Abstract
OBJECTIVES:
To determine the prevalence, risk factors, physician practice patterns and longitudinal hematological outcome of children following screening for non-anemic iron deficiency (NAID).
METHODS:
The present analysis was a longitudinal cohort study invovling healthy children one to five years of age. Descriptive statistics were used to describe the prevalence, risk factors, practice patterns and hematological outcome of children identified with NAID. The association between NAID and potential risk factors were examined using multivariate logistic regression analysis.
RESULTS:
Of 2276 children undergoing screening, 155 had NAID, corresponding to a prevalence of 7% (95% CI 5.95% to 8.05%). Risk factors significantly associated with NAID included: younger age (OR 1.08 [95% CI 1.06 to 1.11]), higher body mass index z-score (OR 1.22 [95% CI 1.01 to 1.48]), longer duration of breastfeeding (OR 1.05 [95% CI 1.01 to 1.08]) and increased volume of cow’s milk intake (OR 1.13 [95% CI 1.01 to 1.26]). An assessment of practice patterns revealed that for 37% of children, an intervention for NAID was documented; and for 8.4% a physician-ordered follow-up laboratory test was completed to re-evaluate iron status. A total of 58 (37%) children underwent a follow-up laboratory test, of whom 38 (65.5%) had resolution of NAID, 15 (25.9%) had persistence of NAID and two (3.4%) had progression of NAID to anemia.
CONCLUSION:
NAID is common in early childhood and is associated with modifiable risk factors. Substantial practice variation exists in the management of NAID. Further research is necessary to understand the benefits of screening for NAID and evidence-informed practice guidelines may reduce practice variation in the management of NAID in early childhood.
Keywords: Hemoglobin, Non-anemic iron deficiency, Preschool children, Screening, Serum ferritin
Abstract
OBJECTIFS :
Déterminer la prévalence, les facteurs de risque, les profils de pratique des médecins, et les résultats hématologiques longitudinaux des enfants après le dépistage diagnostiqué d’une carence en fer sans anémie (CFSA).
MÉTHODOLOGIE :
La présente analyse était une étude de cohorte longitudinale d’enfants en santé de un à cinq ans. Les chercheurs ont utilisé les statistiques descriptives pour décrire la prévalence, les facteurs de risque et les résultats hématologiques d’enfants atteints d’une CFSA diagnostiquée. Ils ont utilisé l’analyse de régression logistique multivariée pour examiner l’association entre la CFSA et les facteurs de risque potentiels.
RÉSULTATS :
Des 2 276 enfants faisant l’objet du dépistage, 155 étaient atteints d’une CFSA, pour une prévalence de 7 % (95 % IC 5,95 % à 8,05 %). Les facteurs de risque qui s’associaient de manière significative à une CFSA incluaient un âge plus jeune (RC 1,08 [95 % IC 1,06 à 1,11]), un écart réduit plus élevé de l’indice de masse corporelle (RC 1,22 [95 % IC 1,01 à 1,48]), un allaitement de plus longue durée (RC 1,05 [95 % IC 1,01 à 1,08]) et une plus grande consommation de lait de vache (RC 1,13 [95 % IC 1,01 à 1,26]). L’évaluation des profils de pratique a révélé que 37 % des enfants avaient subi une intervention consignée pour soigner la CFSA, et que des médecins avaient demandé un test de laboratoire de suivi pour réévaluer le statut en fer de 8,4 % des cas. Au total, 58 enfants (37 %) ont subi un test de laboratoire de suivi, dont 38 (65,5 %) ont guéri, 15 (25,9 %) ont présenté une CFSA persistante et deux (3,4 %) ont vu leur CFSA se transformer en anémie.
CONCLUSION :
La CFSA, courante dans la petite enfance, s’associe à des facteurs de risque modifiables. On remarque une variation importante de la pratique pour la prise en charge de la CFSA. D’autres recherches s’imposent pour comprendre les avantages de son dépistage. De plus, des directives de pratique factuelles réduiraient peut-être les variations dans les pratiques de prise en charge de la CFSA pendant la petite enfance.
Iron deficiency represents a spectrum ranging from non-anemic iron deficiency (NAID) to iron deficiency with anemia (IDA). NAID represents the early latent phase of iron deficiency (1,2). Evidence suggests that NAID may be associated with adverse neurodevelopmental outcomes in young children (2,3). Furthermore, in the absence of treatment, NAID may progress to IDA, which has been found to be associated with irreversible developmental delay in young children (4–6). These attributes of NAID suggest that the early phase of iron deficiency may meet the criteria required for screening of disease (7,8).
The prevalence of iron deficiency and IDA among toddlers in the United States has been reported to be 9.2% and 2.1%, respectively (2,9). In Canada, although there are no data regarding the national prevalence of iron deficiency and IDA in young children, a review of several small regional studies found the prevalence of iron deficiency and IDA to range from approximately 12% to 64% and 1.5% to 79% (10). These prevalence rates do not signify those of NAID, because NAID represents a particular stage of iron deficiency. Due to lack of screening, specifically using iron-specific indicators, the true prevalence of NAID in preschool children is not well understood. Most developed countries other than the United States do not recommend screening for IDA (2,11–13), hence, how best to screen for and manage NAID in primary care practice remains unclear.
Furthermore, certain characteristics have been found to be risk factors for developing IDA in young children. These include children of families of low socioeconomic status, ethnicity, low birth weight, obesity and certain nutritional behaviours (such as current use of bottle in preschool children, excessive cow’s milk intake, whole cow’s milk during the first year of life and longer duration of breastfeeding) (10,14–21). Due to lack of investigation and identification of young children in their early stage of iron deficiency, there is also a gap in evidence regarding risk factors associated with NAID.
The current study took advantage of the unique opportunity presented through screening of young children for NAID using iron indicators. The overall goal of the present study was to evaluate the clinical practice of screening for NAID followed by treatment and follow-up. Specific objectives included: evaluating the prevalence and risk factors associated with NAID in preschool children; describing physician practice patterns associated with the management of NAID in primary care settings and; describing the longitudinal hematological outcome of children identified with NAID.
METHODS
The present analysis was a longitudinal cohort study involving healthy urban children living in Toronto, Ontario. Data were collected from June 2008 to June 2012. Participants included children 12 to 60 months of age who screened positive for NAID. Children diagnosed with IDA or any other type of anemia, C-reactive protein (CRP) level ≥10 mg/L, a previously diagnosed developmental disorder, a genetic, chromosomal or syndromic condition or a previously diagnosed chronic medical condition (exception of asthma and allergy) were excluded from the study.
Participants were recruited from the TARGet Kids! research network, which is a collaboration between child health researchers at The Hospital for Sick Children (Toronto, Ontario), St Michael’s Hospital (Toronto, Ontario) and primary care physicians within the Greater Toronto Area (www.targetkids.ca) (22). Healthy children, during their scheduled health supervision visits, were approached to participate by trained research assistants embedded in each practice. TARGet Kids! practices offer up to 10 scheduled health supervision visits between two months and five years of age. From June 2008 to September 2013, a total of 13,004 children (zero to 72 months of age) were found to be eligible to participate in the TARGet Kids! program and approximately 39% consented to participate (23).
Approval for data collection was received from the Hospital for Sick Children and St Michael’s Hospital Research Ethics Boards, and informed consent was received by parents of participating children. Data related to health, nutrition and sociodemographic characteristics of children were collected using a standardized parent-completed survey instrument based on the Canadian Community Health Survey (23,24). Trained research assistants obtained height and weight of children using standardized instruments, and also collected a blood sample that was analyzed at Mount Sinai Services laboratory (Toronto, Ontario). Blood analysis included serum ferritin and blood hemoglobin concentration. The laboratory results were sent back to the clinic sites in real-time. All data generated from the TARGet Kids! research initiative were entered into a secure web-based data management system, Medidata Rave (Medidata, USA). From this electronic data capture and repository, it was possible to collect children’s hematological data and identify any iron-related disorders, such as NAID and IDA.
At the clinics, consistent with provincial standards, physicians, once notified of the laboratory results, were at liberty to provide management to children with NAID and document their activity in clinic charts (25). A standardized data collection form was used to abstract the following information from the clinic charts of children who were identified with NAID: documentation of any intervention; types of interventions recommended by physicians to treat NAID and; documentation of any physician ordered follow-up laboratory test to re-evaluate iron status. A period of six months was selected as the follow-up duration because this approximates the time for management and follow-up for children with IDA (26).
The TARGet Kids! program offers parents the opportunity to have blood work for their children at their enrollment visit and also at any subsequent scheduled health supervision visits. Therefore, it was possible to longitudinally follow the hematological outcome of children. For the current study, the hematological outcome of children with NAID was identified either through the TARGet Kids! follow-up blood test results or from the physician-prescribed follow-up blood tests results abstracted from the medical records of TARGet Kids! practices.
Iron status was measured using indicators in the American Academy of Pediatrics guideline for assessment of iron deficiency in young children (hemoglobin and serum ferritin with CRP) (2). Values commonly considered to be low for serum ferritin are 10 μg/L to 12 μg/L (9,11,18). For the present study NAID was defined as a serum ferritin level ≤12 μg/L with CRP <10 mg/L and a normal hemoglobin level (≥110 g/L). IDA was defined as a serum ferritin level ≤12 μg/L with CRP <10 mg/L and a low hemoglobin level (<110 g/L). Iron sufficiency was defined as having both a normal serum ferritin and hemoglobin level (serum ferritin >12 μg/L with CRP <10 mg/L and hemoglobin ≥110 g/L) (1,2,9,27). Serum ferritin was measured using a modular platform (Roche Diagnostics, Switzerland) and hemoglobin was measured using another analysis system (Sysmex Corporation, Japan).
Clinically important covariates included child’s age (months), sex, body mass index (BMI) z-score, birth weight (pounds), total breastfeeding duration (months), current bottle use (yes or no), volume of cow’s milk intake (cups/per day) and maternal ethnicity and education (2,20,21). BMI was calculated as weight in kilograms divided by height in metres squared (28,29). BMI z-scores were calculated using WHO growth standards. Maternal ethnicity was dichotomized to European and non-European groups (East Asian, South Asian, Southeast Asian, West Asian and North African, African, Caribbean, Latin American and others). Maternal education was dichotomized to college/university and high school/elementary school.
Statistical analysis
To assess the representativeness of the sample, children who underwent screening laboratory tests were compared with children who did not, using an independent t test or χ2 test where applicable. The prevalence of NAID was described as percentage of children with NAID from the study sample during the specified study period. Descriptive statistics were used to describe the distribution of the clinically important variables in the NAID and iron sufficient groups. Characteristics were compared using either the independent t test for continuous variables or the χ2 test for categorical variables. The association between NAID (reference group) and all clinically important variables were examined using a multivariate logistic regression analysis, in which clinically important and not highly correlated variables (variance inflation factor for all variables was <2.5) were tested simultaneously (30). Validity of the model was tested using influence diagnostics including Pearson residual, Leverage and DfBetas (plotted against all variables) (30). The plots showed no significant influential points in the model and most cases were well accounted for by the model.
To describe physician practice patterns, the proportion of children with NAID was calculated for whom there was documentation of the following: a physician-prescribed intervention to treat NAID (dietary advice, oral iron, both or other) and a physician-ordered follow-up laboratory test within six months. To describe the hematological outcomes of children with NAID at follow-up, the proportion of children with resolution of NAID, persistence of NAID and progression of NAID to IDA, were calculated.
Statistically significant results were those with values of P≤0.05. All statistical analyses were performed using SAS version 9.1 (SAS Institute, USA).
RESULTS
During the study period, a total of 3814 children were enrolled in the TARGet Kids! research network and a total of 2276 children were screened for iron deficiency. Children who did and did not undergo screening laboratory tests, were similar with respect to independent variables. Therefore, the sample for the present study appeared to be representative of the entire TARGet Kids! sample (data not shown).
According to the definition of NAID used for the present study, 155 children 12 to 60 months of age were identified with NAID. Hence, the prevalence rate of NAID within the study period among children 12 to 60 months of age, enrolled in the TARGet Kids! research network was found to be 7% (95% CI 6.0% to 8.1%). Table 1 shows the distribution of the clinically important variables within NAID and iron-sufficient groups.
TABLE 1.
Comparison between non-anemic iron deficiency (NAID) and iron sufficient children (n=2276)
| Variable |
Group
|
P | |
|---|---|---|---|
| NAID (n=155) | Iron sufficient (n=2121) | ||
| Age, months | 24.70±10.80 | 34.88±13.74 | <0.0001 |
| Sex, n (%) | 0.26 | ||
| Male | 77 (57.89) | 899 (52.79) | |
| Female | 56 (42.11) | 804 (47.21) | |
| Body mass index z-score | 0.39±1.00 | 0.24±1.07 | 0.09 |
| Birth weight, pounds | 6.62±1.51 | 6.53±1.74 | 0.56 |
| Maternal ethnicity, n (%) | 0.01 | ||
| European | 101 (78.91) | 1118 (68.55) | |
| Non-European | 27 (21.09) | 513 (31.45) | |
| Maternal education | 0.14 | ||
| College/university | 123 (93.89) | 1488 (89.96) | |
| High school and public school | 8 (6.11) | 166 (10.04) | |
| Duration of breast feeding, months | 12.18±6.11 | 11.30±6.92 | 0.14 |
| Currently use bottle, n (%) | 0.01 | ||
| Yes | 43 (33.08) | 391 (23.34) | |
| No | 87 (66.92) | 1284 (76.66) | |
| Volume of cows milk, cups per day | 4.39±2.14 | 4.35±1.96 | 0.81 |
Data presented as mean ± SD unless otherwise indicated
In the multivariate analysis, factors found to be significantly associated with NAID were: age (each month decrease in age, children had 1.08 times greater odds of NAID); BMI z-score (each unit increase in BMI z-scores, children had 1.22 times greater odds of NAID); duration of breastfeeding (each month increase in breast-feeding duration, children had 1.05 times greater odds of NAID); and volume of cow’s milk intake (each cup/day increase in cow’s milk intake, children had 1.13 greater odds of NAID) (Table 2).
TABLE 2.
Multivariable analyses of factors associated with non-anemic iron deficiency in children 12 to 60 months of age
| Predictor variable | β coefficient | P | OR (95% CI) |
|---|---|---|---|
| Age, months† | 0.0814 | <0.0001 | 1.08 (1.06–1.11) |
| Body mass index z-score | 0.2011 | 0.04 | 1.22 (1.01–1.48) |
| Birth weight, pounds | −0.0166 | 0.79 | 0.98 (0.87–1.12) |
| Duration of breast feeding, months | 0.0475 | 0.01 | 1.05 (1.01–1.08) |
| Volume of cow’s milk, cups per day | 0.1231 | 0.03 | 1.13 (1.01–1.26) |
| Sex | 0.3645 | 0.09 | 1.44 (0.94–2.20) |
| Female | |||
| Male | |||
| Currently use bottle | −0.3539 | 0.17 | 0.70 (0.42–1.17) |
| No | |||
| Yes | |||
| Maternal ethnicity | 0.3064 | 0.23 | 1.36 (0.83–2.23) |
| European | |||
| Non-European | |||
| Maternal education | 0.1100 | 0.79 | 1.12 (0.49–2.56) |
| College/university | |||
| High school and elementary school |
Decreasing age was significantly associated with greater odds of having non-anemic iron deficiency
Clinic chart documentation showed that of the 155 children with NAID, 57 (37%) were recommended an intervention. Table 3 shows the different types and proportion of interventions that were recommended by physicians to treat NAID. Furthermore, only 13 (8.4%) of the 155 children with NAID had a physician prescribed follow-up laboratory test performed to re-evaluate their iron status. This was 23% of all those who were recommended a treatment (n=57).
TABLE 3.
Interventions recommended by physicians to treat non-anemic iron deficiency (n=57)
| Intervention | n (%) |
|---|---|
| Oral iron | 25 (43.9) |
| Dietary advice | 13 (22.8) |
| Oral iron + dietary advice | 6 (10.5) |
| Dietary advice + others | 4 (7) |
| Others (multivitamins with iron) | 2 (3.5) |
| Not recorded | 7 (12.3) |
For hematological outcome of children identified with NAID, a total of 58 children had a follow-up (TARGet Kids! follow-up or physician prescribed) laboratory test to re-evaluate their iron status. The mean follow-up time was one year. Among them, 38 of 58 (65.5%) children had resolution of NAID, 15 of 58 (25.9%) had persistence of NAID, and two of 58 (3.4%) had progression of NAID to IDA (Figure 1). Of the children with documentation of a recommended intervention, 32 had a follow-up laboratory test with the following results: 22 had resolution of NAID, seven had persistence of NAID, one had progression from NAID to IDA and, in two cases, data were not available. Of children with no documentation of an intervention, 26 had a follow-up laboratory test with the following results: 16 had resolution of NAID, eight had persistence of NAID, one had progression from NAID to IDA and in one case data were missing (Figure 1).
Figure 1).
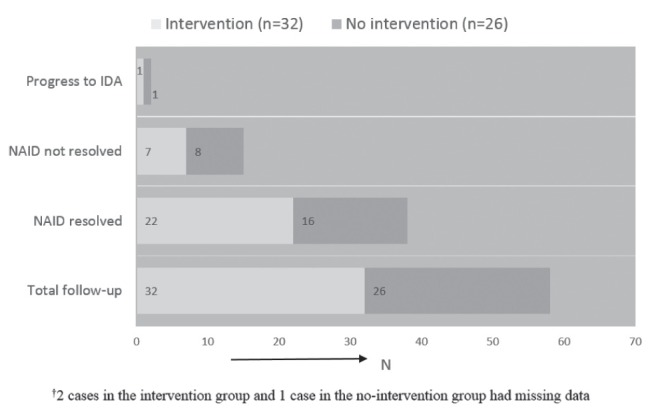
Hematological outcome of children with non-anemic iron deficiency (NAID) categorized as intervention and no intervention groups (n=58). IDA Iron deficiency with anemia
DISCUSSION
The present study showed the prevalence of NAID to be 7% among healthy urban Canadian preschool children (12 to 60 months of age) participating in the TARGet Kids! research network. Furthermore, we identified that children in the younger age group (12 to 36 months), the prevalence of NAID was 12.6%. Our data may be the first to identify the prevalence of NAID in a large sample of urban Canadian children in this age group. The Canadian Health Measures Survey has reported prevalence of anemia (hemoglobin <110 g/L [0.5%]) and low iron stores (serum ferritin <12 μg/L [3.2%]) separately for 487 children three to five years of age (31,32). The lower prevalence in the Canadian Health Measures Survey data compared with our findings may be due to the older age of children in the survey or the different methods of recruitment.
In the present study, we used serum ferritin to screen for NAID in preschool children in the community practice setting; this appears to be feasible, especially in developed countries where the prevalence of IDA is low and sole use of hemoglobin becomes less effective in identifying iron deficiency in children. However, further research is necessary to evaluate and compare the diagnostic properties of serum ferritin with other indicators (such as transferrin receptor and reticulocyte hemoglobin) used to screen NAID in young children (2,33). We also identified risk factors significantly associated with NAID (younger age, higher BMI z-score, longer duration of breastfeeding and greater intake of cow’s milk per day). These modifiable risk factors being associated with the early stage of iron deficiency may be considered by primary care physicians in preventing the development of IDA in young children.
Our study findings also highlight substantial variation in clinical practice with respect to management and follow-up of children identified with NAID on screening. Thirty-seven percent of children identified with NAID on screening had documentation of a recommended intervention, and 8.4% of children had a physician-ordered follow-up laboratory test to re-evaluate their iron status. Also, five different types of interventions were used by clinicians to treat NAID. This level of practice variation suggests substantial physician uncertainty regarding the interventions and follow-up of children identified with NAID. In the absence of clinical guidelines for managing NAID in children, it is possible that physicians extrapolate from clinical guidelines for managing IDA (2,11). Our study, however, showed that approximately one-third of children who had follow-up blood testing had persistent NAID (25.5%) or progressed to IDA (3.4%). The potential for incomplete resolution of NAID emphasizes the need to develop clinical guidelines that not only target IDA, but also NAID, the early latent stage of iron deficiency.
There were several limitations to the present study. First, not all children enrolled in TARGet Kids! underwent laboratory testing. However, comparisons of children who did and did not undergo laboratory testing showed no difference between the two groups. Furthermore, the prevalence of NAID in our study was similar to those reported in other developed countries, supporting the generalizability of our findings (9,11). Second, for the assessment of physician practice, we used clinic chart abstraction and necessarily relied on the presence or absence of documented management as a proxy of actual management. Finally, although we identified 155 children with NAID following screening laboratory testing, only 58 had follow-up laboratory test data. This limits our interpretation of the natural history of NAID and the potential effectiveness of intervention. Furthermore, it is possible that physicians laboratory test-ordering behaviour was influenced by participating in TARGet Kids!, however we are unable to confirm this.
CONCLUSION
We assessed >2000 young children (one to five years of age) following laboratory screening for NAID and identified high prevalence, presence of modifiable risk factors, significant physician practice variation and poor resolution of hematological outcome in children with NAID. To strengthen the findings from the current study, a randomized controlled trial is currently being conducted through our research group to provide further evidence related to screening of NAID, using iron-specific indicators and the effect of NAID on the neurodevelopment of young children (34).
REFERENCES
- 1.World Health Organization . A guide for programme managers. Geneva: World Health Organization; 2001. Iron deficiency anaemia: Assessment, prevention and control. WHO/NHD/01.32001. [Google Scholar]
- 2.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics. 2010;126:1040–50. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 3.Oski FA, Honig AS, Helu B, Howanitz P. Effect on iron therapy on behavior performance in nonanemic, iron-deficienct infants. Pediatrics. 1983;71:877–80. [PubMed] [Google Scholar]
- 4.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 5.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325:687–94. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status. A longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. 2006;160:1108–13. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson JMG, Jungner G. Principles and practices of screening for disease: Public Health paper number 34. Geneva: WHO; 1968. [Google Scholar]
- 8.Criteria for appraising the viability, effectiveness and appropriatness of a screening programme. UK National Screening Committee. < www.screening.nhs.uk/criteria> (Accessed November 3, 2014)
- 9.Centers for Disease Control and Prevention Iron deficiency–United States, 1999–2000. MMWR. 2002;51:897–9. [Google Scholar]
- 10.Hartfield D. Iron deficiency is a public health problem in Canadian infants and children. Pediatric Child Health. 2010;15:347–50. doi: 10.1093/pch/15.6.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domellöf M, Braegger C, Campoy C, et al. Iron requirements of infants and toddlers. JPGN. 2014;58:119–29. doi: 10.1097/MPG.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 12.Screening for iron deficiency anaemia in children under 5 years of age. External review against programme appraisal criteria for the UK National Screening Committee. 2012. < www.screening.nhs.uk/irondeficiency> (Accessed October 2014)
- 13.Feightner JW. Prevention of iron deficiency anemia in infants Canadian task force on the periodic health examination, Canadian guide to clinical preventive health care. Ottawa: Health Canada; 1994. pp. 244–55. [Google Scholar]
- 14.Zlotkin SH, Ste-Marie M, Kopelman H, Jones A, Adam J. The prevalence of iron depletion and iron-deficiency anaemia in a randomly selected group of infants from four Canadian cities. Nutrition Research. 1996;16:729–33. [Google Scholar]
- 15.Christofides A, Schauer C, Zlotkin SH. Iron deficiency anemia among children: Addressing a global public health problem within a Canadian context. Pediatric Child Health. 2005;10:597–601. doi: 10.1093/pch/10.10.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdullah K, Zlotkin S, Parkin P, Grenier D. Iron deficiency anemia in children. Canadian pediatric surveillance program, resource article. 2011. < www.cpsp.cps.ca/publications/resource-articles> (Accessed July 22, 2014)
- 17.Sutcliffe TL, Khambalia A, Westergard S, Jacobson S, Peer M, Parkin PC. Iron depletion is associated with daytime bottle-feeding in the second and third years of life. Arch Pediatr Adolesc Med. 2006;160:1114–20. doi: 10.1001/archpedi.160.11.1114. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JM, Fujii ML, Lamp CL, Lönnerdal B, Dewey KG, Zidenberg-Cherr S. Anemia, iron deficiency, and iron deficiency anemia in 12–36-mo-old children from low-income families. Am J Clin Nutr. 2005;82:1269–75. doi: 10.1093/ajcn/82.6.1269. [DOI] [PubMed] [Google Scholar]
- 19.Soh P, Ferguson EL, McKenzie JE, Homs MYV, Gibson RS. Iron deficiency and risk factors for lower iron stores in 6–24-month-old New Zealanders. Eur J Clin Nutr. 2004;58:71–9. doi: 10.1038/sj.ejcn.1601751. [DOI] [PubMed] [Google Scholar]
- 20.Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: Risk factors and racial/ethnic disparities. Pediatrics. 2007;120:568–75. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- 21.Ohlund I, Lind T, Hornell A, Hernell O. Prediators of iron status in well-nourished 4-y-old children. Am J Clin Nutr. 2008;87:839–45. doi: 10.1093/ajcn/87.4.839. [DOI] [PubMed] [Google Scholar]
- 22. About TARGet Kids! Our Team, Our Research, Participant Resource, Publication. TARGet Kids! collaboration; < www.targetkids.ca> (Accessed March 18, 2014)
- 23.Carsley S, Borkhoff CM, Maguire JL, et al. Cohort Profile: The Applied Research Group for Kids (TARGet Kids!) Int J Epidemiol. 2014 doi: 10.1093/ije/dyu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Statistics Canada SC. Canadian Community Health Survey. 2010. < www.statcan.gc.ca/access_acces/alternative_alternatif.action?l=eng&loc=/imdb-bmdi/instrument/3226_Q1_V1-eng.pdf> (Accessed March 5, 2012)
- 25.Medical Records The College of Physicians and Surgeons of Ontario. < www.cpso.on.ca/policies/policies/default.aspx?ID=1686> (Accessed August 2012)
- 26.Centers for Disease Control and Prevention Recommendations to Prevent and Control Iron Deficiency in the United States. MMWR. 1998;47(RR-3) [PubMed] [Google Scholar]
- 27.Tietz NW, editor. Clinical guide to laboratory tests. Philadelphia: WB Saunders Co; 1995. [Google Scholar]
- 28.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: A validation study. J Pediatr. 1998;132:204–10. doi: 10.1016/s0022-3476(98)70433-0. [DOI] [PubMed] [Google Scholar]
- 29.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other bodycomposition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75:978–85. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 30.Harrell FE. Regression modeling strategies: With application to lenear models, logistic regression, and survival analysis. New York: Springer-Verlag; 2010. [Google Scholar]
- 31.Cooper M, Greene-Finestone L, Lowell H, Levesque J, Robinson S. Iron sufficiency of Canadians. Statistics Canada. 2012;23:1–10. [PubMed] [Google Scholar]
- 32.Tremblay MS, Gorber SC. Canadian Health Measures Survey: Brief overview. Can J Public Health. 2007;98:453–6. doi: 10.1007/BF03405437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas C, Thomas L. Biochemical markers and hematological indices in the diagnosis of functional iron deficiency. Clin Chem. 2002;48:1066–76. [PubMed] [Google Scholar]
- 34.Abdullah K, Thorpe KE, Mamak E, et al. Optimizing early child development for young children with non-anemic iron deficiency in the primary care practice setting (OptEC): Study protocol for a randomized controlled trial. Trials. 2015;16:132. doi: 10.1186/s13063-015-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


