Abstract
Background:
Global cerebral ischemia (GCIR) arises in patients that are shown a variety of clinical difficulty including cardiac arrest, asphyxia, and shock. In spite of advances in understanding of the brain, ischemia and protective effects to improve ischemic injury still remain unknown. The aim of our study was to investigate the effect of ellagic acid (EA) pretreatment in the rat models of global cerebral ischemia reperfusion.
Methods:
This experimental study was conducted in 2014 at the Physiology Research Center of the Ahvaz Jundishapur University of Medical Sciences in Ahvaz, Iran. Adult male Wistar rats (250–300 g) were used in this study. GCIR was induced by bilateral vertebral and common carotid arteries occlusion (4-VO). 32 rats were divided randomly to four groups: 1) So (Sham) received normal saline as vehicle of EA, 2) EA, 3) normal saline + GCIR, and 4) EA + GCIR. After anesthesia (a mix of xylazine and ketamine), animal subjected to 20 minutes of ischemia followed by 30 minutes of reperfusion in related groups. EA (100 mg/kg, dissolved in normal saline) or 1.5 ml/kg normal saline was administered (gavage, 10 days) to the related groups. EEG was recorded from NTS in GCIR treated groups.
Results:
Present data showed that: 1) EEG in GCIR treated groups was flattened; 2) Blood pressure, voltage of QRS and P-R interval were reduced significantly in the ischemic groups compared to before ischemia, and pretreatment with EA prevented this reduction; and 3) MDA level and heart rate was increased by GCIR and pretreatment with EA reduced MDA level and restored the HR to normal level.
Conclusion:
Results indicate that global cerebral ischemia-reperfusion impairs certain heart functions and ellagic acid as an antioxidant can restore these parameters. The results of this study suggest the possible utility of ellagic acid in patients with brain stroke.
Keywords: Global cerebral ischemia, Ellagic acid, Rat, ECG, Blood pressure, MDA
1. Introduction
According to the report of World Health Organization (WHO) in developed countries, stroke is ranked as the second leading cause of death (1). Ischemic stroke is the most wrecked kind of stroke. Among several types of models for ischemia, high level mortality rates are shown for global cerebral ischemia (2), which occurs in patients who have a variety of clinical conditions, such as: shock, cardiac arrest, and in patients undergoing complex cardiac surgery (3). The severity of brain injury is dependent on the magnitude and duration of the interruption in the blood supply and subsequent damage induced by reperfusion (4). The solitary tract conveys afferent information from chemoreceptors and stretch receptors in the walls of the cardiovascular (5).
The role of solitary nucleus (or solitary tract) in cerebral ischemia was recognized in clinical and experimental animal models (6). The entry point for integration center in the brain that control autonomic nervous visceral circulatory, digestive, immune, and the larynx function is the nucleus tractus solitarii (NTS) and also has an important role in relay for vagal reflex (7). A variety of visceral mechanical and chemical signals and nociceptive stimuli travel to the nucleus tractus solitarii via vagus nerve fibers. For that reason, the peripheral nerve is in close contact with the central nucleus tractus solitarius. Hence, acupuncture stimulation on the body and the harmful stimulation to inner organs may converge at the nucleus tractus solitarii. Therefore, inner organ functions may be ultimately influenced with a “comprehensive contribution” (8). Some of the research on this matter has indicated that solitary nucleus is more susceptible to destruction on middle cerebral arteries occlusion (9), but some reports have shown the role of solitary nucleus on global cerebral ischemia patients (10).
Heart rate, blood pressure (BP) and electrocardiograph (EKG) were changed in globally ischemic brain. Previous studies indicated the regulatory role of heart related physiological parameters in cerebral ischemia (11, 12). Altogether, there is no exact clinical drug for stroke, so studies have historically focused on attenuating the destructive role of ischemia in the brain. The level of reactive oxygen species (ROS) increases during ischemia and then leads to oxidative stress (13). Therefore, using antioxidant scavengers such as polyphenol compounds is more useful in reducing the ROS. As the previous study has shown, the effects of these compounds are known in some diseases, such as cardiovascular disease (14). Ellagic acid is a polyphenol compound. Ellagic acid (EA) is one of the natural phenol antioxidant found in numerous fruits such as blackberry, pomegranate, strawberry, grape, apple, kiwi, various vegetables, and nuts (15). Polyphenol administration partially (for malondialdehyde levels) or completely (for superoxide dismutase and total antioxidant capacity) reduced the oxidative stress induced by ischemia/reperfusion injury (16). It was reported that EA enhanced antioxidant related enzymes such as superoxide dismutase, catalase and gluthatione level in ischemic brain (17). Therefore, EA is one of the best candidates for ischemia related researches.
Taken together in the current study, we examined the role of EA in global cerebral ischemia rats. First, severe ischemia group (4-vessel occlusion (4-VO)) underwent near-complete ischemia as showed by electroencephalography acknowledged ischemic neuronal damages. Second, we used ellagic acid to remedy ischemia, and then we measured the heart rate, BP and EKG parameters.
2. Material and Methods
2.1. Study design and setting
This experimental study was conducted in 2014 at the Physiology Research Center of the Ahvaz Jundishapur University of Medical Sciences in Ahvaz, Iran. The present study included 32 male Wistar rats that were selected according to previous studies (18).
2.2. Chemicals
Ketamine and xylazine (Alfasan Co., Holland) were used for anesthesia. Ellagic acid (EA) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Co., USA). EA was dissolved in DMSO/normal saline (10%), buffered to a pH of 7, and gavaged in such way that the rats received 100 mg/kg/day. The solution of DMSO/normal saline (10%) as the solvent was also gavaged at 1.5 ml/kg/day to the related group. The dissolved EA was prepared daily.
2.3. Animals
In the present study, 32 adult male Wistar rats (250–300 g), purchased from Animal House of Ahvaz Jundishapur University of Medical Sciences, were housed in cages under the following conditions: controlled temperature (22±2 °C), 50% humidity, and 12/12- hr light/dark cycle (light on 07:00–19:00 h). The rats had free access to tap water and standard rat pellet diet (Pars Co. IR). Before the commencement of testing; all animals were slightly handled for 5 days (10 minutes daily). The experiments were approved by the Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (No. ajums. B-9348). All efforts were made to minimize animal suffering, and to reduce the number of animals used (mortality rate is 30% in ischemic groups).
2.4. Experimental protocols
Rats were divided randomly into four groups of eight rats (in accordance with previous studies) (18), as follows: 1) sham-operated (SO) were only given 1.5 ml/kg/day of solvent orally by gavage for 10 consecutive days; 2) ellagic acid group (EA); 3) global cerebral ischemic-reperfusion (GCIR) group where animals received 1.5 ml/kg of solvent by gavage for 10 consecutive days and then were exposed to global cerebral ischemia for 20 minute and 30 minute reperfusion (19); and 4) global cerebral ischemic-reperfusion + ellagic acid (EA+GCIR) group where animals received ellagic acid (100 mg/kg/day) in 1.5 ml/kg of solvent orally by gavage for 10 consecutive days and then were exposed to global cerebral ischemia (20 minutes) followed by 30 minutes of reperfusion; animals received only ellagic acid (100 mg/kg/day) dissolved in 1.5 ml/kg solvent by gavage for 10 consecutive days. The dose of EA (100 mg/kg) was selected based on previous reports (20, 21) and our pilot experimental study (22). Induction of ischemic-reperfusion was carried out 24 hours after last gavage.
2.5. Surgery
Rats were anesthetized by intraperitoneal injection of ketamine/xylazine (50/5 mg/kg). The animal’s body temperatures were maintained at 36.5±0.5 °C using heating pads with their heads mounted in a stereotaxic device for electrode implantation surgery. A coated stainless steel Teflon bipolar metal wire electrode (0.005″ bare, 0.008″ coated, A-M systems, Inc. WA) was implanted in the NTS (SolM) with stereotaxic coordination of AP=−14.04 mm; ML= 0.4 mm to bregma, and DV=8 mm from the dura, correspondingly. Implantation of electrodes at the right location was determined by histological verification at the end of experiments. All implants were fixed to the skull by dental acrylic cement and two glass anchor small bolts (23, 24).
2.6. Local EEG recording
After induction of anesthesia, electrical field potentials (local EEG) from the NTS of rats were fed to a ML135 bio-amplifier (AD Instruments, 4-Channels Power Lab, Lab Chart software version 7, Australia) with 1 mV amplification, sample recording 400 Hz, and 0.3–70 Hz band pass filtration for 5 minute. The basic EEG variations period of 5 seconds were compared in all groups. Electrical power of frequency bands were measured as µV2/Hz (24). The local EEG recording was done 1st and 11th days before and after I/R initiation correspondingly. The animals were allowed to recover for ten days before the commencement of experiments.
2.7. Induction of global cerebral ischemic-reperfusion
On the first day, after anesthesia, animals underwent transient forebrain global ischemia, as described by Pulsinelli et al. (25). A neck ventral midline incision was made and the common carotid arteries were exposed and separated from the vagus nerves. Then, a sterile field around each common carotid artery was slowly placed, without interrupting the carotid blood flow; the incision was then sutured. A second incision, 1 cm in length, covering direct occipital bone at the back of the first two cervical vertebrae was then made. The muscles around the spine from the midline, separating the left and right alar hole of the first cervical vertebrae were (26) thus exposed. Vertebral artery spinal canal passed under the alar foramen before the posterior fossa. 0.5 mm diameter electrocautery needles (Bowie Monopolar Electrocautery, Cincinnati, Ohio) were placed through each vertebral artery foramen electrocauterized alar, and were permanently blocked.
On the second day, under anesthesia, both CCA were occluded by microclamps for 20 minutes (19) to produce 4-vessel occlusion (25). This demonstrated that, in this study, the method of 4-vessel occlusion (4VO) was indeed carried out. Reperfusion (30 minute duration) was started by opening the carotid clamps after 20 minutes of ischemia. Those rats with 4VO were only involved in tests if their EEG was flattened during the ischemia period (27). Similar procedures were carried out in SO and EA groups without vertebral arteries electro-coagulation and carotid arteries occlusion. (28). Blood samples were collected from the heart, and after, coagulation at room temperature was centrifuged at 4000 rpm (10 minutes at ambient temperature). The collected serum samples were stored at − 80 °C until analysis.
2.8. Electrocardiogram recording method
Fifteen minutes after anesthesia, a standard bipolar limb lead II electrocardiogram was recorded to determine heart rates (To assess chronotropic effect), P-R interval (To assess dromotropic effect) and voltage of QRS (To assess inotropic effect). ECG was recorded on the first and 11th days (before and after ischemia) by Bio-Amp and monitored by a Power Lab system (AD-Instruments, Australia) (Figure 1) (18).
Figure 1.
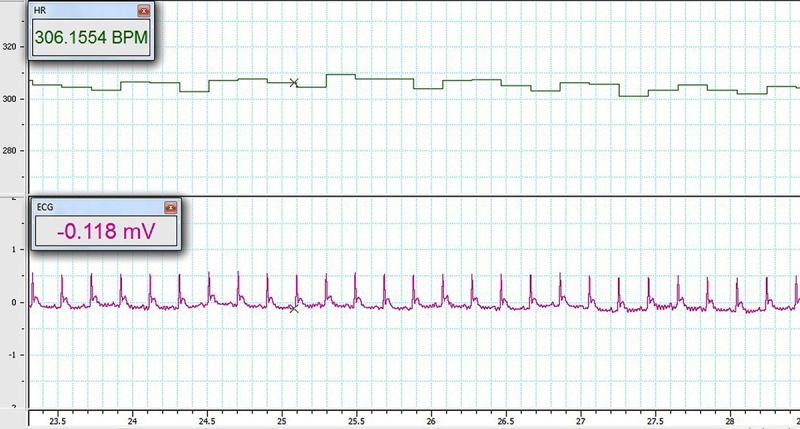
A typical recording of ECG
2.9. Systolic blood pressure measurement
Systolic blood pressure (SBP) was measured in anesthetized rats using a tail-cuff method (Power Lab system, AD-Instruments, Australia). The SBP was recorded on the first and 11th days, before and after ischemia (18).
2.10. Measurement of the malondialdehyde
Malondialdehyde (MDA) levels were measured as a marker of lipid peroxidation, using the double heating method of Satoh (29). The principle of this method is spectrophotometric (Shimadzu UV-1208, at 532 nm, Japan) measurement of the color generated by the reaction of thiobarbituric acid (TBA) with MDA. The concentration of MDA was calculated by the absorbance coefficient of the MDA–TBA complex (absorbance coefficient of 1.56 × 105 cm-1 M-1) and is expressed as μmol/l.
2.11. Statistical analysis
The data obtained for systolic blood pressure and electrocardiogram parameters were analyzed using Paired t-test and one-way analyses of variance (ANOVA), followed by LSD (Least Significant Difference) as a post hoc test for multiple comparisons. The results obtained from MDA measurement were analyzed using one-way analyses of variance (ANOVA). The Data were expressed as the mean ± the standard error of the mean (SEM). P-values less than 0.05 were considered to be statistically significant.
3. Results
3.1. ECG results
A Normal recording of ECG before global cerebral ischemia is shown in figure 1.
3.2. EEG results
The EEG was recorded from nucleus tractus solitaries before and during ischemia (20 minutes) and followed by 30 minutes of reperfusion. The flattening EEG served as the indication of the global cerebral ischemia; this is shown in Figure 2. However, the reperfusion did not return EEG records to normal state.
Figure 2.
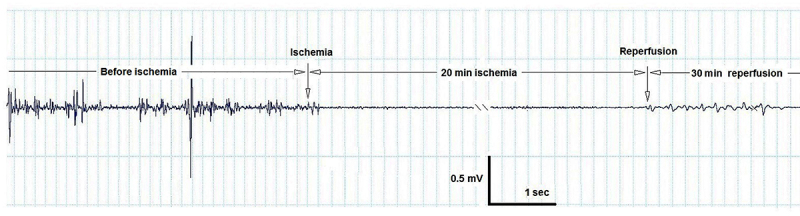
A typical recording of EEG from nucleus tractus solitarius, during global cerebral ischemia and reperfusion periods.
3.3. Effect of EA on impaired BP induced by GCIR
On the 11th day, a comparison of blood pressures (BP) from different groups did not show significant change before induction of ischemia. BP was significantly reduced after ischemia and during reperfusion period in the GCIR and EA+GCIR groups (119.31±4.11vs 83.17±4.64, 120.06±2.92 vs. 99.29±3.51mmHg, p<0.001) (Figure 3). After ischemia, the data analysis showed that those pretreated with EA at dose 100 mg/kg for ten days in EA+GCIR group were significantly higher than the GCIR group (99.29±3.51 vs. 83.17±4.64, p<0.001) (Figure 3).
Figure 3.
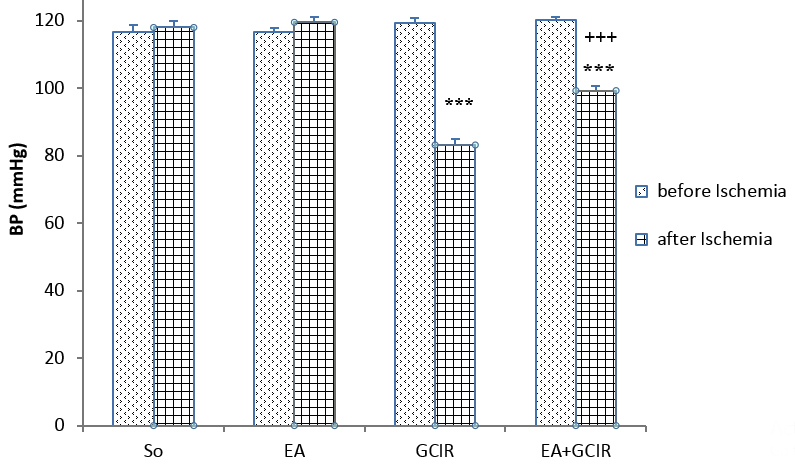
Comparison of blood Pressure before and after ischemia in different groups [SO (Sham), EA (Ellagic Acid 100 mg/kg, 10 days), GCIR (Global Cerebral Ischemia Reperfusion), EA +GCIR (Ellagic Acid 100 mg/kg, 10 days +Global Cerebral Ischemia Reperfusion)]. ***p<0.001, Significant differences before and after ischemia in each group and +++p<0.001 significant compared to GCIR group. (n=8, mean±SEM, paired t-Test or one-way ANOVA followed by LSD).
3.4. Effect of EA on impaired HR (Heart Rate) induced by GCIR
On the 11th day, a comparison of HR from different groups did not show significant change before induction of ischemia. HR was significantly increased after ischemia and during reperfusion period in the GCIR and EA+GCIR groups (257.90±5.79 vs. 352.97±9.30, 258.07±3.88 vs. 295.30±5.63 beats/minutes, p<0.001) (Figure 4). After ischemia, the data analysis showed that those pretreated with EA at dose 100 mg/kg for ten days in EA+GCIR group were significantly lower than the GCIR group (352.97±9.30 vs. 295.30±5.63 beats/min, p<0.001) (Figure 4).
Figure 4.
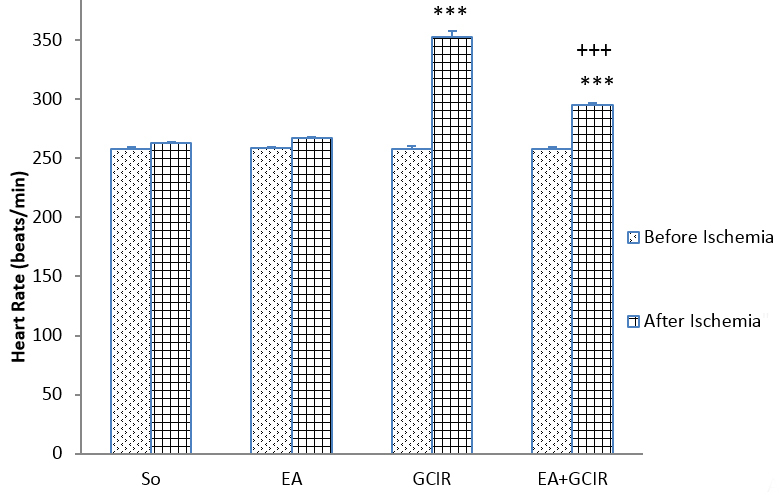
Comparison of Heart rate before and after ischemia in different groups [SO (Sham), EA (Ellagic Acid 100 mg/kg, 10 days), GCIR (Global Cerebral Ischemia Reperfusion), EA +GCIR (Ellagic Acid 100 mg/kg, 10 days + Global Cerebral Ischemia Reperfusion)]. ***P<0.001, Significant differences before and after ischemia in each group and +++p<0.001 significant compared to GCIR group. (n=8, mean±SEM, paired t-Test or one-way ANOVA followed by LSD).
3.5. Effect of EA on impaired Voltage of QRS induced by GCIR
On the 11th day, a comparison of voltage of QRS from different groups did not show a significant change before induction of ischemia. Voltage of QRS significantly reduced after ischemia and during the reperfusion period in the GCIR (0.61±0.031 vs. 0.44±0.041 mv, p<0.001) and EA+GCIR groups (0.62±0.101 vs. 0.67±0.043, p<0.01). After ischemia, the data analysis showed that those pretreated with EA at dose 100 mg/kg for ten days in EA+GCIR group were significantly higher than the GCIR group (0.67±0.043 vs. 0.44±0.041 mv, p<0.001).
3.6. Effect of EA on impaired PR Interval induced by GCIR
On the 11th day, a comparison of PR interval from different groups did not show a significant change before induction of ischemia. PR interval was significantly reduced after ischemia and during reperfusion period in the GCIR and EA+GCIR groups (0.050±0.0013 vs. 0.039±0.0019, 0.051±0.0017 vs. 0.044±0.0015, ms, p<0.001). After ischemia, the data analysis showed that those pretreated with EA at dose 100 mg/kg for ten days in EA+GCIR group were significantly higher than the GCIR group (0.044±0.0015 vs. 0.039±0.0019 ms, p<0.001).
3.7. Effect of EA on impaired MDA activity induced by GCIR
Serum MDA levels in the GCIR group were significantly higher than the SO group (p<0.001). Serum MDA levels in the pretreated group (EA+GCIR) was higher than the SO group without significant change (p>0.05), however was significantly lower than the CGIR group (p<0.001). Serum MDA levels in the EA group, which was only pretreated with EA at dose 100 mg/kg for ten days, was identical to the SO group (Figure 5).
Figure 5.
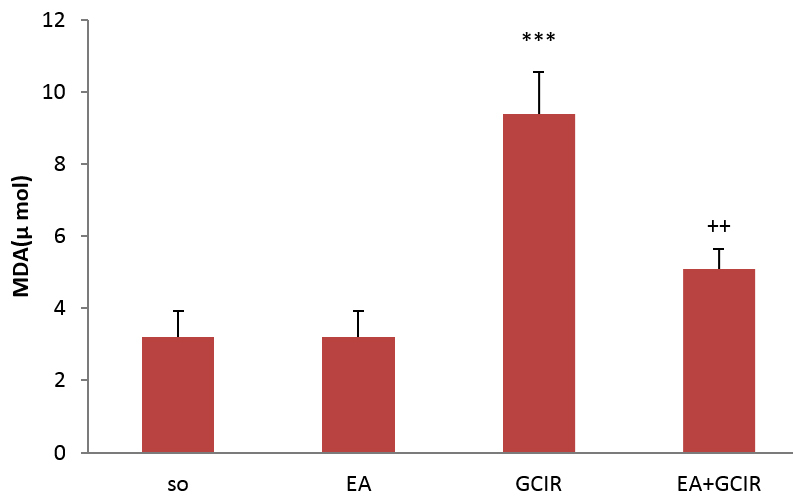
Evaluation of MDA after GCIR in different rats groups [SO (Sham), EA (Ellagic Acid 100 mg/kg, 10 days), GCIR (Global Cerebral Ischemia Reperfusion), EA +GCIR (Ellagic Acid 100 mg/kg, 10 days +Global Cerebral Ischemia Reperfusion)]. ***p<0.001, Significant compared to SO group and ++p<0.001 significant compared to GCIR. Results are expressed as mean±SEM with eight rats per group; one way ANOVA followed by post-hoc LSD test was used.
4. Discussion
The current study’s results demonstrate that global ischemia reperfusion decreased BP and increased HR in the experimental models and pretreatment of animals by EA and improved BP and decreased HR in the ischemic rats. Furthermore, ECG graph analysis indicates that ischemia disrupted the QRS and PR interval and, conversely, treatment of rats by EA improved QRS voltage and enhanced PR interval compared to ischemic group. Finally, through detecting the MDA level activity in the blood plasma, we observed that ischemia increased MDA level and expectedly EA attenuated the MDA level. Patients with severe global cerebral ischemia or cardiac arrest might show different results because the hemodynamic effects of the impediment may need a higher perfusion pressure to maintain BP downstream (30). The level of BP increases during ischemia and, as a result, this phenomenon is likely a defense mechanism against ischemic toxicity. It has been investigated that hypertension will reduce the risk of cerebral ischemia (31). Previous studies have indicated that high levels of BP are caused to protect neuronal cells rather than to lower levels of BP in humans (32). During the reperfusion period (after ischemia), BP starts to decrease; this reduction of BP might be related to production of nitric oxide (33). Hence, some agents that could remain the BP at high levels should be introduced. However, some papers have documented that ischemia increased BP level had no role on BP level (34, 35). These different data might be interpreted by the following: different types of cerebral ischemia, duration of ischemia, and time point of BP estimation. EA is a polyphenol with antioxidant properties that play an important role in reducing of BP, and this is well known (11). Our results parallel previous studies that indicate EA increased BP in a global cerebral ischemia model. One hypothesis articulated by Saenz and co-workers is that EA acts in a protective function in BP regulation by modulating nitric oxide level in the endothelial cells (33, 36).
Global cerebral ischemia, as well as cardiac arrest, resulted in a rapid increase of heart rate. An alternative explanation for the elevated heart rate could be discussed that cardiac arrest or global cerebral ischemia potentially results in diminish in parasympathetic cardiac control, increase in sympathetic activity or intrinsic heart rate (37). Khechinashvili et al, have noted that high level of HR leads to increase toxicity of global cerebral ischemia (38). The present study has shown that pretreatment by EA reduced HR after a global cerebral ischemia model was used. Perhaps EA’s shielding role is related to regulation of thyroid and catecholamine, which subsequently caused reduction in HR (39, 40). Several studies have demonstrated the effect of brain ischemia on cardiac function, but there are few existing research papers about the diagnosis and therapeutic problems for cardiologists and neurologists (41). Our data fill this void, showing that the ECG graph changed during and after cerebral ischemia, QRS shape and voltage were altered and the prolongation of PR interval was disrupted (41). Cojocaru et al., 2014 recognized that occlusion of common carotid and vertebral arteries leads to disruption of QRS in ECG (42). The exact mechanism that leads to the development of this arrhythmia is still unclear, however, increasing evidence suggests that it is mainly due to impaired autonomic nervous system regulation (17, 43). In addition, clinical trials reported serious brain damage as well as sympathetic and parasympathetic outflow in the first hours after the ischemic cope with severe secondary complications (44). One clinical evaluation detected that focal cerebral ischemia changed the QRS complex and the ECG graph in patients without heart disease (45). Another proven result recommended that QRS voltage changed in cerebral infraction (46). Moreover, Smith and colleagues in 2009 documented a shortening of cardiac PR interval in ECG after an experimental model of cerebral stroke (47). The data presented here within illustrate the role of cerebral ischemia on some ECG record changes such as QRS voltage and PR interval. Our analyses parallel the opinions of other research concerning the role of cerebral ischemia on ECG alterations. Furthermore, the protective role of EA on ECG waves was distinguished in the cardiac arrhythmias situation (48, 49). Our data indicated that EA improved QRS voltage and PR interval in ischemic rats. Almost certainly, the role of an autonomic nervous system was unavoidable; meaning that global cerebral ischemia interrupted the sympathetic and parasympathetic system (50). It has been widely accepted that EA improved antioxidant enzyme activity in the cells, so we assessed the MDA level in the plasma. Activation of MDA enzymes inhibited the glutathione peroxidas and other antioxidant enzymes activity (51). Elevated levels of MDA in the brain and plasma of ischemic rats are also well known (52–55). An ameliorative role of EA under toxic condition is detected (56). In parallel with the aforementioned reports, this current investigation has confirmed that EA prevents plasma MDA enhancement in ischemic rats. It has been speculated that reduction of MDA and enhancement of other antioxidant related enzymes could improve ECG and heart hemodynamic function in cardiac arrest models (57). Herein, we generalized that MDA levels decreased through EA pretreatment and, consequently, recovered QRS voltage, P-R interval and improved cardiac hemodynamic function such as HR and PB. The precise mechanism of EA has not been fully detected. Notably, Pang and colleagues in 2014 showed that EA induced progression of focal ischemia in rats (58). They administrated the EA by intravenous injection and then assessed the disruptive function of EA (58). The variety of drug administration has an important role on its function and we suggest here that types of drug injection play a key role in utility of the remedy (59).
5. Conclusions
In summary, this study has shown the protective role of orally administered EA as a supplementary pretreatment diet before induction of global cerebral ischemia. EA improved cardiovascular hemodynamic factors such as BP and HR. Additionally, ECG waves have shown to get better by pre-administration of EA, and EA attenuated MDA enzyme levels in the serum. Nevertheless, there are some concerns about the role of EA.
Acknowledgments
The data used in this paper was from a PhD thesis by Mrs. Khojasteh Hoseiny Nejad, a PhD student in the Abadan Arvand International Division, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The authors gratefully acknowledge the help and financial support of the Physiology Research Center of Ahvaz Jundishapur University of Medical Sciences (grant No.B-9348) (Ahvaz, Iran).
Footnotes
Conflict of Interest:
There is no conflict of interest to be declared.
Authors’ contributions:
All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.WHO Fact sheet N°310 2014. [updated 2014; cited]; Available from: http://www.who.int/mediacentre/factsheets/fs310/en/index.html.
- 2.Schweizer S, Meisel A, Märschenz S. Epigenetic mechanisms in cerebral ischemia. J Cereb Blood Flow Metab. 2013;33(9):1335–46. doi: 10.1038/jcbfm.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salazar J, Wityk R, Grega M, Borowicz L, Doty J, Petrofski J, et al. Stroke after cardiac surgery: short- and long-term outcomes. Ann Thorac Surg. 2001;72(4):1195–1201. doi: 10.1016/s0003-4975(01)02929-0. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Su L, Li X, Di W, Zhang X, Zhang C, et al. Remote limb ischemic postconditioning protects mouse brain against cerebral ischemia/reperfusion Injury via up regulating expression of Nrf2, HO-1, NQO-1 in mice. Int J Neurosci. 2015:1–28. doi: 10.3109/00207454. [DOI] [PubMed] [Google Scholar]
- 5.Stedman TL. Stedman’s Medical Dictionary. 28th ed. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 6.Klein KU, Stadie A, Fukui K, Schramm P, Werner C, Oertel J, et al. Measurement of cortical microcirculation during intracranial aneurysm surgery by combined laser-Doppler flowmetry and photospectrometry. Neurosurgery. 2011;69(2):391–8. doi: 10.1227/NEU.0b013e3182178bc9. [DOI] [PubMed] [Google Scholar]
- 7.McGinnis W, Audhya T, Edelson S. Proposed Toxic and Hypoxic Impairment of a Brainstem Locus in Autism. Int J Environ Res Public Health. 2013;10(12):6955–7000. doi: 10.3390/ijerph10126955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JH, Yan J, Chang XR, et al. Effect of electroacupuncture after electrolytic damage nucleus tractus solitarii. Zhongyiyao Linchuang Zazhi. 2010;22(5):414–416. [Google Scholar]
- 9.Liu AJ, Ling G, Wu J, Shen FM, Wang DS, Lin LL, et al. Arterial baroreflex function is an important determinant of acute cerebral ischemia in rats with middle cerebral artery occlusion. Life Sci. 2008;83(11–12):388–93. doi: 10.1016/j.lfs.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Sottiurai VS, Herbert L, Hatter D. Revascularization of cerebral ischemia after previous bilateral extra cranial-intracranial bypass procedures. J Vasc Surg. 1997;26(1):160–3. doi: 10.1016/s0741-5214(97)70163-0. [DOI] [PubMed] [Google Scholar]
- 11.Li LX, Campbell K, Zhao S, Knuckey NW, Meloni BP. The effect of blood pressure (37 vs 45 mmHg) and carotid occlusion duration (8 vs 10 min) on CA1-4 neuronal damage when using isoflurane in a global cerebral ischemia rat model. Brain Res Bull. 2011;86(5–6):390–4. doi: 10.1016/j.brainresbull.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Pomfy M, Franko J. Validation of a four-vessel occlusion model for transient global cerebral ischemia in dogs. J Hirnforsch. 1999;39(4):465–71. [PubMed] [Google Scholar]
- 13.Zhang F, Iadecola C. Temporal characteristics of the protective effect of aminoguanidine on cerebral ischemic damage. Brain Res. 1998;802(1–2):104–10. doi: 10.1016/s0006-8993(98)00557-5. [DOI] [PubMed] [Google Scholar]
- 14.Kannan MM, Quine SD. Ellagic acid ameliorates isoproterenol induced oxidative stress: Evidence from electrocardiological, biochemical and histological study. Eur J Pharmacol. 2011;659(1):45–52. doi: 10.1016/j.ejphar.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Ascacio-Valdés Juan A, Buenrostro-Figueroa José J, Aguilera-Carbó Antonio, Prado-Barragán Arely, Rodríguez-Herrera Raúl, Aguilar Cristóbal N. Ellagitannins: Biosynthesis, biodegradation and biological properties. Journal of Medical Plants Research. 2011;5(19):4696–4703. Available from: http://www.academicjournals.org/JMPR. [Google Scholar]
- 16.Xue R, He J, Wang N, Yao F, Lv J, Wu G. Relationship between transmembrane signal transduction pathway and DNA repair and the mechanism after global cerebral ischemia-reperfusion in rats. Neurosci Bull. 2009;25(3):115–21. doi: 10.1007/s12264-009-8818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang X, Li T, Feng L, Zhao J, Zhang X, Liu J. Ellagic acid-induced thrombotic focal cerebral ischemic model in rats. J Pharmacol Toxicol Methods. 2014;69(3):217–22. doi: 10.1016/j.vascn.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Dianat M, Veisi A, Ahangarpour A, Fathi Moghaddam H. The effect of hydro-alcoholic celery (Apiumgraveolens) leaf extract on cardiovascularparameters and lipid profile in animal model of hypertension induced by fructose. Avicenna J Phytomed. 2015;5(3):203–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Yonekura I, Kawahara N, Nakatomi H, Furuya K, Kirino T. A model of global cerebral ischemia in C57 BL/6 mice. J Cereb Blood Flow Metab. 2004;24(2):151–8. doi: 10.1097/01.WCB.0000096063.84070.C1. [DOI] [PubMed] [Google Scholar]
- 20.Beltz J, McNeil C, Fisher M, et al. Investigation of the antihyperalgesic effects of the pomegranate extract ellagic acid. AANA J. 2008;76(5):365–366. Abstract A6. [Google Scholar]
- 21.Girish C, Koner B, Jayanthi S, Ramachandra R, Rajesh B, Pradhan S. Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fundam Clin Pharmacol. 2009;23(6):735–45. doi: 10.1111/j.1472-8206.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 22.Mansouri M, Naghizadeh B, Ghorbanzadeh B, Farbood Y. Central and peripheral antinociceptive effects of ellagic acid in different animal models of pain. European Journal of Pharmacology. 2013;707(1–3):46–53. doi: 10.1016/j.ejphar.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Cheng CH, Yi P, Chang H, Tsai Y, Chang F. Kappa-opioid receptors in the caudal nucleus tractus solitarius mediate 100 Hz electro acupuncture-induced sleep activities in rats. Evid Based Complement Alternat Med. 2012;2012:715024. doi: 10.1155/2012/715024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkaki A, Eidypour Z, Motamedi F, keramati K, Farbood Y. Motor disturbances and thalamic electrical power of frequency bands’ improve by grape seed extract in animal model of Parkinson’s disease. Avicenna J Phytomed. 2012;2(4):222–232. [PMC free article] [PubMed] [Google Scholar]
- 25.Pulsinelli W, Brierley J. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.STR.10.3.267. [DOI] [PubMed] [Google Scholar]
- 26.Greene E. Transactions Am Philosophical. Philadelphia, USA: 1935. Anatomy of the rat; pp. 127–9. [Google Scholar]
- 27.Diler A, Ziylan Y, Uzum G, Lefauconnier J, Seylaz J, Pinard E. Passage of spermidine across the blood–brain barrier in short recirculation periods following global cerebral ischemia: effects of mild hyperthermia. Neurosci Res. 2002;43(4):335–342. doi: 10.1016/S0168-0102(02)00059-7. [DOI] [PubMed] [Google Scholar]
- 28.Leeuwenburgh C, Heinecke J. Oxidative stress and antioxidants in exercise. Curr Med Chem. 2001;8(7):829–38. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- 29.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by new colorimetric Method. Clin Chim Acta. 1978;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 30.Sumiyoshi M, Kitazato K, Yagi K, Miyamoto T, Kurashiki Y, Matsushita N, et al. The accumulation of brain water-free sodium is associated with ischemic damage independent of the blood pressure in female rats. Brain Res. 2015;1616:37–44. doi: 10.1016/j.brainres.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 31.Herguido M, Carceller F, Madero R, Roda J. Animal survival related to acute blood pressure response in global cerebral ischemia in rats. Neurol Res. 1997;19(4):417–9. doi: 10.1080/01616412.1997.11740835. [DOI] [PubMed] [Google Scholar]
- 32.Smith J, Baumert M, Nalivaiko E, McEvoy R, Catcheside P. Arousal in obstructive sleep apnoea patients is associated with ECG RR and QT interval shortening and PR interval lengthening. J Sleep Res. 2009;18(2):188–95. doi: 10.1111/j.1365-2869.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 33.Seki Y, Tomizawa T, Khechinashvili N, Soda K. Contribution of solvent water to the solution X-ray scattering profile of proteins. Biophys Chem. 2002;95(3):235–52. doi: 10.1016/s0301-4622(01)00260-5. 28. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Kim SH, Choi H. Dehydroepiandrosterone supplements increases malate dehydrogenase activity and decreases NADPH-dependent antioxidant enzyme activity in rat hepatocellular carcinogenesis. Nutr Res Pract. 2008;2(2):80–4. doi: 10.4162/nrp.2008.2.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters J, Ihle P. ECG-synchronized thoracic vest inflation during autonomic blockade, myocardial ischemia, or cardiac arrest. J Appl Physiol. 1992;73(6):2263–73. doi: 10.1152/jappl.1992.73.6.2263. [DOI] [PubMed] [Google Scholar]
- 36.Chakkarapani E, Dingley J, Aquilina K, Osredkar D, Liu X, Thoresen M. Effects of xenon and hypothermia on cerebrovascular pressure reactivity in newborn global hypoxic-ischemic pig model. J Cereb Blood Flow Metab. 2013;33(11):1752–60. doi: 10.1038/jcbfm.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsanos A, Korantzopoulos P, Tsivgoulis G, Kyritsis AP, Kosmidou M, Giannopoulos S. Electrocardiographic abnormalities and cardiac arrhythmias in structural brain lesions. Int J Cardiol. 2013;167(2):328–34. doi: 10.1016/j.ijcard.2012.06.107. [DOI] [PubMed] [Google Scholar]
- 38.Khechinashvili G, Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovasc Dis. 2002;14(2):67–76. doi: 10.1159/000064733. [DOI] [PubMed] [Google Scholar]
- 39.Lindgren A, Wohlfart B, Pahlm O, Johansson BB. Electrocardiographic changes in stroke patients without primary heart disease. Clin Physiol. 1994;14(2):223–31. doi: 10.1111/j.1475-097x.1994.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 40.Aki H, Fujita M, Yamashita S, Fujimoto K, Kumagai K, Tsuruta R, et al. Elevation of jugular venous superoxide anion radical is associated with early inflammation, oxidative stress, and endothelial injury in forebrain ischemia-reperfusion rats. Brain Res. 2009;1292:180–90. doi: 10.1016/j.brainres.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 41.Kannan M, Quine S. Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism. 2013;62(1):52–61. doi: 10.1016/j.metabol.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Woo M, Choi H, Seo M, Jeon H, Lee B. Ellagic acid suppresses lipid accumulation by suppressing early adipogenic events and cell cycle arrest. Phytother Res. 2015;29(3):398–406. doi: 10.1002/ptr.5264. [DOI] [PubMed] [Google Scholar]
- 43.Sander D, Klingelhofer J. Extent of autonomic activation following cerebral ischemia is different in hypertensive and normotensive humans. Arch Neurol. 1996;53(9):890–4. doi: 10.1001/archneur.1996.00550090096015. [DOI] [PubMed] [Google Scholar]
- 44.Mohagheghi F, Khalaj L, Ahmadiani A, Rahmani B. Gemfibrozil pretreatment affecting antioxidant defense system and inflammatory, but not Nrf-2 signaling pathways resulted in female neuroprotection and male neurotoxicity in the rat models of global cerebral ischemia-reperfusion. Neurotox Res. 2013;23(3):225–37. doi: 10.1007/s12640-012-9338-3. [DOI] [PubMed] [Google Scholar]
- 45.Akil E, Tamam Y, Akil M, Kaplan I, Bilik M, Acar A, et al. Identifying autonomic nervous system dysfunction in acute cerebrovascular attack by assessments of heart rate variability and catecholamine levels. J Neurosci Rural Pract. 2015;6(2):145–50. doi: 10.4103/0976-3147.153216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaz-Velez C, Garcia-Castineiras S, Mendoza-Ramos E, Hernandez-Lopez E. Increased malondialdehyde in peripheral blood of patients with congestive heart failure. Am Heart J. 1996;131(1):146–52. doi: 10.1016/s0002-8703(96)90063-0. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Kim C, Lim K, Kim J, Kim S, Yun YP, et al. Increases in blood pressure and heart rate induced by caffeine are inhibited by (−)-epigallocatechin-3-O-gallate: involvement of catecholamines. J Cardiovasc Pharmacol. 2011;58(4):446–9. doi: 10.1097/FJC.0B013E31822D93CB. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimura M, Watanabe Y, Kasai K, Yamakoshi J, Koga T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci Biotechnol Biochem. 2005;69(12):2368–73. doi: 10.1271/bbb.69.2368. [DOI] [PubMed] [Google Scholar]
- 49.Saenz de Tejada I, Carceller F, Fernandez A, Cuevas B, Ortes L, Giménez-Gallego G, et al. Kinins are implicated of the hypotensive effect of acidic fibroblast growth factor. Eur J Med Res. 1997;2(7):302–4. [PubMed] [Google Scholar]
- 50.Powers W, Clarke W, Grubb RL, Jr, Videen T, Adams HP, Jr, Derdeyn C. Lower stroke risk with lower blood pressure in hemodynamic cerebral ischemia. Neurology. 2014;82(12):1027–32. doi: 10.1212/WNL.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madias J. Mechanism of attenuation of the QRS voltage in heart failure: a hypothesis. Europace. 2009;11(8):995–1000. doi: 10.1093/europace/eup127. [DOI] [PubMed] [Google Scholar]
- 52.Bhargava U, Westfall B. The mechanism of blood pressure depression by ellagic acid. Proc Soc Exp Biol Med. 1969;132(2):754–6. doi: 10.3181/00379727-132-34303. [DOI] [PubMed] [Google Scholar]
- 53.Ishiwata S, Nishiyama S, Nakanishi S, Nishimura S, Yanagishita Y, Kato K, et al. [Natural history of 82 patients with hypertrophic cardiomyopathy: follow-up for over ten years] J Cardiol. 1991;21(1):61–73. [PubMed] [Google Scholar]
- 54.Ito Y, Ohkubo T, Asano Y, Hattori K, Shimazu T, Yamazato M, et al. Nitric oxide production during cerebral ischemia and reperfusion in eNOS- and nNOS-knockout mice. Curr Neurovasc Res. 2010;7(1):23–31. doi: 10.2174/156720210790820190. [DOI] [PubMed] [Google Scholar]
- 55.Shinde R, Shinde S, Makhale C, Grant P, Sathe S, Durairaj M, et al. Occurrence of “J waves” in 12-lead ECG as a marker of acute ischemia and their cellular basis. Pacing Clin Electrophysiol. 2007;30(6):817–9. doi: 10.1111/j.1540-8159.2007.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perlaki G, Orsi G, Schwarcz A, Bodi P, Plozer E, Biczo K, et al. Pain-related autonomic response is modulated by the medial prefrontal cortex: An ECG-fMRI study in men. J Neurol Sci. 2015;349(1–2):202–8. doi: 10.1016/j.jns.2015.01.019. doi: http://dx.doi.org/10.1016/j.jns.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 57.Mari Kannan M, Darlin Quine S. Pharmacodynamics of ellagic acid on cardiac troponin-T, lyosomal enzymes and membrane bound ATPases: mechanistic clues from biochemical, cytokine and in vitro studies. Chem Biol Interact. 2011;193(2):154–61. doi: 10.1016/j.cbi.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt J, Crimmins M, Lantigua H, Fernandez A, Zammit C, Falo C, Agarwal S, Claassen J, Mayer SA. Prolonged elevated heart rate is a risk factor for adverse cardiac events and poor outcome after subarachnoid hemorrhage. Neurocrit Care. 2014;20(3):390–8. doi: 10.1007/s12028-013-9909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang G, Shi B, Luo W, Yang J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav Brain Funct. 2015;11:18. doi: 10.1186/s12993-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


