Abstract
Aims/Introduction
The impact of metabolic syndrome (MetS) on the development of type 2 diabetes has been reported in different ethnic populations. However, whether central obesity is an essential component as a diagnostic criterion for MetS remains a controversial topic. The aim of the present study was to investigate the association between MetS and the incidence of type 2 diabetes with or without central obesity in a Japanese American population.
Materials and Methods
We examined whether MetS predicts incident type 2 diabetes among 928 Japanese American participants who did not have diabetes enrolled in an ongoing medical survey between 1992 and 2007. MetS was defined on the basis of American Heart Association/National Heart, Lung, and Blood Institute criteria. The average follow-up period was approximately 6.8 years.
Results
During the follow-up period, 116 new cases of diabetes were diagnosed. Compared to the participants without MetS, the hazard ratio (HR) for incident type 2 diabetes was significantly higher in participants with MetS, after adjustment for sex, age and impaired glucose tolerance (HR 1.64, 95% CI 1.11–2.42). The risk of type 2 diabetes was found to be significantly higher in participants with MetS but without central obesity (HR 2.07, 95% CI 1.25–3.41), as well as in participants with MetS and with central obesity (HR 2.46, 95% CI 1.51–4.01) than in participants with neither MetS nor central obesity, after adjustment for sex, age and impaired glucose tolerance.
Conclusions
These results show that the presence of MetS, with or without central obesity, could independently predict the development of type 2 diabetes in Japanese Americans.
Keywords: Central obesity, Metabolic syndrome, Type 2 diabetes mellitus
Introduction
Metabolic syndrome (MetS) is well known as a risk factor for cardiovascular disease. Visceral fat as a fundamental characteristic of MetS is associated with an increase in future insulin resistance1. Furthermore, it has been reported that MetS is one of the risk factors for the development of type 2 diabetes in different ethnic populations2–12, but only limited data are available on the association between MetS and the risk of type 2 diabetes among the Japanese population13.
Several organizations have proposed criteria for MetS14–17. Among these, in the criteria developed by the International Diabetes Federation (IDF)14 and the Japanese Society of Internal Medicine (Japanese criteria)15, central obesity is defined as an essential component. In contrast, in the criteria formulated by the World Health Organization16 and the American Heart Association/National Heart, Lung and Blood Institute Scientific Statement (AHA/NHLBI)17, central obesity is not defined as an essential component. Controversy has thus surrounded the decision about whether or not the central obesity component should be regarded as a prerequisite for diagnosis of MetS. In addition, no information has been published regarding the association between MetS and the future development of type 2 diabetes on the basis of the presence or absence of central obesity.
Since 1970, we have carried out a comparative medical survey of Japanese Americans immigrating to the USA and native Japanese living in Japan (called the Hawaii–Los Angeles–Hiroshima Study). This project represents a long-term epidemiological study of Japanese Americans who are genetically equivalent to native Japanese living in Japan, but who have undergone a rapid and intense Westernization of lifestyle18. We have shown through the survey that Japanese Americans have high levels of insulin resistance, and increased total cholesterol and triglycerides, thereby showing that a Westernized lifestyle aggravates atherosclerosis risk factors19. In addition, we have reported that a Westernized lifestyle promotes the development of diabetes as a result of insulin resistance20, and increases the prevalence of MetS among Japanese Americans as compared with native Japanese living in Japan21. These results suggest that MetS might increase the prevalence of type 2 diabetes as well as atherosclerosis in Japanese Americans. We therefore investigated the impact of MetS and its individual components, particularly central obesity, with regard to the incidence of type 2 diabetes among Japanese Americans living in Hawaii and Los Angeles.
Materials and Methods
Participants
Study participants were Japanese Americans enrolled two or more times in the Hawaii–Los Angeles–Hiroshima Study between 1992 and 2007. The study population consisted of 379 men and 549 women who did not have diabetes, as ascertained by a 75-g oral glucose tolerance test (OGTT) at baseline. Participants who had a medical history of gastric resection were excluded from the present study. Written informed consent was obtained from all participants. This study was approved by the ethics committee of Hiroshima University, and the Councils of the Hiroshima Kenjin-Kai Associations in Hawaii and Los Angeles.
Measurements
In the morning after an overnight fast, each participant underwent an interview, physical and blood pressure measurements, and venous blood sampling. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Waist circumference was measured at the level of the umbilicus. An OGTT was then carried out at the time of each follow-up examination, and participants were examined at least twice during the study periods. We used the 2003 American Diabetes Association criteria for diabetes and impaired glucose tolerance (IGT)22. Each blood sample was centrifuged, and the obtained serum samples were immediately frozen and stored until analysis. Serum glucose levels were measured by the hexokinase method, insulin levels by a double-antibody radioimmunoassay, triglyceride levels by an enzymatic method, and total and high density lipoprotein (HDL) cholesterol levels by the immunoinhibition method. Insulin resistance was evaluated by homeostasis model assessment of insulin resistance (HOMA-IR).
Diagnostic Criteria for Metabolic Syndrome
MetS was diagnosed according to the AHA/NHLBI17, with waist circumferences modified for Asian populations in accordance with the IDF definition of MetS14. MetS was diagnosed when three or more of the following components were present: (i) central obesity (waist circumference ≥90 cm in men and ≥80 cm in women); (ii) high blood pressure (systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg and/or current use of medication for hypertension); (iii) high triglyceride (triglyceride ≥150 mg/dL and/or current use of medication for dyslipidemia); (iv) low HDL cholesterol (HDL cholesterol <40 mg/dL in men or <50 mg/dL in women and/or current use of medication for dyslipidemia); and (v) impaired fasting glycemia (IFG; fasting glucose ≥100 mg/dL).
Statistical Analysis
Statistical analysis was carried out using the Statistical Package for Social Science (version 12.0 for Windows; SPSS, Chicago, IL, USA). Data were expressed as means ± standard deviation. Categorized data were analyzed by χ2-test, and unpaired Student's t-tests were used for group comparisons (with MetS vs without MetS). Before analysis, the skewed distribution of fasting insulin, HOMA-IR and triglyceride was normalized by logarithmic transformation. Multivariate-adjusted hazard ratios (HRs) and their 95% CIs were estimated with the use of the Cox proportional hazards model. P < 0.05 was considered statistically significant in all analyses.
Results
Baseline characteristics divided into two groups of individuals with or without MetS are shown in Table 1. There was no significant difference in sex ratio. However, in the group with MetS compared with the group without MetS, age, body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, fasting glucose, 2-h glucose, fasting insulin, 2-h insulin, HOMA-IR, total cholesterol, triglycerides and IGT ratio were significantly higher, and HDL cholesterol was lower.
Table 1.
Baseline characteristics of the subjects divided by individuals with or without metabolic syndrome
| With MetS | Without MetS | P | |
|---|---|---|---|
| Men/women | 137/170 | 242/379 | 0.103 |
| Age (years) | 63.7 ± 11.6 | 59.3 ± 14.1 | <0.001 |
| Body mass index (kg/m2) | 25.8 ± 3.5 | 22.5 ± 3.2 | <0.001 |
| Waist circumference (cm) | 84.6 ± 9.0 | 75.5 ± 8.4 | <0.001 |
| Systolic blood pressure (mmHg) | 139 ± 13.8 | 128.0 ± 17.0 | <0.001 |
| Diastolic blood pressure (mmHg) | 78.1 ± 10.2 | 72.6 ± 9.9 | <0.001 |
| Fasting glucose (mg/dL) | 93.4 ± 11.3 | 85.4 ± 9.3 | <0.001 |
| 2-h glucose (mg/dL) | 129.5 ± 30.6 | 105.5 ± 30.1 | <0.001 |
| Fasting insulin (μU/mL)† | 10.2 ± 6.3 | 6.2 ± 3.7 | <0.001 |
| 2-h insulin (μU/mL)† | 80.4 ± 57.7 | 49.5 ± 42.6 | <0.001 |
| HOMA-IR† | 2.4 ± 1.6 | 1.3 ± 0.9 | <0.001 |
| Total cholesterol (mg/dL) | 230.3 ± 37.8 | 220.5 ± 36.4 | <0.001 |
| Triglycerides (mg/dL)† | 239.1 ± 187.6 | 118 ± 69.5 | <0.001 |
| HDL cholesterol (mg/dL) | 44.1 ± 13.6 | 57.0 ± 13.5 | <0.001 |
| Impaired glucose tolerance (%) | 117 (56.0) | 190 (26.4) | <0.001 |
| Developed to diabetes (%) | 67 (21.8) | 49 (7.9) | <0.001 |
MetS, metabolic syndrome; HOMA-IR, homeostasis model assessment of insulin resistance; HDL, high density lipoprotein. Data are expressed as means ± SD. P values are determined by unpaired t test or χ2 test.
Parameters are transformed logarithmically before analysis.
A total of 116 participants developed type 2 diabetes during the follow-up period (6.8 ± 0.1 years). The Cox proportional hazards model was used to examine the association between MetS or its individual components and the development of type 2 diabetes (Table 2). MetS was a significant risk factor for type 2 diabetes (HR 3.08, 95% CI 2.13–4.46, P < 0.001). All individual components of MetS, except for high triglycerides, were also associated with a significantly increased risk of type 2 diabetes. The multivariate analysis for prediction of type 2 diabetes associated with MetS or its individual components after adjustment for sex, age and IGT is shown in Table 2. Only IFG (HR 2.28, 95% CI 1.51–3.44, P < 0.001) among the five components and MetS (HR 1.64, 95% CI 1.11–2.42, P < 0.001) were significantly associated with incident type 2 diabetes. Other individual components as well as central obesity were not independent risk factors for the development of type 2 diabetes. Baseline characteristics of the participants divided by individuals with or without central obesity and MetS are shown in Table 3. In this analysis, MetS was defined as the presence of at least three metabolic syndrome components, not including the component of central obesity. The participants with central obesity and without MetS had higher body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, fasting glucose, 2-h glucose, fasting insulin, 2-h insulin, HOMA-IR and triglyceride, and had lower HDL cholesterol compared with participants without both central obesity and MetS. The participants with MetS, regardless of central obesity, were significantly older and had higher body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, fasting glucose, 2-h glucose, fasting insulin, 2-h insulin, HOMA-IR, total cholesterol, triglycerides and IGT ratio, and had lower HDL cholesterol compared with participants without both central obesity and MetS. We next examined the combined and separate effects of central obesity and MetS on incident type 2 diabetes. The participants with central obesity without MetS did not have significantly higher HR of incident type 2 diabetes (HR 0.98, 95% CI 0.52–1.83, P = 0.939) compared with those without both central obesity and MetS. However, those with MetS had a significantly higher HR of incident type 2 diabetes with or without central obesity (HR 4.17, 95% CI 2.60–6.70, P < 0.001; HR 3.68, 95% CI 2.25–5.99, P < 0.001), respectively. After adjusting for sex, age and IGT, the participants with central obesity but without MetS did not have a significantly higher HR of incident type 2 diabetes (HR 0.85, 95% CI 0.45–1.60, P = 0.612) compared with those without central obesity and without MetS (Figure 1). However, those with MetS had a significantly higher HR of incident type 2 diabetes with or without central obesity (HR 2.46, 95% CI 1.51–4.01, P < 0.001; HR 2.07, 95% CI 1.25–3.41, P = 0.004), respectively.
Table 2.
HRs for the development of type 2 diabetes associated with metabolic syndrome and its individual components
| Crude HR (95% CI) | P | Multivariate-adjusted HR (95% CI) | P | |
|---|---|---|---|---|
| Presence of MetS | 3.08 (2.13–4.46) | <0.001 | 1.64 (1.11–2.42) | <0.001 |
| Central obesity (waist circumference ≥90 cm [men], ≥80 cm [women]) | 1.96 (1.35–2.85) | <0.001 | 1.28 (0.87–1.88) | 0.214 |
| High blood pressure (BP) (BP ≥ 130/85 mmHg or medication use) | 2.16 (1.41–3.30) | <0.001 | 1.21 (0.76–1.92) | 0.424 |
| High triglycerides (TG) (TG ≥ 150 mg/dL or medication use) | 1.35 (0.94–1.90) | 0.108 | 0.85 (0.58–1.24) | 0.393 |
| Low HDL cholesterol (HDL-C) (HDL-C <40 mg/dL [men], <50 mg/dL [women] or medication use) | 1.61 (1.12–2.32) | 0.010 | 1.27 (0.88–1.83) | 0.207 |
| Impaired fasting glycemia (fasting glucose ≥100 mg/dL) | 5.26 (3.61–7.67) | <0.001 | 2.28 (1.51–3.44) | <0.001 |
Multivariate adjustment was made for sex, age, and IGT. HR, hazards ratio; CI, confidence interval; MetS, metabolic syndrome.
Table 3.
Baseline characteristics of the subjects divided by individuals with or without central obesity and metabolic syndrome
| Without central obesity, without MetS | With central obesity, without MetS | Without central obesity, with MetS | With central obesity, with MetS | |
|---|---|---|---|---|
| Men/Women | 187/283 | 78/112 | 65/77 | 49/77 |
| Age (years) | 59.6 ± 14.5 | 60.2 ± 13.7 | 63.6 ± 11.5* | 62.8 ± 10.3* |
| Body mass index (kg/m2) | 21.9 ± 2.8 | 26.2 ± 3.4* | 22.9 ± 2.6* | 26.9 ± 3.3* |
| Waist circumference (cm) | 74.2 ± 7.8 | 84.7 ± 9.4* | 77.4 ± 7.2* | 86.3 ± 8.8* |
| Systolic blood pressure (mmHg) | 128.4 ± 17.0 | 132.2 ± 16.3* | 139.0 ± 17.4* | 134.8 ± 12.7* |
| Diastolic blood pressure (mmHg) | 72.6 ± 10.1 | 74.5 ± 10.1* | 78.3 ± 10.3* | 76.8 ± 9.8* |
| Fasting glucose (mg/dL) | 85.7 ± 9.9 | 88.2 ± 10.8* | 90.9 ± 11.0* | 93.3 ± 10.4* |
| 2-h glucose (mg/dL) | 105.1 ± 30.4 | 114.2 ± 29.7* | 126.1 ± 32.8* | 129.4 ± 31.7* |
| Fasting insulin (μU/mL)† | 5.9 ± 3.1 | 9.0 ± 5.8* | 7.9 ± 4.6* | 11.1 ± 7.5* |
| 2-h insulin (μU/mL)† | 47.1 ± 36.4 | 68.2 ± 58.7* | 64.2 ± 39.2* | 88.8 ± 71.9* |
| HOMA-IR† | 1.3 ± 0.8 | 2.0 ± 1.4* | 1.8 ± 1.2* | 2.6 ± 1.9* |
| Total cholesterol (mg/dL) | 220.3 ± 37.3 | 223.6 ± 35.4 | 229.1 ± 35.2* | 230.6 ± 39.7* |
| Triglycerides (mg/dL)† | 123.8 ± 86.1 | 157.7 ± 160.7* | 226.4 ± 172.5* | 209.5 ± 146.3* |
| HDL cholesterol (mg/dL) | 56.6 ± 14.2 | 51.2 ± 12.5* | 46.8 ± 15.3* | 47.2 ± 15.7* |
| Impaired glucose tolerance (%) | 68 (14.5) | 38 (20.0) | 55 (38.7)* | 48 (38.1)* |
| Developed to diabetes (%) | 32 (6.8) | 14 (7.4) | 33 (23.2)* | 37 (29.4)* |
Data are expressed as means ± SD. P values are determined by unpaired t-test or χ2 test.
Parameters are transformed logarithmically before analysis. MetS, metabolic syndrome; HOMA-IR, homeostasis model assessment of insulin resistance; HDL, high density lipoprotein.
P < 0.05 compared to by individuals without central obesity and without MetS.
Figure 1.
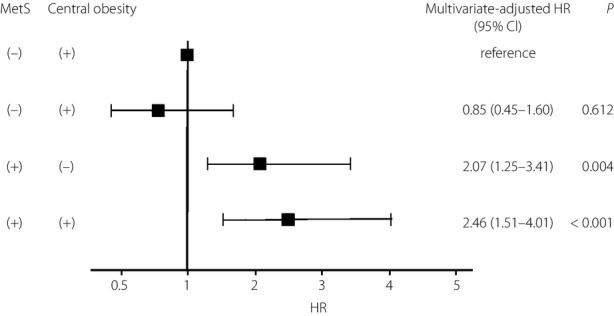
Multivariate-adjusted hazard ratios (HRs) for the development of type 2 diabetes according to the presence or absence of metabolic syndrome (MetS) and central obesity. MetS was defined as the presence of at least three MetS components, not including the component of central obesity. Multivariate adjustment was made for sex, age and impaired glucose tolerance. The centers of the boxes are placed at the estimates of HRs. Error bars indicate 95% confidence intervals (CIs).
Discussion
In the present study, even when participants were stratified by the presence or absence of central obesity, MetS as defined by AHA/NHLBI diagnostic criteria was a significant predictor of type 2 diabetes in a Japanese American population. This result suggests that central obesity is not the indispensable component of MetS for detecting the risk of type 2 diabetes.
In the present study, IFG and MetS itself remained as factors that were significantly associated with an increased risk of incident type 2 diabetes after adjustment for sex, age and IGT. In several studies, IFG was known to be the strongest predictor of type 2 diabetes among the components8,13,23–25. We therefore showed that MetS is an important predictor of type 2 diabetes among Japanese Americans, as is the case with IFG.
Ford et al.23 reported that MetS was a factor in increased risk of incident type 2 diabetes in Europeans, and that central obesity as well as IFG in particular among the components of MetS were strong factors. Central obesity has been reported as an important factor in the development of type 2 diabetes26,27, and is defined as an essential component in some MetS diagnostic criteria, such as those of the IDF14 or the Japanese criteria15. However, in the present study, MetS was not considered a significant risk factor for incident type 2 diabetes after adjustment for sex, age, and IGT when using the IDF and Japanese criteria (P = 0.074 and P = 0.270, respectively; data not shown). The reason for this is that waist circumference might not accurately reflect visceral fat in East Asians. Insulin resistance and risk of type 2 diabetes were reported to be increased by the accumulation of visceral fat, but not subcutaneous fat in Japanese Americans1. However, despite lower levels of overall adiposity, participants in East Asia, mainly Japan, Korea and China, were characterized by a larger relative visceral adipose tissue accumulation than any other ethnic group28. Therefore, central obesity might not accurately express the risk of type 2 diabetes among our study's Japanese American population.
The present study had several limitations. First, we did not evaluate family history, drinking habits, educational status or physical activity, all of which are well-known risk factors for type 2 diabetes. Second, there was a lack of data regarding inflammation and smoking status of the participants. Third, the thresholds of waist circumference for Asians according to the IDF MetS definition used in the present study might not be applicable to a Japanese population. However, the result did not change even when the thresholds of waist circumference in the Japanese criteria (≥85 cm in men and ≥90 cm in women) were used instead of those indicated in the IDF definition for Asians (≥90 cm in men and ≥80 cm in women; data not shown). Further studies are required to determine the appropriate thresholds for assessing the risk of type 2 diabetes in the Japanese population.
In summary, the present study showed that the overlap of multiple risk factors regardless of central obesity was a significant predictor of type 2 diabetes. Accordingly, the AHA/NHLBI diagnostic criteria for MetS could be more useful than other criteria in evaluating the risk of type 2 diabetes in Japanese Americans, and MetS is recognized as a risk factor of cardiovascular disease.
Acknowledgments
We thank the members of the Hiroshima Kenjin-kai organizations of Hawaii and Southern California for their participation and cooperation.
Disclosure
The authors declare no conflict of interest.
References
- 1.Hayashi T, Boyko EJ, McNeely MJ, et al. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–1275. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 2.Wannamethee SG, Shaper AG, Lennon L, et al. Metabolic syndrome vs. Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo C, Williams K, Hunt KJ, et al. The National Cholesterol Education Program-Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 4.Meigs JB, Rutter MK, Sullivan LM, et al. Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care. 2007;30:1219–1225. doi: 10.2337/dc06-2484. [DOI] [PubMed] [Google Scholar]
- 5.Wang JJ, Li HB, Kinnunen L, et al. How well does the metabolic syndrome defined by five definitions predict incident diabetes and incident coronary heart disease in a Chinese population? Atherosclerosis. 2007;192:161–168. doi: 10.1016/j.atherosclerosis.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, D'Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and Metabolic syndrome and incident diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 8.Cheung BM, Wat NM, Man YB, et al. Development of diabetes in Chinese with the metabolic syndrome: a 6-year prospective study. Diabetes Care. 2007;30:1430–1436. doi: 10.2337/dc06-1820. [DOI] [PubMed] [Google Scholar]
- 9.Sattar N, McConnachie A, Shaper AG, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927–1935. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- 10.Cameron AJ, Magliano DJ, Zimmet PZ, et al. The metabolic syndrome as a tool for predicting future diabetes: the AusDiab Study. J Intern Med. 2008;264:177–186. doi: 10.1111/j.1365-2796.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 11.Mannucci E, Monami M, Cresci B, et al. National Cholesterol Education Program and International Diabetes Federation definitions of metabolic syndrome in the prediction of diabetes. Results from the Firenze-Bagno A Ripoli Study. Diabetes Obes Metab. 2008;10:430–435. doi: 10.1111/j.1463-1326.2007.00724.x. [DOI] [PubMed] [Google Scholar]
- 12.Ley SH, Harris SB, Mamakeesick M, et al. Metabolic syndrome and its components as predictors of incident type 2 diabetes mellitus in an Aboriginal community. CMAJ. 2009;180:617–624. doi: 10.1503/cmaj.080972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukai N, Doi Y, Ninomiya T, et al. Impact of metabolic syndrome compared with impaired fasting glucose on the development of type 2 diabetes in a general Japanese population. Diabetes Care. 2009;32:2288–2293. doi: 10.2337/dc09-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 15.Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Definition and the diagnostic standard for metabolic syndrome [in Japanese] Nippon Naika Gakkai Zassi. 2005;94:794–809. [PubMed] [Google Scholar]
- 16.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi S, Okubo M, Yoneda M, et al. A comparison between Japanese-Americans living in Hawaii and Los Angeles and native Japanese: the impact of lifestyle westernization on diabetes mellitus. Biomed Pharmacother. 2004;58:571–577. doi: 10.1016/j.biopha.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Egusa G, Watanabe H, Ohshita K, et al. Influence of the extent of westernization of lifestyle on the progression of preclinical atherosclerosis in Japanese subjects. J Atheroscler Thromb. 2002;9:299–304. doi: 10.5551/jat.9.299. [DOI] [PubMed] [Google Scholar]
- 20.Hara H, Egusa G, Yamakido M, et al. The high prevalence of diabetes mellitus and hyperinsulinemia among the Japanese-Americans living in Hawaii and Los Angeles. Diabetes Res Clin Pract. 1994;24(Suppl):S37–S42. doi: 10.1016/0168-8227(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 21.Yoneda M, Yamane K, Jitsuiki K, et al. Prevalence of metabolic syndrome compared between native Japanese and Japanese-Americans. Diabetes Res Clin Pract. 2008;79:518–522. doi: 10.1016/j.diabres.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 22.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Schulze MB, Pischon T, et al. Metabolic syndrome and risk of incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Cardiovasc Diabetol. 2008;7:35. doi: 10.1186/1475-2840-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols GA, Moler EJ. Diabetes incidence for all possible combinations of metabolic syndrome components. Diabetes Res Clin Pract. 2010;90:115–121. doi: 10.1016/j.diabres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Zeng P, Zhu X, Zhang Y, et al. Metabolic syndrome and the development of type 2 diabetes among professionals living in Beijing, China. Diabetes Res Clin Pract. 2011;94:299–304. doi: 10.1016/j.diabres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Ledoux M, Lambert J, Reeder BA, et al. Correlation between cardiovascular disease risk factors and simple anthropometric measures: Canadian Heart Health Surveys Research Group. Can Med Assoc J. 1997;57(Suppl. 1):S46–S53. [PubMed] [Google Scholar]
- 27.Mamtani MR, Kulkarni HR. Predictive performance of anthropometric indexes of central obesity for the risk of type 2 diabetes. Arch Med Res. 2005;36:581–589. doi: 10.1016/j.arcmed.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 28.Nazare JA, Smith JD, Borel AL, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-abdominal adiposity. Am J Clin Nutr. 2012;96:714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]


