Early detection and diagnosis of gastric cancer has been aided by technological advances in endoscopy such as chromoendoscopy and narrow-band imaging. Although endoscopic mucosal dissection has established itself as a preferred, less-invasive treatment, the remaining gastric mucosa after endoscopic resection is considered to be a high-risk microenvironment, especially in individuals with Helicobacter pylori infection. This study aimed to investigate risk factors for the emergence of metachronous – as opposed to primary – cancers in 155 patients who were treated with endoscopic mucosal dissection.
Keywords: Endoscopic submucosal dissection, Helicobacter pylori, Intestinal metaplasia, Neutrophil infiltration, Stomach neoplasms
Abstract
BACKGROUND:
Endoscopic submucosal dissection (ESD) of early gastric cancer is a minimally invasive procedure. However, the risk for metachronous cancers after successful cancer treatment remains high and the risk factors for metachronous cancers have not been elucidated.
OBJECTIVE:
To evaluate the risk factors for metachronous gastric cancers after ESD with a long-term follow-up.
METHODS:
A total of 155 consecutive patients (119 men, 36 women, mean age 68.9 years) were treated with ESD between September 2000 and September 2009. Biopsy specimens were obtained from the greater curvature of the antrum and middle corpus to evaluate gastric mucosal status, including Helicobacter pylori, intestinal metaplasia (IM) and neutrophil infiltration (NI) before ESD. Follow-up endoscopy after ESD was scheduled at two and six months, one year and annually thereafter. H pylori eradication was recommended when possible.
RESULTS:
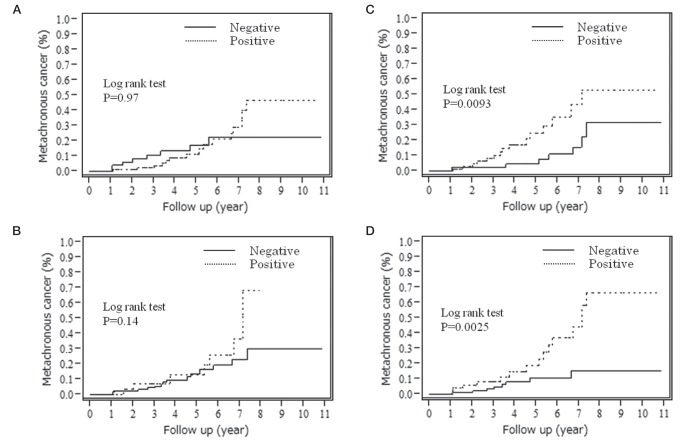
The median follow-up period was 4.2 years. Metachronous gastric cancers were found in 23 of 155 patients (3.5% per year). No local recurrences were observed. The cumulative incidence of metachronous gastric cancer was significantly high in IM and NI in the corpus (P=0.0093 and P=0.0025, respectively [log-rank test]). The ORs for IM and NI in the corpus were 2.65 and 3.06, respectively, according to the Cox proportional hazards model (P=0.024 and P=0.0091, respectively).
CONCLUSIONS:
The presence of IM and NI in the corpus was closely related to the development of metachronous gastric cancer after ESD.
Abstract
HISTORIQUE :
La dissection endoscopique des tissus sous-muqueux (DETS) du cancer gastrique précoce est une intervention à effraction minimale. Cependant, le risque de cancers métachrones demeure élevé après un traitement fructueux du cancer, et les facteurs de risque de cancers métachrones ne sont pas établis.
OBJECTIF :
Évaluer les facteurs de risque des cancers gastriques métachrones après une DETS au moyen d’un suivi à long terme.
MÉTHODOLOGIE :
Au total, 155 patients consécutifs (119 hommes et 36 femmes, d’un âge moyen de 68,9 ans) ont subi une DETS entre septembre 2000 et septembre 2009. Les échantillons de biopsie ont été prélevés dans la grande courbure de l’antre et le corps moyen pour évaluer l’état de la muqueuse gastrique, y compris l’Helicobacter pylori, la métaplasie intestinale (MI) et l’infiltration de neutrophiles (IN) avant la DETS. Une endoscopie de suivi après la DETS était planifiée au bout de deux mois, de six mois, d’un an, puis tous les ans par la suite. Dans la mesure du possible, l’éradication de l’H pylori était recommandée.
RÉSULTATS :
La période de suivi médiane était de 4,2 ans. Les médecins ont découvert des cancers gastriques métachrones chez 23 des 155 patients (3,5 % par année). Ils n’ont observé aucune récurrence locale. L’incidence cumulative de cancers gastriques métachrones était très élevée en présence de MI et d’IN du corps de l’estomac (P=0,0093 et P=0,0025, respectivement [test de Mantel-Haenszel]). Les rapports de cote de MI et d’IN dans le corps de l’estomac correspondaient à 2,65 et à 3,06, respectivement, conformément au modèle des risques proportionnels de Cox (P=0,024 et P=0,0091, respectivement).
CONCLUSIONS :
La présence de MI et d’IN dans le corps de l’estomac était étroitement liée à l’apparition de cancer gastrique métachrone après une DETS.
The rate of diagnosis of early gastric cancer has increased due to improved diagnostic procedures such as endoscopic examinations including chromoendoscopy and narrow-band imaging (1,2). Endoscopic resection (ER), which includes endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), has been established as a less-invasive treatment for early gastric cancer without concomitant lymph node metastasis (3–5). ESD has become accepted for en bloc resection, which is beneficial for curative treatment to avoid local recurrence (5–10). Although ER contributes to preservation of the majority of the stomach, metachronous cancers developing at other sites in the stomach may occur more frequently after ER than those after surgical partial gastrectomy (11). Several studies have reported that the incidence of metachronous cancers after ER is 2.4% to 14% (12,13). The residual gastric mucosa after ER is believed to be a high-risk microenvironment.
Helicobacter pylori is one of the most important risk factors for gastric cancer (14–17). Inflammation caused by H pylori infection may play an initiating role in gastric carcinogenesis (18). Based on this hypothesis, intestinal-type gastric adenocarcinoma develops through multistep mucosal changes from superficial gastritis to atrophic gastritis, intestinal metaplasia (IM) and dysplasia (18). Eradication of H pylori is a highly useful method of preventing primary and metachronous gastric cancers (19–21). We have reported previously that IM and neutrophil infiltration (NI) are high-risk microenvironments and risk factors for primary gastric cancers (22). However, the risk factors for metachronous cancers are unclear. Thus, the aim of the present study was to evaluate the risk factors for metachronous gastric cancers after ESD in a long-term follow-up.
METHODS
Patients
A total of 201 consecutive patients were treated using ESD at the University of Tokyo Hospital (Tokyo, Japan) between September 2000 and September 2009. All demographic data and endoscopic findings obtained before ESD were evaluated retrospectively from patient records. The location, macroscopic types and histological findings of the tumours were categorized according to the Japanese classification of gastric carcinoma (23). Expanded ESD criteria have been proposed by Gotoda et al (24). All patients were followed up for at least one year. Forty-six patients were excluded from the study: 19 did not meet the ESD specimen histology criteria and required additional surgery, five had remnant stomach cancers, nine were followed for up to one year, four did not have H pylori information and, in nine patients, ESD was performed before enrollment. The present study was approved by the Institutional Review Board of the University of Tokyo.
Disease assessment
Experienced endoscopists performed the gastrointestinal endoscopy procedures, and gastric cancer was diagnosed by histology. Biopsy specimens were obtained from the greater curvature of the antrum and middle corpus to evaluate gastric mucosal status including H pylori, IM and NI before ESD (22). Both a rapid urease test (Helicocheck, Otsuka Pharmaceuticals, Japan) and histopathological examination were performed. A positive result on at least one test was deemed to be evidence of H pylori infection. Blood samples were also collected before ESD to measure serum levels of pepsinogen I and II using ELISA kits (LS test Eiken Kagaku, Inc, Japan). ESD was performed as described previously (8,9). Follow-up endoscopy after ESD for detection of metachronous gastric cancers was scheduled at two and six months, one year and annually thereafter. Lesions detected within one year of initial ESD were regarded as synchronous multiple lesions because microcancers may have been missed at the time of ESD (12,25). Metachronous gastric cancers were defined as new gastric cancers in different areas from the initial lesion and occurring at least one year after the initial ESD. Eradication of H pylori was recommended when possible. Metachronous gastric cancer was defined as the development of a new carcinoma in areas other than the primary gastric cancer site at least one year after ESD. H pylori-positive patients were treated with triple therapy consisting of 200 mg clarithromycin, 750 mg amoxicillin and 30 mg lansoprazole twice daily for one week after ER. Patients in whom H pylori was not eradicated were treated with second-line therapy consisting of 250 mg metronidazole instead of clarithromycin (26). Eradication was confirmed by a negative 13C-urea breath test (8,9).
Statistical analyses
Study subjects were categorized according to the presence of the metachronous gastric cancer. All analyses were performed using JMP version 9 (SAS Institute, USA). Student’s t tests were used for intergroup comparison of mean age, follow-up period and tumour size. The other patient clinical characteristics and histopathological characteristics of the gastric mucosa and cancers were compared using the χ2 test or Fisher’s exact test, as appropriate. Relative risks for metachronous gastric cancer were calculated using the Cox proportional hazards model. Differences were considered to be statistically significant at the 5% probability level.
RESULTS
Baseline characteristics of the study subjects
Baseline clinical characteristics of the patients are summarized in Table 1. A total of 155 patients (119 men, 36 women, mean age 68.9 years) were followed for up to 10.8 years (mean 4.2 years). Six patients died during the study period, but none of the deaths were related to gastric cancer. Twenty-three (14%) patients developed metachronous gastric cancers (3.5% per year).
TABLE 1.
Baseline characteristics of 155 patients
| Total | Metachronous (n=23) | No recurrence (n=132) | P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Sex, male/female, n | 119/36 | 20/3 | 99/33 | 0.19 |
| Age, years, mean (range) | 68.9 (50–83) | 69.2 (57–80) | 68.8 (50–83) | 0.84 |
| Follow-up period, years (range) | 4.23 (1.02–10.8) | 4.11 (1.05–7.35) | 4.25 (1.02–10.8) | 0.78 |
| Outcome (alive/dead) | 149/6 | 23/0 | 126/6 | 0.16 |
| Helicobacter pylori status after endoscopic submucosal dissection, n (%) | 0.17 | |||
| Negative | 25 (16.1) | 1 (4.3) | 24 (18.1) | |
| Eradication | 100 (64.5) | 17 (74.0) | 83 (62.9) | |
| Persistent | 30 (19.4) | 5 (21.7) | 25 (19.0) | |
| Histological characteristics, n (%) | ||||
| Intestinal metaplasia in the antrum | 101 (65.2) | 15 (65.2) | 86 (65.2) | 0.99 |
| Intestinal metaplasia in the corpus | 74 (47.7) | 15 (65.2) | 59 (44.7) | 0.068 |
| Neutophil infiltration in the antrum | 30 (19.4) | 8 (34.8) | 22 (16.7) | 0.056 |
| Neutophil infiltration in the corpus | 50 (32.3) | 15 (65.2) | 35 (26.5) | 0.0004 |
| Pepsinogen, ng/mL, mean (n=127) | ||||
| I | 44.1 | 27.2 | 47.2 | 0.17 |
| II | 16.8 | 13.3 | 17.5 | 0.24 |
| I/II | 2.61 | 2.10 | 2.71 | 0.15 |
| Tumour characteristics | ||||
| Location, upper/middle/lower, n | 14/65/76 | 1/13/9 | 13/52/67 | 0.27 |
| Size, mm, mean | 15.7 | 20.3 | 14.9 | 0.0048 |
| Macroscopic type, protruded/depressed, n | 88/67 | 12/11 | 76/56 | 0.63 |
| Pathology, adenoma/cancer, n | 31/124 | 5/18 | 26/106 | 0.82 |
Of the 155 patients, 25 were H pylori negative, 27 had H pylori eradicated before ESD, 73 received eradication therapy and 30 experienced continuing H pylori infection. IM at the greater curvature of the antrum and middle corpus was observed in 101 (65.1%) and 74 (47.7%) patients, respectively. NI was observed in 30 (19.4%) and 50 (32.3%) patients, respectively.
Factors related to metachronous gastric cancers
The cumulative incidence of metachronous gastric cancer is summarized in Table 2, and Figures 1 and 2. The ORs for metachronous gastric cancers in IM and NI in the corpus were 3.00 (P=0.010) and 3.47 (P=0.0034). IM and NI in the corpus and tumour size were risk factors. Successful H pylori eradication did not contribute significantly to the reduction of risk for metachronous gastric cancer in the present analysis (OR 2.56 [95% CI 0.52 to 46.3]; P=0.30).
TABLE 2.
Cumulative incidence of metachronous gastric cancer, univariate analysis*
| OR (95% CI) | P | |
|---|---|---|
| Demographic characteristics | ||
| Sex | 1.78 (0.61–7.55) | 0.32 |
| Age | 1.02 (0.96–1.08) | 0.60 |
| Helicobacter pylori status after ESD | 0.64 | |
| Negative | 1 | |
| Eradication | 2.56 (0.52–46.3) | 0.30 |
| Persistent | 2.50 (0.40–48.3) | 0.36 |
| Histological characteristics | ||
| Intestinal metaplasia in the antrum | 0.99 (0.43–2.46) | 0.97 |
| Intestinal metaplasia in the corpus | 3.00 (1.29–7.51) | 0.010 |
| Neutrophil infiltration in the antrum | 1.89 (0.76–4.39) | 0.16 |
| Neutrophil infiltration in the corpus | 3.47 (1.51–8.63) | 0.0034 |
| Pepsinogen (measured in 127 patients) | ||
| I | 0.98 (0.96–1.01) | 0.11 |
| II | 0.96 (0.92–1.02) | 0.18 |
| I/II | 0.83 (0.59–1.17) | 0.28 |
| Tumour characteristics | ||
| Location | 0.29 | |
| Upper | 1 | |
| Middle | 2.26 (0.45–41.2) | 0.38 |
| Lower | 1.21 (0.23–22.4) | 0.85 |
| Tumour size | 1.05 (1.01–1.09) | 0.021 |
| Macroscopic type (protruded/depressed) | 0.79 (0.35–1.83) | 0.58 |
| Pathology (cancer/adenoma) | 1.18 (0.47–3.59) | 0.73 |
Cox proportional hazards model. ESD Endoscopic submucosal dissection
Figure 1).
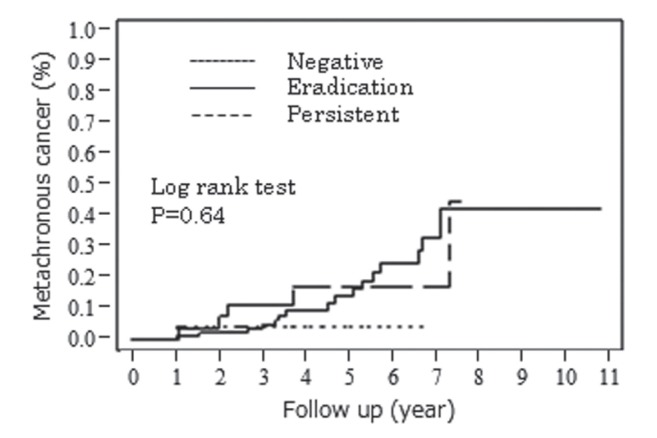
The cumulative incidence of metachronous gastric cancer. Helicobacter pylori status after endoscopic submucosal dissection
Figure 2).
Cumulative incidence of metachronous gastric cancer. A Intestinal metaplasia in the antrum. B Intestinal metaplasia in the corpus. C Neutrophil infiltration in the antrum. D Neutrophil infiltration in the corpus
Multivariate analysis for metachronous gastric cancer
The multivariate analysis of tumour size, IM and NI in the corpus showed that IM and NI in the corpus were independent risk factors for metachronous gastric cancer (Table 3). The ORs for metachronous gastric cancer were 2.65 (95% CI 1.13 to 6.66; P=0.024) in the IM-positive group in the corpus and 3.06 (95% CI 1.32 to 7.64; P=0.0091) in the NI-positive group in the corpus.
TABLE 3.
Multivariate analysis* for metachronous gastric cancer
| Variable | OR (95% CI) | P |
|---|---|---|
| Tumour size | 1.03 (0.99–1.07) | 0.20 |
| Intestinal metaplasia in the corpus | 2.65 (1.13–6.66) | 0.024 |
| Neutrophil infiltration in the corpus | 3.06 (1.32–7.64) | 0.0091 |
Cox proportional hazards model
DISCUSSION
The results of the present single-centre study involving 155 consecutive patients showed that IM and NI in the corpus were related to metachronous gastric cancer. IM and NI were high-risk microenvironments and risk factors for primary and metachronous gastric cancers. Not only H pylori eradication but also annual follow-up endoscopy may be important for improving the prognosis of patients with metachronous gastric cancer.
Although many studies of residual gastric cancer have been conducted (27,28), few reports of metachronous gastric cancers after ESD for early gastric cancer are available. The rate of recurrence of early gastric cancer in the gastric stump has been estimated to be 1% to 3% (13,29). An increased recurrence rate would be expected in patients treated with ESD, in proportion to the larger area of gastric mucosa remaining in these patients. Other studies have reported that the annual incidence of metachronous gastric cancer after EMR is 2.5% to 4% (12,20). In the present study, the annual incidence was approximately 3.5%.
In their randomized controlled study, Fukase et al (20) reported that eradicating H pylori reduced the risk for developing metachronous gastric cancer in patients treated with EMR. In our previous study, we reported that IM, NI and the gastritis pattern are related to developing primary gastric cancer (22). In the present study, our data revealed that IM and NI were also related to metachronous gastric cancer; however, no significant difference in H pylori status was observed. It has been suggested that a larger sample size would demonstrate a significant reduction in the incidence of metachronous gastric cancer after eradicating H pylori. Some studies have reported a significant reduction in cancer development following H pylori eradication in subgroups with mild atrophy or antrum-dominant atrophy (30,31). The preventive effect of H pylori eradication against gastric cancer may depend on the degree of baseline gastric mucosal atrophy and IM. Many patients in our study had IM, which may represent a point of no return at which the development of gastric cancer can no longer be prevented by eradicating H pylori.
In a previous study, we reported that NI in the corpus was related to prevalent diffuse-type gastric cancer (22). In that study, the diffuse type of the metachronous cancers were found in only two cases. A prospective study with a larger number is needed to elucidate the difference between diffuse type and intestinal type cancers.
The annual incidence of metachronous gastric cancer in the present study was 3.5%. The cumulative prevalence of gastric remnant cancer after surgical partial gastrectomy for early gastric cancer is reported to be 2.4% at five years. Patients with early gastric cancer after ESD treatment have been identified as a high-risk group for metachronous gastric cancer. If H pylori is eradicated, the NI status of gastric mucosa improves (32). However, IM of the gastric mucosa cannot easily be improved (33–36). Endoscopists should recognize the characteristics of metachronous gastric cancer and special attention should be devoted to detection of early gastric cancer.
Six patients died during the study period, but none of these deaths were related to gastric cancer. All patients with metachronous gastric cancer were treated completely with ESD or surgery. Annual endoscopy follow-up may be useful for detection of metachronous gastric cancer during the early stage and prevention of gastric cancer death.
A limitation of our study may be a retrospective cohort study in consecutive patients. A prospective study is needed to determine whether baseline histological characteristics can be predictive factors for metachronous gastric cancer and be used to determine the intensity of surveillance endoscopy among this patient population.
CONCLUSION
The presence of IM and NI in the corpus was closely related to the development of metachronous gastric cancer after ESD.
Acknowledgments
The authors thank the gastroenterologists at the University of Tokyo Hospital for their endoscopic procedure. They are grateful to all the pathologists for their histopathological examinations on our subjects.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Curvers WL, van den Broek FJ, Reitsma JB, et al. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach (with video) Gastrointest Endosc. 2009;69:307–17. doi: 10.1016/j.gie.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 2.Polkowski M. Endoscopic diagnosis and treatment of upper gastrointestinal tumors. Endoscopy. 2008;40:862–7. doi: 10.1055/s-2008-1077588. [DOI] [PubMed] [Google Scholar]
- 3.Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;21:2962–7. doi: 10.3748/wjg.14.2962. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto H. Technology insight: Endoscopic submucosal dissection of gastrointestinal neoplasms. Nat Clin Pract Gastroenterol Hepatol. 2007;4:511–20. doi: 10.1038/ncpgasthep0906. [DOI] [PubMed] [Google Scholar]
- 5.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 6.Soetikno R, Kaltenbach T, Yeh R, et al. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–8. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 7.Takenaka R, Kawahara Y, Okada H, et al. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:887–94. doi: 10.1016/j.gie.2008.03.1089. [DOI] [PubMed] [Google Scholar]
- 8.Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: A large-scale feasibility study. Gut. 2009;58:331–6. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto T, Okamoto M, Mitsuno Y, et al. Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: A multicenter feasible study. J Clin Gastroenterol. 2012;46:124–9. doi: 10.1097/MCG.0b013e31822f3988. [DOI] [PubMed] [Google Scholar]
- 10.Jang JS, Choi SR, Qureshi W, et al. Long-term outcomes of endoscopic submucosal dissection in gastric neoplastic lesions at a single institution in South Korea. Scand J Gastroenterol. 2009;44:1315–22. doi: 10.3109/00365520903254304. [DOI] [PubMed] [Google Scholar]
- 11.Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942–8. doi: 10.1016/j.gie.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Nasu J, Doi T, Endo H, et al. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37:990–3. doi: 10.1055/s-2005-870198. [DOI] [PubMed] [Google Scholar]
- 13.Hosokawa O, Kaizaki Y, Watanabe K, et al. Endoscopic surveillance for gastric remnant cancer after early cancer surgery. Endoscopy. 2002;34:469–73. doi: 10.1055/s-2002-32007. [DOI] [PubMed] [Google Scholar]
- 14.Nomura A, Stemmermann GN, Chyou PH, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–6. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 15.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 16.Eslick GD, Lim LL, Byles JE, et al. Association of Helicobacter pylori infection with gastric carcinoma: A meta-analysis. Am J Gastroenterol. 1999;94:2373–9. doi: 10.1111/j.1572-0241.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 17.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 18.Correa P. Human gastric carcinogenesis: A multistep and multifactorial process – First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 19.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA. 2004;291:187–94. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 20.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet. 2008;372:392–7. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 21.Ogura K, Hirata Y, Yanai A, et al. The effect of Helicobacter pylori eradication on reducing the incidence of gastric cancer. J Clin Gastroenterol. 2008;42:279–83. doi: 10.1097/01.mcg.0000248006.80699.7f. [DOI] [PubMed] [Google Scholar]
- 22.Sakitani K, Hirata Y, Watabe H, et al. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol. 2011;26:1570–5. doi: 10.1111/j.1440-1746.2011.06767.x. [DOI] [PubMed] [Google Scholar]
- 23.Association JGC. Japanese classification of gastric carcinoma: Third English edition. Gastric Cancer. 2011;14:101–12. [Google Scholar]
- 24.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–25. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 25.Mitsudomi T, Watanabe A, Matsusaka T, et al. A clinicopathological study of synchronous multiple gastric cancer. Br J Surg. 1989;76:237–40. doi: 10.1002/bjs.1800760308. [DOI] [PubMed] [Google Scholar]
- 26.Maehata Y, Nakamura S, Fujisawa K, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39–46. doi: 10.1016/j.gie.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko K, Kondo H, Saito D, et al. Early gastric stump cancer following distal gastrectomy. Gut. 1998;43:342–4. doi: 10.1136/gut.43.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohashi M, Katai H, Fukagawa T, et al. Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg. 2007;94:92–5. doi: 10.1002/bjs.5538. [DOI] [PubMed] [Google Scholar]
- 29.Takeda J, Toyonaga A, Koufuji K, et al. Early gastric cancer in the remnant stomach. Hepatogastroenterology. 1998;45:1907–11. [PubMed] [Google Scholar]
- 30.Shiotani A, Uedo N, Iishi H, et al. Predictive factors for metachronous gastric cancer in high-risk patients after successful Helicobacter pylori eradication. Digestion. 2008;78:113–9. doi: 10.1159/000173719. [DOI] [PubMed] [Google Scholar]
- 31.Yanaoka K, Oka M, Ohata H, et al. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer. 2009;125:2697–703. doi: 10.1002/ijc.24591. [DOI] [PubMed] [Google Scholar]
- 32.Tari A, Kitadai Y, Sumii M, et al. Basis of decreased risk of gastric cancer in severe atrophic gastritis with eradication of Helicobacter pylori. Dig Dis Sci. 2007;52:232–9. doi: 10.1007/s10620-006-9411-y. [DOI] [PubMed] [Google Scholar]
- 33.Ito M, Haruma K, Kamada T, et al. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: A 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002;16:1449–56. doi: 10.1046/j.1365-2036.2002.01311.x. [DOI] [PubMed] [Google Scholar]
- 34.Kodama M, Murakami K, Okimoto T, et al. Helicobacter pylori eradication improves gastric atrophy and intestinal metaplasia in long-term observation. Digestion. 2012;85:126–30. doi: 10.1159/000334684. [DOI] [PubMed] [Google Scholar]
- 35.Forbes GM, Warren JR, Glaser ME, et al. Long-term follow-up of gastric histology after Helicobacter pylori eradication. J Gastroenterol Hepatol. 1996;11:670–3. doi: 10.1111/j.1440-1746.1996.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 36.Satoh K, Kimura K, Takimoto T, et al. A follow-up study of atrophic gastritis and intestinal metaplasia after eradication of Helicobacter pylori. Helicobacter. 1998;3:236–40. [PubMed] [Google Scholar]