Abstract
Introduction
Platelet activation, thrombin generation and fibrin formation play important roles in intracoronary thrombus formation, which may lead to acute myocardial infarction.
We investigated whether the prothrombotic markers D-dimer, pro-thrombin fragment 1 + 2 (F1 + 2) and endogenous thrombin potential (ETP) are associated with myocardial necrosis assessed by Troponin T (TnT), and left ventricular impairment assessed by left ventricular ejection fraction (LVEF) and N-terminal pro b-type natriuretic peptide (NT-proBNP).
Materials/Methods
Patients (n = 987) with ST-elevation mycardial infarction (STEMI) were included. Blood samples were drawn at a median time of 24 h after onset of symptoms.
Results
Statistically significant correlations were found between both peak TnT and D-dimer (p < 0.001) and F1 + 2 (p < 0.001), and between NT-proBNP and D-dimer (p = 0.001) and F1 + 2 (p < 0.001). When dividing TnT and NT-proBNP levels into quartiles there were significant trends for increased levels of both markers across quartiles (all p < 0.001) D-dimer remained significantly associated with NT-proBNP after adjustments for covariates (p = 0.001) whereas the association between NTproBNP and F1 + 2 was no longer statistically significant (p = 0.324).
A significant inverse correlation was found between LVEF and D-dimer (p < 0.001) and F1 + 2 (p = 0.013). When dichotomizing LVEF levels at 40 %, we observed significantly higher levels of both D-dimer (p < 0.001) and F1 + 2 (p = 0.016) in the group with low EF (n = 147).
Summary/conclusion
In our cohort of STEMI patients we demonstrated that levels of D-dimer and F1 + 2 were significantly associated with myocardial necrosis as assessed by peak TnT. High levels of these coagulation markers in patients with low LVEF and high NTproBNP may indicate a hypercoagulable state in patients with impaired myocardial function.
Keywords: Myocardial infarction, D-dimer, Prothrombin fragment 1 + 2, Myocardial function
Introduction
Coronary artery disease (CAD) is a progressive atherosclerotic condition and is, together with thrombus formation, the most important underlying mechanism of an acute myocardial infarction (AMI) [1–4].
In addition to platelet activation, thrombin generation and fibrin formation play an important role in the development of an intracoronary thrombus, which may lead to an acute coronary occlusion [2, 5, 6].
The generation of thrombin through the tissue factor pathway is essential in the haemostatic process [1, 2, 7]. It is crucial in normal physiology, whereas an inappropriate generation of thrombin may contribute to vascular occlusions such as in myocardial infarction. Increased thrombin generation, as an expression of activation of the coagulation system, was previously shown in patients with acute coronary syndrome and unstable angina pectoris [8, 9].
When prothrombin is converted to activated thrombin, prothrombin fragment 1 + 2 (F1 + 2) is formed, thus indicating thrombin generation in vivo, with subsequent fibrin formation. From the fibrinolytic system, plasmin converted from plasminogen degrades fibrin, resulting in degredation products like D-dimer. Elevated D-dimer levels therefore indicate both ongoing coagulation and fibrinolytic activation. Both markers have been shown to be persistantly elevated for months after the acute myocardial infarction [5], whereas an early decrease in D-dimer levels has been shown to be associated with improved prognosis [10].
The endogenous thrombin potential (ETP) has been proposed as an informative method to determine the degree of hypercoagulability, measuring the potential to generate thrombin ex vivo [11].
Several studies have shown increased levels of prothrombotic markers in patients with myocardial infarction [10, 12] and also association to clinical outcome [10, 13, 14]. There is, however, limited knowledge about activation of the coagulation cascade in the acute phase of ST-elevation myocardial infarction (STEMI) and also limited data on the degree of hypercoagulability in relation to the degree of myocardial injury and severity of the disease in these patients.
The aim of the present substudy was therefore to investigate whether circulating levels of selected prothrombotic markers were associated with the degree of myocardial necrosis assessed by peak troponin T (TnT) and with left ventricular impairment assessed by left ventricular ejection fraction (LVEF) and N-terminal pro b-type natriuretic peptide (NT-proBNP) in STEMI patients. Furthermore, the degree of hypercoagulability was studied in relation to traditional risk factors and baseline characteristics of the STEMI population.
Material and methods
A total of 987 percutaneous coronary intervention (PCI)-treated STEMI patients from a cross sectional cohort study were included, all admitted to Oslo University Hospital Ullevål, Oslo, Norway in the period from June 2007 to August 2011. STEMI was defined as ST segment elevation of >2 mm in two or more contiguous chest leads or > 1 mm in two or more limb leads or left bundle branch block, together with typical chest pain and elevated troponin levels above the recommended diagnostic threshold. Patients on warfarin treatment, below 18 years of age and patients unable or unwilling to give written informed consent were excluded. Blood samples were collected at median time of 24 h after symptoms and 18 h after the PCI procedure, between 8 and 10 a.m. the following morning. In order to standardize blood sampling, and also to avoid any influence of diurnal variations and food intake, all samples were taken after an overnight fast. Routine blood samples were drawn at hospital admission, and samples for TnT were measured after standardized time intervals.
Citrated blood (0.129 M trisodium citrate in dilution 1:10) was centrifuged within 30 min at 2500 × g at 4 °C and kept frozen at ÷80 °C until analyzed. D-dimer and F1 + 2 were determined by ELISA (Asserachrom D-dimer, Stago Diagnostica, Ansiere, France and Enzygnost F1 + 2, Siemens, Marburg, Germany, respectively). Coefficients of variation (CV) were for D-dimer 6.5 % and F1+ 2 5.4 %.
ETP was determined by the Calibrated Automated Thrombogram (CAT) assay according to the manufacturer’s instructions (Thrombinoscope BV, Maastricht, The Netherlands) and thrombin generation was measured on the Fluoroscan Ascent fluorometer (Thermo Fisher Scientific OY, Vantaa, Finland). A reagent mixture of rTF and phospholipids in addition to a thrombin-specific fluorogenic substrate in Hepes buffer containing CaCl2 was added to the plasma to obtain a final concentration of 5 pM, 4 μM and 416.7 μM, respectively. In order to calculate the final results, plasma was measured along with a thrombin calibrator. The software (version 3.0.0.29; Thrombinoscope BV) enabled the calculation of the lag time (LT), peak thrombin generation (pTG), ETP and time to peak (TTP). Further, VT (Velocity Index) = TP/(TTP-LT), indicating the average net rate of prothrombin activation during the propagation phase, was calculated. All experiments were run in duplicates and the interassay coefficients of variation for the different CAT parameters were 14.2, 4.6, 5.0 and 8.0 %, respectively.
CRP was measured with kits from DRG Instruments (Marburg/Lahn, Germany), CV <5 %.
Electrochemiluminescence technology for quantitative measurement was used for repeated measures of TnT (3rd generation cTroponinT, Elecsys 2010, Roche, Mannheim, Germany). The lower detection limit of the assay is 10 ng/L with a recommended diagnostics threshold of 30 ng/L. The inter-assay coefficient of variation was 7 %. NT-ProBNP was measured in serum using Elecsys proBNP sandwich immunoassay on Elecsys 2010 (Roche Diagnostics, Indianapolis, USA). The inter-assay coefficient of variation was 7 %.
Left ventricular ejection fraction (LVEF) was measured by echocardiography before hospital discharge or at a clinical follow-up within 3 months after the AMI (n = 767).
Diabetes was defined according to the American Diabetes Association criteria [15] and hypertension (HT) was defined as previously diagnosed and treated hypertension. Smokers were defined as current smokers or quit within the last month.
Clinical information was collected from hospital records and questionnaires acquired at the time of inclusion. Patients on warfarin were not included in this patient cohort.
The study was approved by The Regional Ethics Commitee and all patients gave written informed consent.
Statistical analysis
Continous variables are presented as median values with 25,75 percentiles and categorial variables as number or proportions. As most of the variables were skewed, correlation analyses were performed using Spearman’s method. Differences between groups were tested by Mann–Whitney U test for continuous variables. Associations between prothrombotic markers and peak TnT and left ventricular impairment were tested in multivariate regression models, adjusting for relevant covariates. As the markers are strongly inter-related they were analyzed in separate models. Skewed data were log-transfomed before entered in the model. P values < 0.05 were considered statistically significant. The statistical analyses were performed with SPSS software version 18.0 (SPSS Inc, Chicago, USA).
Results
Baseline characteristics of the total population are given in Table 1. The cohort was a typical STEMI population of relatively young, predominantly male patients (81 %) with medium size infarction (measured by peak TnT). Only 23 % with previous CVD, 12 % with known diabetes and half of the patients were smokers.
Table 1.
Characteristics of the study population (n = 987)
| Age (years) (range) | 61 (24–94) |
|---|---|
| Male sex | 800 (81) |
| Current smokers | 474 (48) |
| Previous CVD | 229 (23) |
| Treated hypertension | 334 (34) |
| Treated diabetes mellitus | 124 (12) |
| BMI (kg/m2) | 26.6 (24.3,29.2) |
| Prehospital thrombolysis | 119 (12) |
| Aspirin | 231 (23) |
| Statins | 233 (23) |
| Total cholesterol (mmol/L) | 4.9 (4.1,5.6) |
| HDL (mmol/L) | 1.06 (0.88,1.30) |
| Triglycerides (mmol/L) | 1.25 (0.89,1.78) |
| CRP (mg/L) | 13.4 (7.0,31.3) |
| Admission glucose (mmol/L) | 7.4 (6.3,9.0) |
| Fasting glucose (mmol/L) | 5.8 (5.2,6.6) |
| HbA1c (%) | 5.9 (5.6,6.3) |
| Peak Troponin T (ng/L) | 3850 (1710,7250) |
| NT-ProBNP (pmol/L) | 31 (10,118) |
| LV Ejection fraction (%) | 50 (44,55) |
| D-dimer (ng/mL) | 456 (287,796) |
| F1 + 2 (pmol/L) | 246 (178,356) |
| ETP (nM⋅min) | 1564 (1366,1743) |
| Time from onset of symptoms to blood sampling (hours) (range) | 24 (5–118) |
Number (proportions) or median (25,75 percentiles) are given
BMI Body mass index, CVD Cardiovascular Disease, HDL High Density lipoprotein cholesterol, CRP C-reactive Protein, ETP endogenous thrombin potential
Levels of the haemostatic variables in the total population are shown in Table 1.
There were strong inter-correlations between D-dimer and F1 + 2 (r = 0.504, p < 0.001), and a weaker, inverse correlation between D-dimer and ETP (r = −0-.102, p < 0.001).
As visualized in Table 2, age was significantly correlated with F1 + 2 and D-dimer, inversely to ETP (all p < 0.001) and further weakly correlated to lag time, time to peak, peak hight and velocity index (all p < 0.05).
Table 2.
Correlations between prothrombotic markers and selected variables
| D-dimer | F1 + 2 | ETP | LT | TTP | pTG | VT | ||
|---|---|---|---|---|---|---|---|---|
| Age | r | 0.412 | 0.277 | −0.229 | −0.075 | −0.155 | −0.086 | 0.088 |
| p | <0.001 | <0.001 | <0.001 | 0.021 | <0.001 | 0.008 | 0.006 | |
| BMI | r | −0.184 | −0.203 | 0.218 | 0.115 | 0.103 | 0.183 | 0.070 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.032 | |
| NT-ProBNP | r | 0.243 | 0.120 | −0.118 | 0.072 | 0.003 | −0.033 | 0.066 |
| p | <0.001 | <0.001 | 0.002 | 0.026 | 0.92 | 0.30 | 0.044 | |
| HbA1c | r | 0.063 | −0.015 | 0.033 | 0.025 | 0.006 | 0.055 | 0.048 |
| p | 0.141 | 0.765 | 0.161 | 0.44 | 0.85 | 0.09 | 0.146 | |
| Fasting glucose | r | 0.006 | 0.062 | −0.054 | 0.042 | −0.031 | 0.061 | 0.104 |
| p | 0.844 | 0.074 | 0.115 | 0.19 | 0.34 | 0.06 | 0.001 | |
| Peak TnT | r | 0.260 | 0.364 | −0.072 | −0.012 | −0.059 | 0.001 | 0.065 |
| p | <0.001 | <0.001 | 0.015 | 0.70 | 0.062 | 0.97 | 0.044 | |
| LVEF | r | −0.160 | −0.090 | 0.022 | −0.040 | 0.024 | −0.065 | −0.107 |
| p | <0.001 | 0.013 | 0.553 | 0.27 | 0.51 | 0.077 | 0.003 |
TnT Troponin T, LVEF left ventricular ejection fraction, BMI Body mass index, LT lag time, TTP Time to peak, pTG peak thrombin generation, V T Velocity index
r-values refer to Spearman’s rank correlation coefficient
Prothrombotic markers and association with myocardial injury
Statistically significant correlations were found between peak TnT and D-dimer and F1 + 2 (both p < 0.001) (Table 2). Linear trend analysis across quartiles of peak TnT revealed increased levels of both markers with increasing quartiles (p for trend < 0.001). When adjusting for relevant covariates as visualized in Table 4a both D-dimer and F1 + 2 remained significantly associated with peak TnT (both p < 0.001) (Table 4a and Fig. 1a). Weak, but statistically significant correlations were observed between TnT and ETP and velocity index (Table 2).
Table 4.
Determinants of peak Troponin T (a) and NT-proBNP (b)
| Determinants | Standardized beta | 95 % CIc | p-value | Determinants | Standardized beta | 95 % CI | p-value |
|---|---|---|---|---|---|---|---|
| a) | |||||||
| D-dimer | 0.138 | <0.001– < 0.001 | <0.001 | F1 + 2 | 0.216 | 0.001–0.001 | <0.001 |
| Age | −0.054 | −0.012–0.002 | 0.156 | Age | −0.052 | −0.012–0.002 | 0.157 |
| Sex | 0.025 | −0.117–0.261 | 0.457 | Sex | 0.020 | −0.128–0.244 | 0.542 |
| BMI | −0.026 | −0.025–0.012 | 0.466 | BMI | −0.013 | −0.22–0.015 | 0.706 |
| HT | −0.006 | −0.179–0.152 | 0.870 | HT | −0.013 | −0.194–0.132 | 0.710 |
| Time framea | −0.076 | −0.018– − 0.001 | 0.032 | Time framea | −0.071 | −0.017– < −0.001 | 0.042 |
| CRPb | 0.081 | 0.031–0.333 | 0.018 | CRPb | 0.083 | 0.039–0.335 | 0.013 |
| NT-ProBNP | 0.187 | 0.183–0.423 | <0.001 | NT-ProBNP | 0.196 | 0.199–0.435 | <0.001 |
| b) | |||||||
| D-dimer | 0.063 | <−0.001– < 0.001 | <0.001 | F1 + 2 | 0.024 | <−0.001– < 0.001 | 0.446 |
| Age | 0.180 | 0.011–0.023 | 0.050 | Age | 0.190 | 0.012–0.024 | <0.001 |
| Sex | −0.083 | −0.405– − 0.061 | <0.001 | Sex | −0.085 | −0.412– − 0.067 | 0.007 |
| BMI | −0.086 | −0.039– − 0.006 | 0.008 | BMI | −0.087 | −0.040– − 0.006 | 0.008 |
| HT | 0.125 | 0.145–0.445 | <0.001 | HT | 0.126 | 0.148–0.449 | <0.001 |
| Time framea | 0.307 | 0.031–0.046 | <0.001 | Time framea | 0.307 | 0.031–0.046 | <0.001 |
| CRPb | 0.035 | −0.059–0.217 | 0.261 | CRPb | 0.043 | −0.041–0.233 | 0.169 |
Multivariable regression analysis adjusted for age, sex, BMI, HT, Time frame, CRP and NT-proBNP
For abbreviations, see text
aTime from symptoms to blood sampling bLogtransformed c Confidence Intervals
Fig. 1.
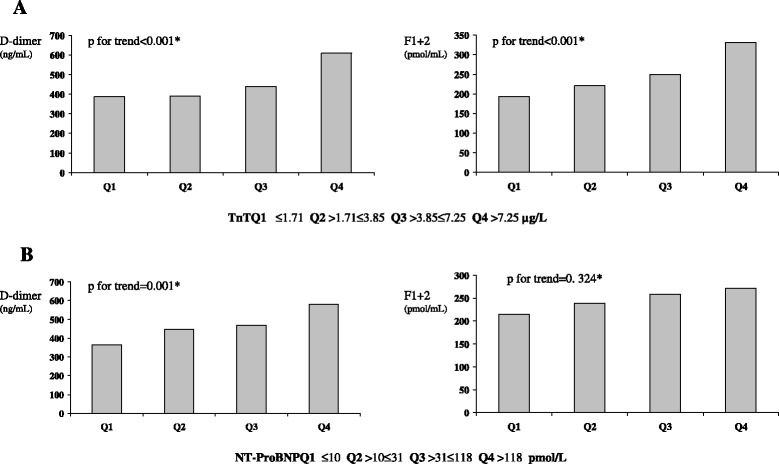
D-dimer and F1 + 2 (medians) in quartiles of peak TnT (a) and NT-ProBNP (b). a * = adjusted for age, sex, BMI, hypertension, time from symptoms to blood sampling, CRP and NT-ProBNP; b * = adjusted for age, sex, BMI, hypertension, time from symptoms to blood sampling and CRP
Prothrombotic markers and association with myocardial function
Significant correlations were found between D-dimer and F1 + 2 and NT-ProBNP (both p = 0.001). Weak, but statistically significant correlations were also observed for the CAT-variables ETP, lagtime and velocity index (Table 2). When dividing NT-ProBNP levels into quartiles there were significant trends for increased levels of D-dimer and F1 + 2 across quartiles (both p < 0.001). D-dimer remained significantly associated with NT-proBNP after adjusting for covariates as visualized in Table 4b (p = 0.001), whereas the association between NT-proBNP and F1 + 2 was no longer statistically significant (p = 0.446) (Table 4b, Fig. 1b).
A weak, but significant inverse correlation was found between LVEF and D-dimer (p < 0.001), F1 + 2 (p = 0.013) and velocity index (p = 0.003) (Table 2). When dichotomizing LVEF levels at 40 % we observed significantly higher levels of all variables (p < 0.001, p = 0.016 and p = 0.004, respectively) in patients with LVEF below (n = 147), compared to above 40 % (Table 3). After adjustments for the covariates visualized in Table 4b the difference in D-dimer levels remained statistically significant (p = 0.003) whereas the association between LVEF and F1 + 2 and velocity index was no longer significant (p = 0.552 and p = 0.084, respectively).
Table 3.
Levels of the prothrombotic markers according to group characteristics of the population
| n | D-dimer (ng/mL) | F1 + 2 (pmol/L) | ETP (nM⋅min) | ||
|---|---|---|---|---|---|
| Sex | Male | 800 | 424 (275,755) | 238 (175,350) | 1576 (1401,1748) |
| Female | 187 | 609 (399,1008) | 287 (203,395) | 1494 (1284,1702) | |
| p | 0.001 | 0.001 | 0.001 | ||
| Smoking | + | 474 | 448 (275,796) | 245 (179,347) | 1573 (1364,1762) |
| - | 513 | 467 (295,795) | 250 (178,382) | 1557 (1368,1726) | |
| p | 0.675 | 0.594 | 0.269 | ||
| Previous CVD | + | 229 | 484 (286,798) | 244 (177,332) | 1569 (1348,1733) |
| - | 758 | 452 (286,796) | 247 (180,369) | 1563 (1370,1745) | |
| p | 0.553 | 0.386 | 0.503 | ||
| HT | + | 553 | 553 (355,952) | 275 (197,398) | 1563 (1366,1730) |
| - | 418 | 418 (273,773) | 235 (174,346) | 1565 (1366,1745) | |
| p | 0.001 | 0.001 | 0.381 | ||
| Diabetes | + | 870 | 454 (246,848) | 232 (169,324) | 1490 (1261,1754) |
| - | 117 | 456 (292,795) | 249 (180,365) | 1572 (1373,1754) | |
| p | 0.285 | 0.142 | 0.004 | ||
| LVEF (%) | >40 | 147 | 440 (285,755) | 242 (174,363) | 1573 (1371,1735) |
| ≤40 | 620 | 679 (408,1156) | 297 (189,397) | 1488 (1293,1691) | |
| p | <0.001 | 0.016 | 0.017 |
Median (25,75 percentiles) values are given
HT hypertension, CVD cardiovascular disease, LVEF left ventricular ejection fraction
Prothrombotic markers and traditional risk factors
Levels of D-dimer and F1 + 2 were significantly higher in women (p < 0.001, both), while ETP was higher in men (p = 0.001) (Table 3). There were no significant sex differences in other CAT variables (data not shown).
No difference in any of the prothrombotic markers between smokers and non-smokers or patients with or without previous CVD was observed.
In patients with hypertension, D-dimer, F1 + 2 and velocity index levels were significantly higher compared to the group without hypertension (all p < 0.001), however the association weakened after adjustments for covariates (p = 0.018, p = 0.015 and p = 0.026, respectively).
Diabetic patients had significantly lower ETP levels compared to non diabetics (p = 0.004) without any differences in other CAT variables (data not shown) or D-dimer and F1 + 2. There were also limited correlations between the haemostatic markers and HbA1c and fasting glucose, except for velocity index which correlated weakly to fasting glucose (Table 2).
Significant inverse correlations were observed between BMI and D-dimer and F1 + 2 (both p < 0.001), whereas all CAT variables were positively correlated with BMI (all p < 0.05) (Table 2). There were significant trends for decreased levels of D-dimer and F1 + 2 and increased ETP across quartiles of BMI (adjusted p = 0.011, p = <0.001, p < 0.001, respectively).
Discussion
In this large cohort of STEMI patients we found that levels of D-dimer and F1 + 2 were significantly associated with the extent of myocardial injury as measured by peak TnT. Significant associations between these coagulation markers and myocardial function, assessed by LVEF and NT-ProBNP, were further demonstrated.
We observed an inverse pattern for the in vivo thrombin generation and ex vivo potential to generate thrombin, which confirm previous findings in patients with stable CAD [16]. It might be speculated that this is due to an increased in vivo production of thrombin in the acute phase, resulting in reduced potential to generate thrombin ex vivo, as an exhaustion phenomenon.
Patients with STEMI admitted to primary PCI, receive heparin before or during the procedure. Heparin could potentially influence the results. However, as heparin was given only during the procedure, any effect on the measured variables was most likely not present when the blood samples were drawn 18 h (median time) after the procedure.
There was a clear association between the variables and myocardial necrosis measured by peak TnT. This association was also present after adjustments for potential covariates including CRP. Thus the prothrombotic state, to some degree also reflected in CAT parameters, was probably not a result of inflammation in the acute phase. We have previously reported similar results in another AMI population [17]. In that particular study the prothrombotic markers were measured 3–4 days after the acute event, probably reflecting a more stable situation. Nevertheless, the results clearly indicate that patients with larger infarctions are in an increased hypercoagulable state. It might therefore be discussed if patients with large infarctions are sufficiently protected by use of double antiplatelet therapy [18]. Use of warfarin has been shown to reduce both D-dimer and F1 + 2 after AMI [19], and randomized, clinical studies have shown beneficial effects on clinical outcome by use of warfarin as anticoagulation after acute MI [20, 21].
The findings of a significant association between procoagulant activity, shown especially by D-dimer and F1 + 2, but also by CAT variables, and impaired myocardial function in the acute phase of a STEMI, has to our knowledge, not been reported before. Elevated prothrombotic markers in the early phase of AMI are known to identify patients with incrased risk of subsequent cardiac death, but such associations have so far been reported to appear independent of LVEF [22]. Elevated levels of D-dimer and F1 + 2 were shown along with impaired myocardial function in another population not suffering from CAD [23]. An association between elevated D-dimer and heart failure has also been demonstrated [24]. Although there is no convincing evidence that oral anticoagulant therapy reduces mortality and vascular events in patients with heart failure and sinus rhythm [25, 26], prolonged anticoagulant treatment of such patients may be discussed after an AMI.
Diabetes is generally associated with elevated levels of prothrombotic markers [27], also in diabetic patients without coronary heart disease [28]. In our population, diabetes and glucometabolic disturbances were limited associated with a prothrombotic state, other than lower levels of ETP and a significant correlation between fasting glucose and velocity index. The latter may indicate glucose per se to play a role for the propagation phase of thrombin generation. Our results differ from some other studies showing enhanced thrombin generation in diabetics [28–30]. However, in the study by Tripodi et al. ETP levels were higher in diabetics versus controls only in the presence of added thrombomodulin [29]. Difference in the populations investigated may also be of importance. The limited findings in our study may be explained by the elevated levels of prothrombotic markers in the acute situation of an AMI, thus masking any difference. In addition, the levels of fasting glucose and HbA1C indicate adequate treatment of diabetes in the present population. Similar results have also been shown in another study on stable patients with CAD [16].
The inverse correlations between BMI and D-dimer and F1 + 2 indicating a less hypercoagulable state in overweight individuals, are in accordance with previous findings in a population of stable CAD patients [16] and is not easily explained. In contrast, all CAT variables were positively associated with BMI, indicative of an increased potential to thrombin generation. Other studies have shown positive correlation between BMI and D-dimer, however, only in patients not diagnosed with CVD [31]. In one study on healthy, obese individuals D-dimer values were found not to be correlated to BMI [32].
Increased levels of prothrombotic markers in patients with hypertension is well known [33–35]. This was also present in our population of STEMI patients when evaluated in the acute phase, showing elevated levels of D-dimer, F1 + 2 and velocity index in the group of hypertensive patients, although highly dependent of related factors.
Limitations
Single bloodsampling prevented us from studying the time-course of the measured markers. We are not sure to have measured peak values of the variables or transient changes due to the variability in the time frame from onset of symptoms to blood sampling. However, the results did not change when taken this into account in the multivariate models. The blood samples were centrifuged at 4° C, thus any contact activation cannot be ruled out. We have also not included an extra centrifugation step before the analysis. The measure of LVEF by echo cardiography was performed at different time points from hospital discharge until 3 months after the index infarct, and our cohort of STEMI patients was a low risk population with few complications and just slightly reduced LVEF, and this fact may have influenced the results. As we do not have follow-up information of this cohort, any impact of the results on future clinical endpoints cannot be explored.
Conclusion
In our cohort of STEMI patients we could demonstrate a significant association between levels of D-dimer and F1 + 2 and the extent of myocardial necrosis as assessed by TnT. The high levels of these markers in patients with low LVEF and high NT-ProBNP may indicate a hypercoagulable state in patients with impaired myocardial function. The inverse relation between BMI and procoagulant activity is not easily explainable, and has to be further explored.
Acknowledgements
This work was supported by the Stein Erik Hagen Foundation for Clinical Heart Research, Oslo, Norway. We thank the study nurses and the staff at the Coronary Intensive Care Unit and Center for Clinical Heart Research for excellent assistance and medical technologist Beate Vestad for laboratory analysis. The study was a part of the Biobanking in myocardial infarction (BAMI) project at Oslo University Hospital, Ullevål, which is lead by a steering committee including Mangschau, A and the following authors: Seljeflot, Arnesen (Chair), Eritsland, Halvorsen, Bjørnerheim and Andersen.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CHH wrote the manuscript and participated in the coordination of the study, performed analysis and interpretation of data, contributed to acqusition of data , VR contributed to acqusition of data, SH participated in the design of the study and helped to draft the manuscript, GØA participated in the design of the study and helped to draft the manuscript, RB participated in the design of the study, JE participated in the design of the study, HA participated in the design of the study and helped to draft the manuscript, IS helped perform the statistical analysis, participated in the design and coordination of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
C. H. Hansen, Phone: +47 22119943, Email: uxchho@ous-hf.no
V. Ritschel, Email: uxvino@ous-hf.no
S. Halvorsen, Email: sighalvo@ous-hf.no
G. Ø. Andersen, Email: uxgend@ous-hf.no
R. Bjørnerheim, Email: uxrejr@ous-hf.no
J. Eritsland, Email: jeritsla@ous-hf.no
H. Arnesen, Email: uxhaar@ous-hf.no
I. Seljeflot, Email: uxinlj@ous-hf.no
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Robbie L, Libby P. Inflammation and atherothrombosis. Ann N Y Acad Sci. 2001;947:167–79. doi: 10.1111/j.1749-6632.2001.tb03939.x. [DOI] [PubMed] [Google Scholar]
- 3.Corti R, Farkouh ME, Badimon JJ. The vulnerable plaque and acute coronary syndromes. Am J Med. 2002;113:668–80. doi: 10.1016/S0002-9343(02)01344-X. [DOI] [PubMed] [Google Scholar]
- 4.Brener SJ. Insights into the pathophysiology of ST-elevation myocardial infarction. Am Heart J. 2006;151:S4–10. doi: 10.1016/j.ahj.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Merlini PA, Bauer KA, Oltrona L, Ardissino D, Cattaneo M, Belli C, et al. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation. 1994;90:61–8. doi: 10.1161/01.CIR.90.1.61. [DOI] [PubMed] [Google Scholar]
- 6.James SK, Siegbahn A, Armstrong P, Barnathan E, Califf R, Simoons ML, et al. Activation of the inflammation, coagulation, and fibrinolysis systems, without influence of abciximab infusion in patients with non-ST-elevation acute coronary syndromes treated with dalteparin: a GUSTO IV substudy. Am Heart J. 2004;147:267–74. doi: 10.1016/j.ahj.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Rapaport SI, Rao LV. Initiation and regulation of tissue factor-dependent blood coagulation. Arterioscler Thromb. 1992;12:1111–21. doi: 10.1161/01.ATV.12.10.1111. [DOI] [PubMed] [Google Scholar]
- 8.Merlini PA, Ardissino D, Oltrona L, Broccolino M, Coppola R, Mannucci PM. Heightened thrombin formation but normal plasma levels of activated factor VII in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1995;15:1675–9. doi: 10.1161/01.ATV.15.10.1675. [DOI] [PubMed] [Google Scholar]
- 9.Kruskal JB, Commerford PJ, Franks JJ, Kirsch RE. Fibrin and fibrinogen-related antigens in patients with stable and unstable coronary artery disease. N Engl J Med. 1987;317:1361–5. doi: 10.1056/NEJM198711263172201. [DOI] [PubMed] [Google Scholar]
- 10.Christersson C, Oldgren J, Bylock A, Siegbahn A, Wallentin L. Early decrease in coagulation activity after myocardial infarction is associated with lower risk of new ischaemic events: observations from the ESTEEM Trial. Eur Heart J. 2007;28:692–8. doi: 10.1093/eurheartj/ehl564. [DOI] [PubMed] [Google Scholar]
- 11.Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006;96:553–61. [PubMed] [Google Scholar]
- 12.Kikkert WJ, Claessen BE, Stone GW, Mehran R, Witzenbichler B, Brodie BR, et al. D-dimer levels predict ischemic and hemorrhagic outcomes after acute myocardial infarction: a HORIZONS-AMI biomarker substudy. J Thromb Thrombolysis. 2014;37:155–64. doi: 10.1007/s11239-013-0953-5. [DOI] [PubMed] [Google Scholar]
- 13.Ardissino D, Merlini PA, Gamba G, Barberis P, Demicheli G, Testa S, et al. Thrombin activity and early outcome in unstable angina pectoris. Circulation. 1996;93:1634–9. doi: 10.1161/01.CIR.93.9.1634. [DOI] [PubMed] [Google Scholar]
- 14.Seljeflot I, Hurlen M, Hole T, Arnesen H. Soluble tissue factor as predictor of future events in patients with acute myocardial infarction. Thromb Res. 2003;111:369–72. doi: 10.1016/j.thromres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35 Suppl 1:S64-71. [DOI] [PMC free article] [PubMed]
- 16.Bratseth V, Pettersen AA, Opstad TB, Arnesen H, Seljeflot I. Markers of hypercoagulability in CAD patients. Effects of single aspirin and clopidogrel treatment. Thromb J. 2012;10:12. doi: 10.1186/1477-9560-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halvorsen S, Seljeflot I, Weiss T, Bohmer E, Arnesen H. Inflammatory and thrombotic markers in patients with ST-elevation myocardial infarction treated with thrombolysis and early PCI: a NORDISTEMI substudy. Thromb Res. 2012;130:495–500. doi: 10.1016/j.thromres.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs289. [DOI] [PubMed] [Google Scholar]
- 19.Seljeflot I, Hurlen M, Arnesen H. Increased levels of soluble tissue factor during long-term treatment with warfarin in patients after an acute myocardial infarction. J Thromb Haemost. 2004;2:726–30. doi: 10.1111/j.1538-7836.2004.00676.x. [DOI] [PubMed] [Google Scholar]
- 20.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–74. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 21.van Es RF, Jonker JJ, Verheugt FW, Deckers JW, Grobbee DE. Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet. 2002;360:109–13. doi: 10.1016/S0140-6736(02)09409-6. [DOI] [PubMed] [Google Scholar]
- 22.Li YH, Teng JK, Tsai WC, Tsai LM, Lin LJ, Guo HR, et al. Prognostic significance of elevated hemostatic markers in patients with acute myocardial infarction. J Am Coll Cardiol. 1999;33:1543–8. doi: 10.1016/S0735-1097(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 23.Saito T, Yamamoto Y, Matsumura T, Nozaki S, Fujimura H, Shinno S. Coagulation system activated in Duchenne muscular dystrophy patients with cardiac dysfunction. Brain Dev. 2005;27:415–8. doi: 10.1016/j.braindev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Jug B, Vene N, Salobir BG, Sebestjen M, Sabovic M, Keber I. Prognostic impact of haemostatic derangements in chronic heart failure. Thromb Haemost. 2009;102:314–20. doi: 10.1160/TH08-11-0721. [DOI] [PubMed] [Google Scholar]
- 25.Lip GY, Shantsila E. Anticoagulation versus placebo for heart failure in sinus rhythm. Cochrane Database Syst Rev. 2014;3:CD003336. doi: 10.1002/14651858.CD003336.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aispuru GR, Clavier MM, Cardone AJ, Gilberto DO, Barousse AP. Thrombotic biomarkers and left ventricle characteristics as short-term predictors of thrombotic events in patients hospitalized for acute decompensated heart failure. Eur J Intern Med. 2012;23:545–51. doi: 10.1016/j.ejim.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Morel O, Jesel L, Abbas M, Morel N. Prothrombotic changes in diabetes mellitus. Semin Thromb Hemost. 2013;39:477–88. doi: 10.1055/s-0033-1343888. [DOI] [PubMed] [Google Scholar]
- 28.Beijers HJ, Ferreira I, Spronk HM, Bravenboer B, Dekker JM, Nijpels G, et al. Impaired glucose metabolism and type 2 diabetes are associated with hypercoagulability: potential role of central adiposity and low-grade inflammation–the Hoorn Study. Thromb Res. 2012;129:557–62. doi: 10.1016/j.thromres.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Tripodi A, Branchi A, Chantarangkul V, Clerici M, Merati G, Artoni A, et al. Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis. 2011;31:165–72. doi: 10.1007/s11239-010-0506-0. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Kim JE, Park SH, Kim YI, Nam-Goong IS, Kim ES. High coagulation factor levels and low protein C levels contribute to enhanced thrombin generation in patients with diabetes who do not have macrovascular complications. J Diabetes Complications. 2014;28:365–9. doi: 10.1016/j.jdiacomp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Michalska M, Iwan-Zietek I, Gnilka W, Dabrowiecki S, Goralczyk B, Goralczyk K, et al. PAI-1 and alpha2-AP in patients with morbid obesity. Adv Clin Exp Med. 2013;22:801–7. [PubMed] [Google Scholar]
- 32.Franco CK, Silva DR, Barreto SS. Relationship of body mass index and waist-to-hip ratio with fibrinolytic activity measured as D-dimer. Obes Res Clin Pract. 2011;5:e1–78. doi: 10.1016/j.orcp.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Lip GY, Blann AD, Jones AF, Lip PL, Beevers DG. Relation of endothelium, thrombogenesis, and hemorheology in systemic hypertension to ethnicity and left ventricular hypertrophy. Am J Cardiol. 1997;80:1566–71. doi: 10.1016/S0002-9149(97)00749-2. [DOI] [PubMed] [Google Scholar]
- 34.Makris TK, Tsoukala C, Krespi P, Hatzizacharias A, Gialeraki A, Papargyriou J, et al. Haemostasis balance disorders in patients with essential hypertension. Thromb Res. 1997;88:99–107. doi: 10.1016/S0049-3848(97)00222-3. [DOI] [PubMed] [Google Scholar]
- 35.Coban E, Ozdogan M, Akcit F. Levels of plasma fibrinogen and D-dimer in subjects with white-coat hypertension. J Hum Hypertens. 2004;18:291–2. doi: 10.1038/sj.jhh.1001670. [DOI] [PubMed] [Google Scholar]


