interspersed with normoxia, hyperventilation and increased sympathetic activity are sustained over an hour or more i.e. so called long term facilitation [83]. Several mechanisms appear to contribute to the sustained activity following removal of the chemoreceptor stimulus. First, with brief intermittent hypoxia, carotid sinus nerve activity stayed elevated upon return to normoxia; no morphologic changes at the level of the carotid body were apparent. Increased reactive oxygen species and inflammatory cytokines have been implicated in the sustained carotid body sensitization [104]. Increased carotid body AT1 receptors have also been shown to result from prolonged intermittent hypoxia and in animal models of CHF [80]. In turn, the carotid body sensitization in CHF models has been attributed to reduced cardiac output and reduced carotid chemoreceptor blood flow (i.e. “stagnant” hypoxia) [30]. Secondly, central adaptive responses also occur following intermittent hypoxia as seen in the persistent elevation of tonic hyperactivity of neurons of the level of the paraventricular nucleus (PVN)[115] and the RVLM [116]. This after– effect phenomena in the CNS likely contributes significantly to the daytime elevation of sympathetic activity and the hypertension observed during the daytime in patients with OSA and nocturnal intermittent hypoxia [28].
Given these recent understandings of the mechanisms underlying these types of enhanced chemosensitivity and their after-effects on ventilation and sympathetic activity further studies have used pharmacologic means to attenuate this plasticity. Thus, anti-inflammatory medications [64], blockade of increased ROS [107] prevention of up-regulation of angiotensin receptors [42] will all attenuate IH or low blood flow effects on chemosensitivity. Further, increasing cardiac output via habitual physical training will also attenuate the increased chemoreceptor sensitivity in CHF animal models [77]. These approaches all offer as yet untried treatments for chemo-hypersensitivity and its sequelae attending OSA and autonomic imbalances in humans.
Finally, the aftereffects or long-term facilitation of both phrenic and hypoglossal nerve activity elicited by even a few sessions of intermittent hypoxia (for example 5 minutes of normoxia followed by 5 minutes of hypoxia for three days/week for 10 weeks) elicited increased serotonin, immunoreactive BDNF and endothelial growth factor at the level of the phrenic motor neurons) [20]. This type of moderate, brief IH also upregulated growth and trophic factors in non-respiratory motor neurons, suggesting that this type of adaptation to IH represents a general feature of motor systems [20]. It is important to note that this moderate, short-lived type of IH – unlike the persistent cyclical and long-lived nature of the IH attending severe sleep apnea – probably has little if any persistent daytime effects on chemosensitivity or negative consequences for the cardiovascular system. Accordingly, some investigators are recommending IH in promoting synaptic plasticity and spontaneous ventilation following selected types of spinal cord injury [126].
Exercise hyperpnea in health and disease
In healthy humans of all ages the ventilatory response to exercise of up to some 10 to 20 fold greater than resting levels is achieved with remarkable precision and efficiency in terms of both CO2, O2 and pH regulation of arterial blood and economy of effort on the part of the respiratory muscles. The key primary drivers of this hyperpnea which is so tightly and mysteriously linked to respiratory CO2 exchange has been narrowed to a central command, feedforward stimulus with parallel recruitment of both locomotor and respiratory muscles and a feedback stimulus involving thinly myelinated afferents from contracting locomotor muscles [44]. Only recently however have new insights been gained into these mechanisms in humans, with implications for the regulation of exercise hyperpnea in health, COPD and CHF.
Central command
Several lines of evidence in the past decade have demonstrated the importance of feedforward central command to exercise hyperpnea in the human as originally hypothesized by Krogh and Lindhard one century ago [73], based upon their observation of anticipatory hyperventilation prior to exercise and the immediate increase in ventilation at exercise onset. First, hyperventilation and cardiovascular responses were shown to occur in the hypnotized human at rest in response to “suggested exercise” [129, 142]. This observation extended older ones which showed that the increased drive to recruit motor units of locomotor muscles during exercise – as triggered by either weakening of the rhythmically contracting muscles via partial curarization [46] or epidural lidocaine [5, 59] or inhibiting central motor command via tendon vibration [49] – was accompanied by increased heart rate and ventilatory responses to a given level of exercise.
Where in the CNS does the central command originate? Animal studies using electrical or pharmacological simulation of subthalamic and mesencephalic locomotor regions have triggered cardiovascular and ventilatory responses in parallel with locomotion – even in the absence of muscle contraction (i.e. fictive locomotion) [39]. In addition, these regions were shown to be activated in intact exercising animals [65]. However, recent human studies clearly point to the motor cortex and midbrain as key sites of central command. First, electrical or magnetic transcranial stimulation [47], deep brain stimulation [50] and stimulation of the primary cortex [103] all elicited diaphragmatic contractions. Secondly, PET imaging in the “suggested” exercise paradigm mentioned above revealed increased blood flow to the motor control regions of the cortex and cerebellum [128, 141]. Most recently, the use of deep brain stimulating electrodes with recording of field potentials in human neurosurgical patients has been used to specifically identify the periaqueductal gray (PAG) and the subthalamic nucleus (STN) has major sites of central command of cardiorespiratory responses to stress [10, 11, 128]. The PAG receives inputs from prefrontal cortex, hypothalamus and nociceptive pathways and has outputs to the brainstem medullary cardiorespiratory control areas. Simulation of muscle afferent inputs in humans also elicited excitation of PAG neuronal activity [11]. Paterson et al. [100] propose that the PAG area is a key ”command center” of functional connectivity to higher centers and to the STN as well as receiving sensory input from the periphery. These findings have also promoted the concept that the essential nature of the control system for exercise hyperpnea resides in the central command centers. However, as summarized below this regulation also appears to require feedback.
Muscle afferent feedback
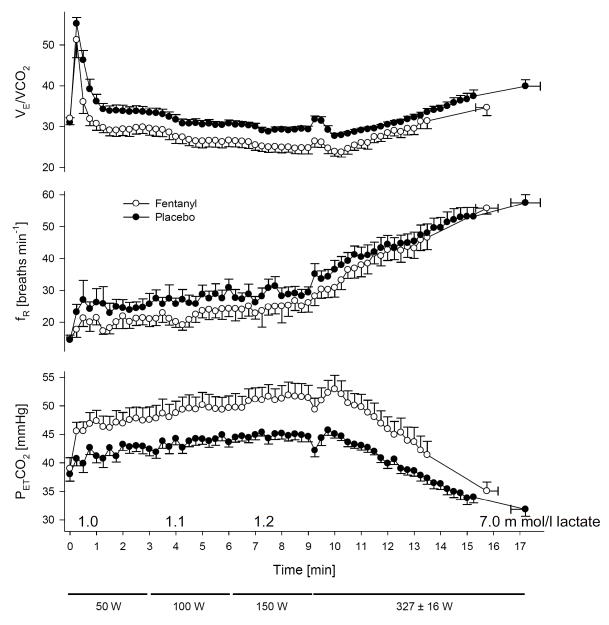
When studied in isolation using direct stimulation of muscle, substantial evidence exists for thinly myelinated afferents – responding to the mechanical distortion of muscle contraction and/or metabolite accumulation. Their sensory pathway ascends via the dorsal horn of the spinal cord to the nucleus of the solitary tract, to cardiorespiratory neurons of the ventral lateral medulla – having a major effect on the cardiorespiratory response to muscle contraction. What is in doubt is whether these afferents play a significant role in exercise hyperpnea in the normally exercising human i.e. when central command and other mechanisms sensitive to respiratory CO2 exchange are also operative. A straight forward approach to determining whether this feedback mechanism is ”essential” to the normal hyperpnea is to block it during steady-state, rhythmic exercise i.e. when all potential competing stimuli are present. This has been accomplished several times in humans with epidural lidocaine injection – all with negative evidence i.e. showing no effect or even an increase in the ventilatory or heart rate response to exercise in the presence of epidural blockade with only a small reduction in blood pressure response [5, 44, 60]. However, this approach has been shown to also block efferents as well as afferents causing limb muscle weakness. Thus, as with the curare experiments (see above) this intervention would likely elicit a compensatory response from central command to recruit more motor units in order to maintain force output with corresponding increases in cardiorespiratory responses. Another approach to block these afferents but without affecting the efferent pathway is to take advantage of their sensitivity to μ-opioids [58]. Accordingly, we used intrathecal administration of Fentanyl at the lumbar level as a partial blockade of muscle afferents and demonstrated that this drug did not influence leg strength, nor did it have cardioventilatory effects at rest breathing room air or CO2 or during arm exercise [3]. However, this blockade did cause substantial hypoventilation and CO2 retention as well as significant reductions in blood pressure and heart rate in healthy subjects during rhythmic leg cycling exercise (see Fig. 3:A). Similar cardiorespiratory effects of fentanyl were also observed in constant load and time trial cycling exercise [4, 6]. We caution that these data do not mean that feedback chemoreception – secondary to sustained CO2 retention of 4–8 mmHg PaCO2 observed with afferent blockade – is ineffective. To the contrary, when the ventilatory equivalent of the heightened chemoreceptor activity secondary to the Fentanyl-induced CO2 retention was accounted for it was estimated that the total effect of the Fentanyl block approached 40 to 50% of the total hyperpnea during mild and moderate steady state exercise [26].
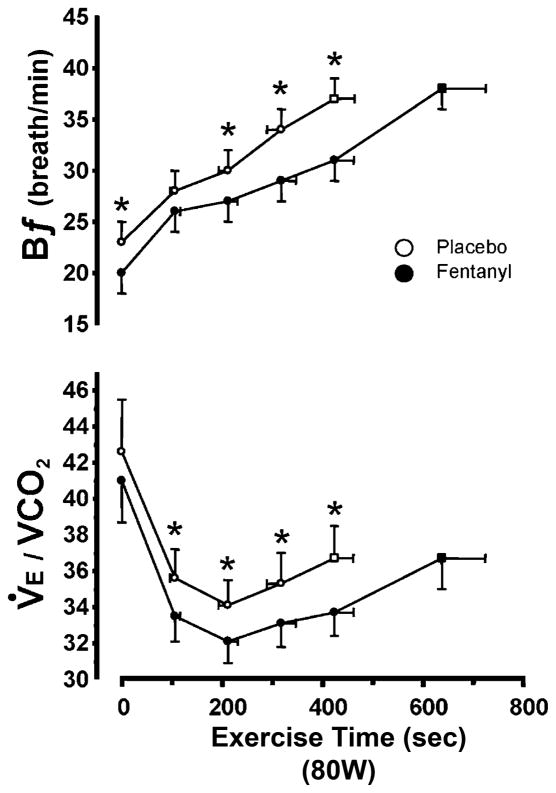
Figure 3.
Reduced steady-state ventilation (V̇E/V̇CO2) and breathing frequency (fR), and the resultant CO2 retention, resulting from type III–IV muscle afferent blockade via intrathecal fentanyl in healthy humans at mild to heavy exercise intensities. Fentanyl had no effect on mean SaO2 except at the 327 W work rate where SaO2 was 97.7% in placebo and 95% with fentanyl. Note the persistence of the hypoventilatory response in the presence of type III–IV afferent blockade – especially during mild and moderate intensity exercise – despite the presence of increased CO2-induced chemoreceptor stimulation. Plasma lactate levels were within 0.5 mmol l−1 of resting values (0.9 ± 0.1 mmol l−1) during 50–150 W exercise and rose to 7-fold > rest during exercise at 325 W in both the placebo and fentanyl trials. Data from Amann et al. [3].
It is especially surprising that muscle afferent blockade affected the cardioventilatory responses even under conditions of mild to moderate exercise intensities where O2 supply to contracting muscle met O2 demand. Such findings are consistent with newer concepts which point to muscle “metaboreceptor” activation in response to venous distention [54] – a mechanism which is especially appealing because the proposed stimulus i.e. increased muscle blood flow, is a major determinant of respiratory CO2 exchange and by regulating breathing is participating in its own control. Finally we need to emphasize that we cannot distinguish whether this substantial contribution of muscle afferents to the cardiorespiratory response found with opioid agonist infusion is secondary to the blockade of the supraspinal pathway from the dorsal horn via the NTS to the medullary rhythm generator neurons and/or whether we have interfered with the interactive effects of ascending afferents on the integrative function of the cortical “central command” centers (see section above). What seems clear from the blockade data (see Fig 3) is that the concept of a purely central, adaptive feedforward control of the cardioventilatory response to exercise is not tenable. Rather, muscle afferent feedback provides critical information deciding both the cardiorespiratory as well as locomotor muscle effort [4, 6, 25].
Healthy aging
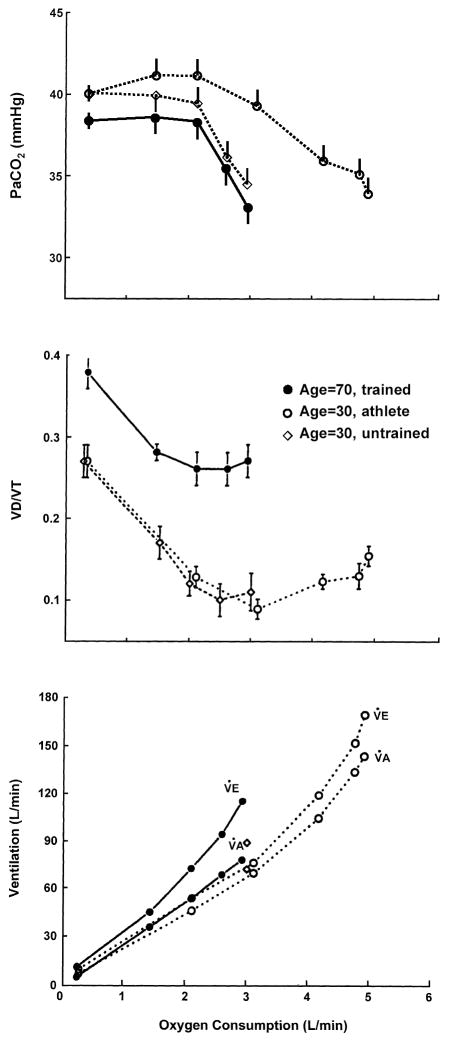
The major change affecting exercise hyperpnea and its limitations with healthy aging are the marked reductions in lung elastic recoil leading to airway narrowing/closure at high lung volumes, a reduced maximum expiratory flow : volume loop, maldistribution of ventilation and increased dead space ventilation [70, 71, 81]. These changes have no discernable effect on resting eupneic ventilation or arterial PCO2 but during exercise there are two major consequences. First, expiratory flow limitation occurs at a level of hyperpnea which would not elicit these limitations in the younger adult and this in turn will cause hyperinflation, increased work of breathing and dyspnea. Secondly, even though the Vd/VT is increased with age PaCO2 is maintained near resting normocapnic levels throughout moderate exercise intensities because the elderly subject increases total V̇E (and V̇E/V̇CO2) above that in the young, so as to maintain V̇A/V̇CO2 comparable to that in the young adult (see Fig. 4). We do not know exactly how respiratory CO2 exchange is sensed to promote this precise regulation of alveolar ventilation relative to V̇CO2, however these types of evidence confirm the importance of this humoral mechanism at least as a “fine tuner” of the hyperpneic response [26, 140]. On the other hand this augmented (total) ventilatory response combined with the age-diminished max flow : volume envelope results in a greater work of breathing in the exercising elder [81].
Figure 4.
Steady-state ventilatory response to treadmill walking in young and elderly healthy subjects. PaCO2 is determined by the relationship of alveolar ventilation (V̇A) to CO2 production (V̇CO2) so that: PaCO2 = V̇CO2/V̇E − V̇d × k where V̇E is total minute ventilation, V̇d is dead space minute ventilation and k is a constant [26]. Note in the elderly that their dead space ventilation (Vd/VT) is greater than in younger subjects at rest and exercise. The older subjects adjust their total V̇E/V̇CO2 higher than in the young during mild to moderate exercise intensities resulting in similar V̇A/V̇CO2 and isocapnic hyperpnea. Data from Johnson et al. [71].
COPD
represents an extreme example of a highly compliant lung and a compromised expiratory flow : volume loop which precipitates expiratory flow limitation with only modest increases in flow rate above resting levels. The ensuing progressive hyperinflation with mild to moderate exercise intensities appears as the major contributor to dyspnea and to exercise limitation [94]. Three approaches to reducing the expiratory flow limitation have resulted in improved exercise performance and decreased limb fatigue. First, inhalation of low density He : O2 expands the maximum flow : volume envelope in most patients thereby reducing the expiratory flow limitation during exercise and also reducing the rate of development of limb fatigue during exercise [7]. Secondly, supplemental inspired O2 reduced chemoreceptor drive and exercise V̇E and improved exercise performance [94]. Thirdly, intrathecal fentanyl was used (see Fig. 5) to reduce muscle afferent input in COPD patients resulting in reduced breathing frequency, which in turn reduced V̇d and total ventilation (but not alveolar ventilation), flow limitation and hyperinflation [45]. So, as in health (see Fig. 3) muscle afferent input in exercising COPD patients contributes significantly to exercise hyperpnea but with a negative – rather than positive – influence on excise performance [26]. Given the markedly diminished aerobic capacity and reduced fatigue resistance [7] of limb locomotor muscles in the sedentary COPD patient [78, 131], specific resistance training of the legs [111] might result in a reduced muscle metaboreflex, and therefore less tachypnea and hyperpneic response to exercise.
Figure 5.
Effects of intrathecal fentanyl blockade on breathing frequency and V̇E/V̇CO2 in COPD patients cycling at 80 W (80% of max). Fentanyl block resulted in a reduced fR and V̇E/V̇CO2 which persisted throughout the exercise. Vd/VT during exercise was also reduced with fentanyl (not shown). Dyspnoeic sensations were reduced and exercise time prolonged as V̇E and expiratory flow limitation were reduced with fentanyl blockade. Data from Gagnon et al. [45].
Congestive Heart Failure
patients commonly respond to exercise with a tachypneic hyperventilation and even occasionally cyclic periodic breathing – the severity of which is prognostic of morbidity and mortality in CHF patients [72, 95]. Deadspace ventilation and V̇E/V̇CO2 are high owing primarily to the increased breathing frequency but so is V̇A/V̇CO2 – thus arterial hypocapnia is common [144]. There are several potential reasons for the hyperventilatory response. First, carotid chemoreceptors are substantially hypersensitized in CHF, owing to the chronic ”stagnant hypoxia” at the level of carotid body created by the low cardiac output, low blood flow and reduced shear stress [30]. This chemo-hypersensitization will also increase control system loop gain (see Sleep Apnea section below) and contribute to the periodic breathing [24]. Secondly, muscle mechanoreceptors are also hypersensitized in CHF in combination with a depressed muscle metaboreceptor sensitivity [106, 120]. Accordingly, intrathecal fentanyl-induced blockade of muscle afferents in human CHF patients resulted in substantial hypoventilation and CO2 retention over a wide range of exercise intensities as compared to age matched controls [98]. Thirdly, high pulmonary vascular pressures are common in CHF, especially during exercise and in the presence of pulmonary edema this would precipitate pulmonary C fiber stimulation and a tachypneic ventilatory response and would also be expected to contribute to an unstable, periodic breathing [95, 96].
These hyperventilatory responses as well as the underlying hypersensitivity of muscle afferents and chemoreceptors in CHF contribute importantly to exercise performance limitation – primarily because of the augmented intrathoracic pressures and increased work of breathing as well as high sympathetic vasoconstrictor outflow effects on limb perfusion. Thus, when pressure support mechanical ventilation was used to reduce respiratory muscle work in CHF patients, ratings of limb discomfort were reduced and exercise performance improved [93]. Further, pressure support elicited substantial increases in limb muscle blood flow and muscle oxygenation [15, 97, 97] in both CHF patients and an animal model [82] due to both an increase in stroke volume and cardiac output in combination with a greater local vasodilation of locomotor muscle vasculature2. Similarly, transient inhibition of hypersensitized carotid chemoreceptors in CHF animal models also reduced locomotor muscle vascular resistance and increased limb blood flow both at rest and during exercise [121].
Chronic exercise training [132] as well as specific respiratory muscle training [16, 66] in CHF animal models and in human patients reduces the hypersensitivity of the carotid chemoreceptors, limb muscle mechanoreceptors and the respiratory muscle metaboreflex. These “desensitizing” effects on multiple feedback regulators result in a reduced work of breathing and reduced sympathetic vasoconstriction, thereby improving O2 transport to contracting locomotor muscle and exercise performance.
Obstructive sleep apnea (OSA) and the ventilatory control system
Significant amounts of sleep apnea and sleep disordered breathing exist in the general population with obesity, male gender, age, and craniofacial structure as major risk factors [28]. Severe cases (> 20–30 apneas/hypopneas (AHI)/HbO2 desaturations per hour) commonly leading to high chemosensitivity, elevated sympathetic vasoconstrictor activity, and endothelial dysfunction – all eliciting both nocturnal and daytime systemic and often pulmonary hypertension [28]. A form of daytime hypoventilation and CO2 retention in the obese is also tightly linked – perhaps via chemoreceptor “resetting” – to carryover effects from nocturnal hypoventilation and CO2 retention and is often effectively eliminated via the use of nasal positive pressure ventilation to correct the nocturnal hypoventilation [105]. In many CHF patients and sojourners to high altitude the ventilatory control system and enhanced chemosensitivity clearly play a major role in the pathogenesis of “central” or mixed (obstructive plus central) type of repetitive apneas (see ref [68] for review). But what role might these control mechanisms play in the more prevalent condition of cyclical obstructive sleep apnea? Certainly the popular view that OSA is a problem of an anatomically compromised upper airway has merit – but accumulating evidence now recognizes that repetitive airway obstructions in sleep are also often a function of other important characteristics of the ventilatory control system [29, 36, 147].
Anatomical : functional links in OSA
First, we know that central respiratory motor output recruits, first the hypoglossal, then (msec later), the phrenic motor neurons serving the upper airway dilators and respiratory pump musculature, respectively [56, 61]. Secondly, the fundamental effects of the loss of “wakefulness” includes both the withdrawal of tonic input to the upper airway dilator muscles thereby increasing airway compliance and collapsibility plus an unmasking of a critical dependence of ventilatory control and its stability on chemoreceptor and mechanoreceptor feedback. Thirdly, in subjects with moderately collapsible airways there is a tight link between CO2–induced central ventilatory instability and airway calibre. So, inducing central output instability by administering brief hypoxic episodes in snoring subjects with mildly collapsible airways precipitated airway closure at the nadir of the oscillating drive [62, 134]; conversely, preventing oscillations in central respiratory motor output via preventing transient hypocapnia also prevented airway obstructions – at least in those subjects with a relatively high chemosensitivity and sensitive apneic threshold [145, 145]. Finally, the passive collapsibility of the upper airway – by itself – in sleeping humans accounts for only a relatively small portion of the variability in apnea : hypopnea index in OSA [63, 146]. Some recent studies of substantial numbers of OSA patients with moderate to severe OSA reveal that more than 80% have a highly collapsible airway – but 30–50% also showed key characteristics of instability including high control system “loop gain”, sensitive arousal thresholds and/or sluggish responsiveness of upper airway dilator muscles to chemoreceptor stimuli [36, 37, 138, 145]. These characteristics are sometimes inherent to the patient, but are also acquired and intensified via the repeated intermittent hypoxemia, transient arousals and obstructions3.
OSA pathogenesis
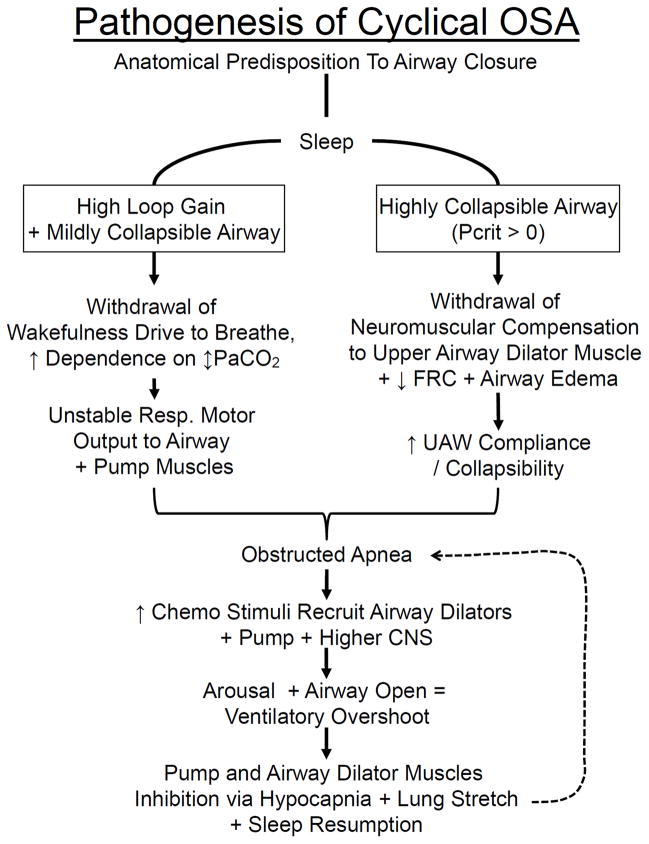
In Fig. 6:A we suggest two overlapping scenarios for the pathogenesis of cyclical OSA, based on the influences of airway collapsibility, neurochemical influences over pharyngeal dilators and respiratory pump musculature and on sleep stage stability. In Fig. 6:B right, a patient with a highly collapsible airway often experiences complete airway collapse when the compensatory tonic input to the upper airways are removed with sleep onset. On the left side of Fig. 5:B a patient with a high chemosensitivity plus a mildly collapsible airway is likely to experience airway obstruction during sleep at the nadir of the oscillating central respiratory motor output. In either case, whether the obstruction is repeated and becomes cyclical will depend upon how the patient’s respiratory control system responds to the obstruction as outlined in Fig. 6:A. The key ingredients to regaining respiratory stability are the ability to recruit airway muscles dilators and to effectively open the airway to restore airflow prior to arousal, because the transient arousal accentuates the ventilatory overshoot and hypocapnia leading to subsequent hypopneas, apneas and obstructions. Accordingly, how the chemoreceptor control system and the airway dilator musculature responds to accumulating CO2 and HbO2 desaturation during the apnea as well as the sensitivity of a patient’s arousal threshold and the effectiveness of dilator muscle recruitment will determine whether initial obstructive events are followed by stable breathing, slowly evolving hypopneas with occasional arousals or repetitive obstructions (94).
Figure 6.
A. Schematic illustrating the interactive effects of airway anatomy with neurochemical control on the magnitude and stability of central respiratory motor output, airway muscle dilator recruitment, and arousability in the pathogenesis of cyclical OSA. Patients with an anatomical predisposition to pharyngeal collapse may experience two types of overlapping scenarios leading to cyclical OSA in sleep. Right: progression initiated by an airway obstruction at sleep onset in a patient with a severely collapsible upper airway; left: progression to airway obstruction (at the nadir of the respiratory cycle) initiated by an unstable central respiratory motor output in a patient with elevated loop gain and a mildly collapsible airway. Bottom: factors that determine the consequences of airway obstruction and accumulating chemoreceptor stimuli on subsequent, postapneic ventilation, airway patency and EEG arousal. These control system characteristics include the responsiveness of both the upper airway and chest wall pump muscles and of central nervous system (CNS) arousability to the rising chemoreceptor stimuli (also see text and Fig. 5:A). UAW, upper airway; FRC, functional residual capacity. Used with permission from Dempsey et al. [29].
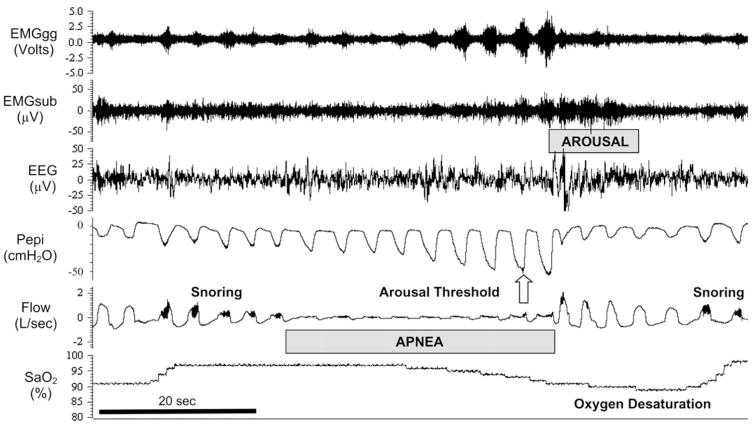
Figure 6. B. Polysomnographic tracing of an obstructed apneic event (between the dotted vertical lines in A) to illustrate the compensatory events occurring during and following the obstruction (apnea/hypopnea index = 56/hour). The cessation and resumption of flow defines the apneic event. Note the progressive increase in inspiratory effort (Pepi) and dilator muscle EMG (EMGgg) during the apnea, the transient arousal coincident with airway opening, and ventilatory overshoot at apnea termination. As the patient returns to sleep, note the gradual reduction in breathing frequency and flow rate, and increased pharyngeal pressure (signifying increased airway resistance) leading to the next obstruction. Evidence of snoring is shown on the flow tracing. Progressive increases in EMGgg activity occurred throughout the obstructive event, although in this instance they were not sufficient to restore flow, which occurred only upon arousal. Pharyngeal pressure serves as a measure of the inspiratory effort made against the obstructed airway, thereby reflecting the magnitude of central respiratory motor output in response to chemoreceptor stimuli accumulated during the obstructed apnea. Arousal threshold is determined by the pharyngeal pressure achieved through respiratory pump muscle contractions during an airway obstruction at the point of EEG arousal. EMGgg, electromyogram of the genioglossus muscle (intramuscular); EMGsub, EMG of the submental muscle (surface); EEG, electroencephalogram (C3-A2); Pepi, pressure at the level of the epiglottis; Flow, airflow measured via nasal mask and pneumotachograph; SaO2, arterial blood oxygen saturation measured via pulse oximetry at the finger. Reprinted with permission of the American Thoracic Society. Copyright © 2013 American Thoracic Society. Eckert DJ and Malhotra A. 2008. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5: 144–153.
Treatment implications
Given the critical contributions of a collapsible airway to all types of OSA it is not surprising that CPAP is a highly effective treatment. However, significant numbers of OSA patients are unable to tolerate CPAP or greatly under utilize it [135]. Three types of alternative treatments have shown promise in significantly reducing AHI in some OSA patients. These include the following:
Use of supplemental O2 to reduce chemoreceptor gain [139, 145] and acetazolamide [37, 67] or preventing hypocapnia via selective increments in FICO2 – to reduce plant gain [145].
Raising the arousal threshold using sedatives to prevent ventilatory overshoot by helping maintain sleep state during an obstructive apneic event until airway dilator muscle recruitment restores patency prior to arousal [12, 14, 35].
Reducing airway collapsibility via recruitment of airway muscle dilators using small increments in PaCO2 via a deadspace rebreathing system [145].
Combining treatments to reduce loop gain or raise arousal threshold together with small reductions in airway collapsibility via moderate weight loss or mandibular advancement [17, 48, 127].
These new approaches have produced mixed results to date with the most consistent success in reducing obstructive AHI achieved when the treatment was tailored to an individual patient’s specific deficiency; for example, lowering loop gain in the patient with high chemosensitivity or raising arousal threshold in those with high arousability. A challenge in using these approaches for treatment purposes is to simplify our ability to recognize specific risk factors in OSA populations so that therapy can be individualized and targeted [29, 36]. Recently, Wellman et al. [137] have proposed a promising screening tool using the routine clinical polysomnogram to characterize these risk factors in individual OSA patients. We also need to continue to explore new agents for reducing loop gain and arousability and especially for effective stimulation of upper airway muscle dilators without invoking confounding side effects on chemoreceptor gain or sleep state continuity or excessive sympathetic activation.
These principles of individualizing therapy for OSA by phenotyping patients have also been recently applied to an exciting, novel treatment for moderate to moderately severe OSA which utilizes hypoglossal nerve stimulation via implanted electrodes during inspiration triggered by an implanted transducer which senses intrathoracic pressure [34, 123]. This therapy – recently receiving FDA approval in the USA – was shown to be safe and highly effective over six month periods in substantially reducing AHI in most of a selected group of CPAP intolerant OSA patients. Importantly, in keeping with the concept of tailoring treatments to individual characteristics, these patients were pre-screened to include only those with a site of upper airway collapse most likely to be prevented via forward movement of the tongue, achieved via hypoglossal nerve stimulation; and b) to exclude those with a significant prevalence of central and mixed apneas [123]. Based on current knowledge of the pathophysiology of OSA, we would predict that the patients included in this latter category would likely have high chemosensitivity and loop gain and might well benefit from a combined therapy of hypoglossal stimulation plus reduced chemoreflex gain via supplemental O2 or a pharmacologic-induced blockade of hyperchemosensitivity.
Carotid body denervation as “treatment” for autonomic imbalances/OSA?
Throughout this review we have emphasized the important contributions of carotid chemoreceptors and their central projections and carotid body hypersensitivity to ventilatory control during exercise and sleep and to excessive sympathetic nerve activity in such diseases as chronic hypertension and heart failure and OSA. Does it follow that bilateral carotid body denervation (CBX) should be considered as a treatment to correct this autonomic imbalance in such diseases as drug-resistant hypertension or CHF or to prevent (some forms) of sleep apnea [91, 101]? There is support for carotid body denervation: a) some older studies of CBX in asthmatic humans showed significant sustained reductions in blood pressure [87]; b) in the rabbit model of CHF, CBX reduced renal sympathetic nerve activity and BP and prevented periodic breathing [79], and in rodent models of spontaneous hypertension CBX caused substantial reductions in systemic blood pressure [1]; c) CBX prevented the development of insulin resistance and hypertension induced via hypercaloric diets [112], substantially increased survival following myocardial infarction in rodents [22] and prevented hypertension induced by chronic intermittent hypoxemia in rats [76]; and d) using an irreversible pharmacologic inhibitor of the enzyme responsible for the gaseous transmitter H2S in the carotid body in a rodent model of severe CHF, almost completely normalized the heightened carotid chemosensitivity as well as the accompanying breathing instability and sympathoexcitatory state [23]. On the other hand there are concerns, including: a) whether selective chemo-denervation can be achieved without including baroreceptor denervation [130]; b) to what extent will long term compensation for CBX normalize CO2 chemosensitivity and eupneic ventilation in humans [19]; c) in the absence of carotid chemoreceptors will patients with airway disease, V̇A/Q maldistribution and high Vd/VT mount sufficient compensatory hyperpnea to prevent chronic hypercapnia and will patients who develop OSA with aging and/or weight gain experience apnea prolongation and more severe hypoxemia and its sequelae [29, 117]? Of course, following CBX, any sojourn to even moderately high altitudes will exacerbate the usual level of arterial hypoxemia. Alternatively, we need to determine if therapies which acutely inhibit carotid chemoreceptors or chronically reduce carotid chemoreceptor hypersensitivity (see above) present effective, safe and especially reversible alternatives and in what subpopulation of patients these approaches are likely to be effective. A strong case may also be made for the well documented sympathoinhibitory effects of habitual exercise training – especially interval-type training – in CHF and hypertension [21, 69, 86, 124].
Summary
The major take home message of our brief review is that recent advances in our basic understanding of ventilatory control – especially those chemoreceptor and extra-chemoreceptor mechanisms controlling breathing and its plasticity during exercise and in sleep – have important implications for understanding the pathophysiology of breathing abnormalities and their consequences in such diseases as COPD, CHF and OSA. A major benefit to these newfound insights is that they are beginning to allow some innovative, meaningful inroads into treatment strategies.
Acknowledgments
Original research findings of the authors reported in this review were supported by NHLBI. We thank Anthony Jacques for his expert assistance with manuscript preparation.
Footnotes
Other views using other preparations with isolated carotid and/or brainstem perfusions also show an interdependence between the chemoreceptors but opinions vary as to whether this interdependence is hypo- or hyperadditive in its effect on ventilation and central respiratory drive [32, 125, 143].
This dual effect i.e. increased CO and limb vascular conductance during pressure support was attributed to: a) a mechanical effect of a reduced intrathoracic pressure on the left ventricle in the highly afterload-dependent CHF patient an effect which is in the opposite direction to the decreased exercise stroke volume observed with positive pressure support in health [55, 82]; and b) a reduced reflex feedback effect from respiratory muscle metaboreceptors.
The tendency toward ventilatory instability depends upon “loop gain”, an engineering term defining the gain of the negative feedback loop which regulates how ventilation responds to transient disturbances in breathing and the accompanying disruption of arterial blood gases. Chemosensitive gain is defined by the slope of the ventilatory response to hypercapnia and hypocapnia i.e. Δ V̇E/Δ PaCO2. Plant gain is determined by the magnitude of the reduction in PaCO2 resulting from a given change in ventilation, (Δ PaCO2/Δ V̇E) i.e. the efficiency with which CO2 is eliminated. These concepts and their effects on ventilatory stability and the apneic threshold may be more readily appreciated when presented in graphical form (see ref [24] and [139]).
Reference List
- 1.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol. 2011;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol. 2008;105:1714–1724. doi: 10.1152/japplphysiol.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol. 2010;299:R314–R324. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badr MS, Skatrud JB, Dempsey JA. Determinants of poststimulus potentiation in humans during NREM sleep. J Appl Physiol. 1992;73:1958–1971. doi: 10.1152/jappl.1992.73.5.1958. [DOI] [PubMed] [Google Scholar]
- 9.Barna BF, Takakura AC, Moreira TS. Acute exercise-induced activation of Phox2b-expressing neurons of the retrotrapezoid nucleus in rats may involve the hypothalamus. Neuroscience. 2014;258:355–363. doi: 10.1016/j.neuroscience.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Basnayake SD, Green AL, Paterson DJ. Mapping the central neurocircuitry that integrates the cardiovascular response to exercise in humans. Exp Physiol. 2012;97:29–38. doi: 10.1113/expphysiol.2011.060848. [DOI] [PubMed] [Google Scholar]
- 11.Basnayake SD, Hyam JA, Pereira EA, Schweder PM, Brittain JS, Aziz TZ, Green AL, Paterson DJ. Identifying cardiovascular neurocircuitry involved in the exercise pressor reflex in humans using functional neurosurgery. J Appl Physiol (1985 ) 2011;110:881–891. doi: 10.1152/japplphysiol.00639.2010. [DOI] [PubMed] [Google Scholar]
- 12.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–454. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 13.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2) J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–45. doi: 10.1164/arrd.1985.131.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Borghi-Silva A, Carrascosa C, Oliveira CC, Barroco AC, Berton DC, Vilaca D, Lira-Filho EB, Ribeiro D, Nery LE, Neder JA. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H2465–H2472. doi: 10.1152/ajpheart.91520.2007. [DOI] [PubMed] [Google Scholar]
- 16.Chiappa GR, Roseguini BT, Vieira PJ, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol. 2008;51:1663–1671. doi: 10.1016/j.jacc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhuri S, Ghabsha A, Sinha P, Kadri M, Narula S, Badr MS. Treatment of central sleep apnea in U.S. veterans. J Clin Sleep Med. 2012;8:555–563. doi: 10.5664/jcsm.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curran AK, Rodman JR, Eastwood PR, Henderson KS, Dempsey JA, Smith CA. Ventilatory responses to specific CNS hypoxia in sleeping dogs. J Appl Physiol. 2000;88:1840–1852. doi: 10.1152/jappl.2000.88.5.1840. [DOI] [PubMed] [Google Scholar]
- 19.Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med. 2007;4:e239. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dale EA, Ben MF, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda ) 2014;29:39–48. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, Lough F, Taylor RS. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12:706–715. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol. 2013;62:2422–2430. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Rio R, Marcus NJ, Schultz HD. Inhibition of hydrogen sulfide restores normal breathing stability and improves autonomic control during experimental heart failure. J Appl Physiol (1985 ) 2013;114:1141–1150. doi: 10.1152/japplphysiol.01503.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 25.Dempsey JA. New perspectives concerning feedback influences on cardiorespiratory control during rhythmic exercise and on exercise performance. J Physiol. 2012;590:4129–4144. doi: 10.1113/jphysiol.2012.233908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempsey JA, Blain GM, Amann M. Are type III–IV muscle afferents required for a normal steady-state exercise hyperpnoea in humans? J Physiol. 2014;592:463–474. doi: 10.1113/jphysiol.2013.261925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dempsey JA, Powell FL, Bisgard GE, Blain GM, Poulin MJ, Smith CA. Role of Chemoreception in Cardio-Respiratory Acclimatization to and Deacclimatization from Hypoxia. J Appl Physiol. 1985;2013 doi: 10.1152/japplphysiol.01126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dempsey JA, Xie A, Patz DS, Wang D. Physiology in medicine: obstructive sleep apnea pathogenesis and treatment--considerations beyond airway anatomy. J Appl Physiol (1985 ) 2014;116:3–12. doi: 10.1152/japplphysiol.01054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding Y, Li YL, Schultz HD. Role of blood flow in carotid body chemoreflex function in heart failure. J Physiol. 2011;589:245–258. doi: 10.1113/jphysiol.2010.200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffin J. The role of the central chemoreceptors: a modeling perspective. Respir Physiol Neurobiol. 2010;173:230–243. doi: 10.1016/j.resp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Duffin J, Mateika JH. Cross-Talk opposing view: peripheral and central chemoreflexes have additive effects on ventilation in humans. J Physiol. 2013;591:4351–4353. doi: 10.1113/jphysiol.2013.256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dwinell MR, Powell FL. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J Appl Physiol. 1999;87:817–823. doi: 10.1152/jappl.1999.87.2.817. [DOI] [PubMed] [Google Scholar]
- 34.Eastwood PR, Barnes M, Walsh JH, Maddison KJ, Hee G, Schwartz AR, Smith PL, Malhotra A, McEvoy RD, Wheatley JR, O’Donoghue FJ, Rochford PD, Churchward T, Campbell MC, Palme CE, Robinson S, Goding GS, Eckert DJ, Jordan AS, Catcheside PG, Tyler L, Antic NA, Worsnop CJ, Kezirian EJ, Hillman DR. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34:1479–1486. doi: 10.5665/sleep.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, White DP, Malhotra A. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, Malhotra A, Wellman A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eldridge FL. Central neural respiratory stimulatory effect of active respiration. J Appl Physiol. 1974;37:723–735. doi: 10.1152/jappl.1974.37.5.723. [DOI] [PubMed] [Google Scholar]
- 39.Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981;211:844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- 40.Engwall MJ, Smith CA, Dempsey JA, Bisgard GE. Ventilatory afterdischarge and central respiratory drive interactions in the awake goat. J Appl Physiol. 1994;76:416–423. doi: 10.1152/jappl.1994.76.1.416. [DOI] [PubMed] [Google Scholar]
- 41.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol. 2002;92:627–633. doi: 10.1152/japplphysiol.00152.2001. [DOI] [PubMed] [Google Scholar]
- 43.Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol (1985 ) 2003;94:784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- 44.Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Compr Physiol. 2012;2:743–777. doi: 10.1002/cphy.c100045. [DOI] [PubMed] [Google Scholar]
- 45.Gagnon P, Bussieres JS, Ribeiro F, Gagnon SL, Saey D, Gagne N, Provencher S, Maltais F. Influences of Spinal Anesthesia on Exercise Tolerance in Patients with COPD. Am J Respir Crit Care Med. 2012;186:606–615. doi: 10.1164/rccm.201203-0404OC. [DOI] [PubMed] [Google Scholar]
- 46.Galbo H, Kjaer M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol. 1987;389:557–568. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandevia SC, Rothwell JC. Activation of the human diaphragm from the motor cortex. J Physiol. 1987;384:109–118. doi: 10.1113/jphysiol.1987.sp016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilmartin G, McGeehan B, Vigneault K, Daly RW, Manento M, Weiss JW, Thomas RJ. Treatment of positive airway pressure treatment-associated respiratory instability with enhanced expiratory rebreathing space (EERS) J Clin Sleep Med. 2010;6:529–538. [PMC free article] [PubMed] [Google Scholar]
- 49.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green AL, Wang S, Owen SL, Xie K, Liu X, Paterson DJ, Stein JF, Bain PG, Aziz TZ. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport. 2005;16:1741–1745. doi: 10.1097/01.wnr.0000183904.15773.47. [DOI] [PubMed] [Google Scholar]
- 51.Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol. 2000;121:147–162. doi: 10.1016/s0034-5687(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 52.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003;546:921–929. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haouzi P, Chenuel B, Huszczuk A. Sensing vascular distension in skeletal muscle by slow conducting afferent fibers: neurophysiological basis and implication for respiratory control. J Appl Physiol. 2004;96:407–418. doi: 10.1152/japplphysiol.00597.2003. [DOI] [PubMed] [Google Scholar]
- 55.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 56.Haxhiu MA, van Lunteren E, Mitra J, Cherniack NS. Comparison of the response of diaphragm and upper airway dilating muscle activity in sleeping cats. Respir Physiol. 1987;70:183–193. doi: 10.1016/0034-5687(87)90049-1. [DOI] [PubMed] [Google Scholar]
- 57.Heymans JF, Bouckaert JJ. Les chemorecepteurs du sinus carotidien. Ergeb Physiol. 1939;41:28–55. [Google Scholar]
- 58.Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol. 1990;68:2466–2472. doi: 10.1152/jappl.1990.68.6.2466. [DOI] [PubMed] [Google Scholar]
- 59.Hornbein TF, Sorensen SC. Ventilatory response to hypoxia and hypercapnia in cats living at high altitude. J Appl Physiol. 1969;27:834–836. doi: 10.1152/jappl.1969.27.6.834. [DOI] [PubMed] [Google Scholar]
- 60.Hornbein TF, Sorensen SC, Parks CR. Role of muscle spindles in lower extremities in breathing during bicycle exercise. J Appl Physiol. 1969;27:476–479. doi: 10.1152/jappl.1969.27.4.476. [DOI] [PubMed] [Google Scholar]
- 61.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO(2) in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 62.Hudgel DW, Chapman KR, Faulks C, Hendricks C. Changes in inspiratory muscle electrical activity and upper airway resistance during periodic breathing induced by hypoxia during sleep. Am Rev Respir Dis. 1987;135:899–906. doi: 10.1164/arrd.1987.135.4.899. [DOI] [PubMed] [Google Scholar]
- 63.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 64.Iturriaga R, Moya EA, Del RR. Carotid body potentiation induced by intermittent hypoxia: implications for cardiorespiratory changes induced by sleep apnoea. Clin Exp Pharmacol Physiol. 2009;36:1197–1204. doi: 10.1111/j.1440-1681.2009.05213.x. [DOI] [PubMed] [Google Scholar]
- 65.Iwamoto GA, Wappel SM, Fox GM, Buetow KA, Waldrop TG. Identification of diencephalic and brainstem cardiorespiratory areas activated during exercise. Brain Res. 1996;726:109–122. [PubMed] [Google Scholar]
- 66.Jaenisch RB, Hentschke VS, Quagliotto E, Cavinato PR, Schmeing LA, Xavier LL, Dal LP. Respiratory muscle training improves hemodynamics, autonomic function, baroreceptor sensitivity, and respiratory mechanics in rats with heart failure. J Appl Physiol. 2011;111:1664–1670. doi: 10.1152/japplphysiol.01245.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 68.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3:141–163. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 69.Johnson BD, Joyner MJ. Carotid body denervation: too soon to get breathless about heart failure? J Am Coll Cardiol. 2013;62:2431–2432. doi: 10.1016/j.jacc.2013.08.718. [DOI] [PubMed] [Google Scholar]
- 70.Johnson BD, Reddan WG, Pegelow DF, Seow KC, Dempsey JA. Flow limitation and regulation of functional residual capacity during exercise in a physically active aging population. Am Rev Respir Dis. 1991;143:960–967. doi: 10.1164/ajrccm/143.5_Pt_1.960. [DOI] [PubMed] [Google Scholar]
- 71.Johnson BD, Reddan WG, Seow KC, Dempsey JA. Mechanical constraints on exercise hyperpnea in a fit aging population. Am Rev Respir Dis. 1991;143:968–977. doi: 10.1164/ajrccm/143.5_Pt_1.968. [DOI] [PubMed] [Google Scholar]
- 72.Johnson RL., Jr Gas exchange efficiency in congestive heart failure. Circulation. 2000;101:2774–2776. doi: 10.1161/01.cir.101.24.2774. [DOI] [PubMed] [Google Scholar]
- 73.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar P, Bin-Jaliah I. Adequate stimuli of the carotid body: more than an oxygen sensor? Respir Physiol Neurobiol. 2007;157:12–21. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Lalley PM. The preBotzinger Complex is not essential for the respiratory depression following systemic administration of opioid analgesics. J Physiol. 2014 doi: 10.1113/jphysiol.2013.261974. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- 77.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 78.Maltais F, Simard AA, Simard C, Jobin J, Desgagnes P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med. 1996;153:288–293. doi: 10.1164/ajrccm.153.1.8542131. [DOI] [PubMed] [Google Scholar]
- 79.Marcus NJ, Del RR, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2014;592:391–408. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller JD, Dempsey JA. Lung Development and Regeneration. Massaro D. Marcel Dekker; 2004. Pulmonary Limitations to Exercise Performance: The Effects of Healthy Ageing and COPD; pp. 525–571. [Google Scholar]
- 82.Miller JD, Smith CA, Hemauer SJ, Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in health and chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H580–H592. doi: 10.1152/ajpheart.00211.2006. [DOI] [PubMed] [Google Scholar]
- 83.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985 ) 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell RA, LOESCHCKE HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol. 1963;18:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- 85.Montandon G. The preBotzinger Complex is essential for the respiratory depression following systemic administration of opioid analgesics. J Physiol. 2014 doi: 10.1113/jphysiol.2013.261974. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mousa TM, Liu D, Cornish KG, Zucker IH. Exercise training enhances baroreflex sensitivity by an angiotensin II-dependent mechanism in chronic heart failure. J Appl Physiol (1985 ) 2008;104:616–624. doi: 10.1152/japplphysiol.00601.2007. [DOI] [PubMed] [Google Scholar]
- 87.Nakayama K. Surgical removal of the carotid body for bronchial asthma. Dis Chest. 1961;40:595–604. doi: 10.1378/chest.40.6.595. [DOI] [PubMed] [Google Scholar]
- 88.Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol. 2012;2:221–254. doi: 10.1002/cphy.c100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol (1985 ) 2004;96:367–374. doi: 10.1152/japplphysiol.00831.2003. [DOI] [PubMed] [Google Scholar]
- 90.Nielsen AM, Bisgard GE, Vidruk EH. Carotid chemoreceptor activity during acute and sustained hypoxia in goats. J Appl Physiol. 1988;65:1796–1802. doi: 10.1152/jappl.1988.65.4.1796. [DOI] [PubMed] [Google Scholar]
- 91.Niewinski P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W, Hart EC, Paton JF, Ponikowski P. Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol. 2013;168:2506–2509. doi: 10.1016/j.ijcard.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 92.Nolan PC, Dillon GH, Waldrop TG. Central hypoxic chemoreceptors in the ventrolateral medulla and caudal hypothalamus. Adv Exp Med Biol. 1995;393:261–266. doi: 10.1007/978-1-4615-1933-1_49. [DOI] [PubMed] [Google Scholar]
- 93.O’Donnell DE, D’Arsigny C, Raj S, Abdollah H, Webb KA. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med. 1999;160:1804–1811. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- 94.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 95.Olson LJ, Arruda-Olson AM, Somers VK, Scott CG, Johnson BD. Exercise oscillatory ventilation: instability of breathing control associated with advanced heart failure. Chest. 2008;133:474–481. doi: 10.1378/chest.07-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olson TP, Frantz RP, Snyder EM, O’Malley KA, Beck KC, Johnson BD. Effects of acute changes in pulmonary wedge pressure on periodic breathing at rest in heart failure patients. Am Heart J. 2007;153:104–107. doi: 10.1016/j.ahj.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol. 2010;588:2487–2501. doi: 10.1113/jphysiol.2009.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol. 2014;99:414–426. doi: 10.1113/expphysiol.2013.075937. [DOI] [PubMed] [Google Scholar]
- 99.Ono S, Takahashi K, Jinnai K, Kanda F, Fukuoka Y, Kurisaki H, Mitake S, Inagaki T, Yamano T, Shimizu N, Nagao K. Loss of catecholaminergic neurons in the medullary reticular formation in myotonic dystrophy. Neurology. 1998;51:1121–1124. doi: 10.1212/wnl.51.4.1121. [DOI] [PubMed] [Google Scholar]
- 100.Paterson DJ. Defining the neurocircuitry of exercise hyperpnoea. J Physiol. 2014;592:433–444. doi: 10.1113/jphysiol.2013.261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61:5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 102.Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010;174:292–298. doi: 10.1016/j.resp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 103.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 104.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- 105.Piper AJ, Grunstein RR. Big breathing: the complex interaction of obesity, hypoventilation, weight loss, and respiratory function. J Appl Physiol. 2010;108:199–205. doi: 10.1152/japplphysiol.00713.2009. [DOI] [PubMed] [Google Scholar]
- 106.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104:2324–2330. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 107.Porzionato A, Macchi V, De CR, Di GC. Inflammatory and immunomodulatory mechanisms in the carotid body. Respir Physiol Neurobiol. 2013;187:31–40. doi: 10.1016/j.resp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 108.Prabhakar NR, Semenza GL. Gaseous messengers in oxygen sensing. J Mol Med (Berl) 2012;90:265–272. doi: 10.1007/s00109-012-0876-1. [DOI] [PubMed] [Google Scholar]
- 109.Read DJ. A clinical method for assessing the ventilatory response to carbon dioxide. Australas Ann Med. 1967;16:20–32. doi: 10.1111/imj.1967.16.1.20. [DOI] [PubMed] [Google Scholar]
- 110.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol. 2005;289:R789–R797. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- 111.Ribeiro F, Theriault ME, Debigare R, Maltais F. Should all patients with COPD be exercise trained? J Appl Physiol (1985 ) 2013;114:1300–1308. doi: 10.1152/japplphysiol.01124.2012. [DOI] [PubMed] [Google Scholar]
- 112.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 2013;62:2905–2916. doi: 10.2337/db12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91:328–335. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- 114.Schwarzacher SW, Rub U, Deller T. Neuroanatomical characteristics of the human pre-Botzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134:24–35. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- 115.Sharpe AL, Calderon AS, Andrade MA, Cunningham JT, Mifflin SW, Toney GM. Chronic intermittent hypoxia increases sympathetic control of blood pressure: role of neuronal activity in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol. 2013;305:H1772–H1780. doi: 10.1152/ajpheart.00592.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol. 2011;589:1463–1476. doi: 10.1113/jphysiol.2010.200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Skatrud JB, Dempsey JA, Bhansali P, Irvin C. Determinants of chronic carbon dioxide retention and its correction in humans. J Clin Invest. 1980;65:813–821. doi: 10.1172/JCI109732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- 121.Stickland MK, Miller JD, Smith CA, Dempsey JA. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res. 2007;100:1371–1378. doi: 10.1161/01.RES.0000266974.84590.d2. [DOI] [PubMed] [Google Scholar]
- 122.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strollo PJ, Jr, Soose RJ, Maurer JT, de VN, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 124.Tasoulis A, Papazachou O, Dimopoulos S, Gerovasili V, Karatzanos E, Kyprianou T, Drakos S, Anastasiou-Nana M, Roussos C, Nanas S. Effects of interval exercise training on respiratory drive in patients with chronic heart failure. Respir Med. 2010;104:1557–1565. doi: 10.1016/j.rmed.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 125.Teppema LJ, Smith CA. CrossTalk opposing view: peripheral and central chemoreceptors have hyperadditive effects on respiratory motor control. J Physiol. 2013;591:4359–4361. doi: 10.1113/jphysiol.2013.256818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med. 2014;189:57–65. doi: 10.1164/rccm.201305-0848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–2234. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 128.Thornton JM, Aziz T, Schlugman D, Paterson DJ. Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol. 2002;539:615–621. doi: 10.1113/jphysiol.2001.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B, Paterson DJ. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol. 2001;533:823–836. doi: 10.1111/j.1469-7793.2001.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. J Physiol. 2003;553:3–11. doi: 10.1113/jphysiol.2003.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van den Borst B, Slot IG, Hellwig VA, Vosse BA, Kelders MC, Barreiro E, Schols AM, Gosker HR. Loss of quadriceps muscle oxidative phenotype and decreased endurance in patients with mild-to-moderate COPD. J Appl Physiol (1985 ) 2013;114:1319–1328. doi: 10.1152/japplphysiol.00508.2012. [DOI] [PubMed] [Google Scholar]
- 132.Wang HJ, Li YL, Zucker IH, Wang W. Exercise training prevents skeletal muscle afferent sensitization in rats with chronic heart failure. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1260–R1270. doi: 10.1152/ajpregu.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang ZY, Olson EB, Jr, Bjorling DE, Mitchell GS, Bisgard GE. Sustained hypoxia-induced proliferation of carotid body type I cells in rats. J Appl Physiol. 2008;104:803–808. doi: 10.1152/japplphysiol.00393.2007. [DOI] [PubMed] [Google Scholar]
- 134.Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol. 1987;62:2201–2211. doi: 10.1152/jappl.1987.62.6.2201. [DOI] [PubMed] [Google Scholar]
- 135.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Rand CM. Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008;164:38–48. doi: 10.1016/j.resp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 137.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, Passaglia CL, Jackson AC, Malhotra A, White DP. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114:911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–151. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Whipp BJ. Control of exercise hyperpnea: the unanswered question. In: Poulin M, Wilson R, editors. Integration of Respiratory Control. Springer; 2008. pp. 16–24. [DOI] [PubMed] [Google Scholar]
- 141.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol (1985 ) 2001;90:1392–1399. doi: 10.1152/jappl.2001.90.4.1392. [DOI] [PubMed] [Google Scholar]
- 142.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Brain activation by central command during actual and imagined handgrip under hypnosis. J Appl Physiol. 2002;92:1317–1324. doi: 10.1152/japplphysiol.00939.2001. [DOI] [PubMed] [Google Scholar]
- 143.Wilson RJ, Day TA. CrossTalk opposing view: peripheral and central chemoreceptors have hypoadditive effects on respiratory motor output. J Physiol. 2013;591:4355–4357. doi: 10.1113/jphysiol.2013.256578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Woods PR, Olson TP, Frantz RP, Johnson BD. Causes of breathing inefficiency during exercise in heart failure. J Card Fail. 2010;16:835–842. doi: 10.1016/j.cardfail.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie A, Teodorescu M, Pegelow DF, Teodorescu MC, Gong Y, Fedie JE, Dempsey JA. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J Appl Physiol. 2013;115:22–33. doi: 10.1152/japplphysiol.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 147.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105:1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]