Abstract
Magnesium-based implants exhibit various advantages such as biodegradability and potential for enhanced in vivo bone formation. However, the cellular mechanisms behind this possible osteoconductivity remain unclear. To determine whether high local magnesium concentrations can be osteoconductive and exclude other environmental factors that occur during the degradation of magnesium implants, magnesium salt (MgCl2) was used as a model system. Because cell lines are preferred targets in studies of non-degradable implant materials, we performed a comparative study of 3 osteosarcoma-derived cell lines (MG63, SaoS2 and U2OS) with primary human osteoblasts. The correlation among cell count, viability, cell size and several MgCl2 concentrations was used to examine the influence of magnesium on proliferation in vitro. Moreover, bone metabolism alterations during proliferation were investigated by analyzing the expression of genes involved in osteogenesis. It was observed that for all cell types, the cell count decreases at concentrations above 10 mM MgCl2. However, detailed analysis showed that MgCl2 has a relevant but very diverse influence on proliferation and bone metabolism, depending on the cell type. Only for primary cells was a clear stimulating effect observed. Therefore, reliable results demonstrating the osteoconductivity of magnesium implants can only be achieved with primary osteoblasts.
Keywords: gene expression, magnesium, MG63, osteoblasts, SaoS2, U2OS
Abbreviations
- OB
osteoblasts
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HPSE
Heparanase
- ALP
Alkaline phosphatase
- RANKL
Receptor Activator of NF-κB Ligand
- BSP
Bone sialoprotein
- Cbfa1
Runt-related transcription factor 2
- OC
Osteocalcin
- OPN
Osteopontin
- OPG
Osteoprotegerin
- Col
Collagen
- PCR
Polymerase chain reaction
Introduction
Magnesium-based implants have been studied since the 19th century for different applications.1 Because of their biodegradability, a second operation to remove the implant is not necessary. Despite promising clinical results in humans, this material is not the clinical standard because the degradation mechanisms are not understood in vitro or in vivo. Several factors, such as the microstructure of magnesium or its alloys, pH, temperature, composition of the corrosion medium or body environment, buffering capacity of the solutions, presence of proteins in the medium or adjustment of flow rate of the environmental medium, induce changes in metal degradation behavior.2-5 Moreover, cells also have a direct impact on the corrosion rate.2
However, it has been shown that magnesium-based implants may enhance bone formation in vivo.3,4 Again, the possible underlying cellular mechanisms, such as magnesium storage or cell type-specific changes in metabolism induced by degradation, remain unclear. In this work, we intend to take the first step toward analyzing the effect of excess magnesium on specific bone markers.
Mg2+ is a co-factor for many different enzymes and is important for cell proliferation, protein synthesis and apoptosis.5 Diseases that are correlated with magnesium depletion (i.e., hypomagnesaemia6) are well studied, but very little is known about the effect of locally high magnesium concentrations. It was hypothesized that increased intracellular Mg2+ serves as a primary regulator of the cell cycle by increasing the protein synthesis.7 Here, we studied different parameters that provide hints about the possible osteoconductivity of magnesium. In addition to cell number and viability, we focused on the size of the cells, which is an important factor for cell behavior regarding adhesion and differentiation.8 Moreover, we determined changes in bone-specific gene expression that occur during proliferation and are induced by magnesium. To keep the system as simple as possible, MgCl2 was used.
Cell lines are often used in cell culture experiments because they are usually fast-growing, well-defined cells. Cell lines are highly useful, particularly for non-degradable implant materials. However, the question remains: do they also deliver comparable results in the case of biodegradable magnesium implants? In recent years, the MG63 cell line has become a standard model for bone research in addition to primary human osteoblasts. A literature research using the relevant keywords of this study (e.g., magnesium, primary human osteoblasts, MG63, SaoS2, U2OS) revealed that more than 20% of the publications found were performed with MG63. In addition to this cell line, other human osteosarcoma-derived cell lines such as SaoS2 or U2OS are used as bone models. Altogether, one of these cell lines was used in approximately 60% of the publications. Although cell lines are often used as “bone mimics,” it is questionable whether they are truly representative cells for the study of degrading magnesium.
It is already known that immortalised cell lines and cells that are directly isolated from the tissue can exhibit different characteristics. Primary cells such as human osteoblasts are still able to differentiate on their own, e.g., due to confluency. Moreover, primary human osteoblasts inherently possess senescence control; after a few generations, cells stop division because of telomerase shortening.9 In contrast, the cell lines used in this study were isolated from osteosarcoma and thus showed different characteristics: (1) MG63 cells are capable of producing high levels of fibroblast-like interferon.10 They are not able to form a calcified matrix.11 Recently, it was shown that these cells are capable of producing action potentials similar to primary osteoblasts.12 As mentioned, they are quite frequently used for the examination of different biomaterials.13-16 (2) U2OS was the first cell line to be established from an osteosarcoma in 1964 (e.g., Pautke et al.17). U2OS cells are also not able to differentiate and form a calcified matrix.18,19 Additionally, their proteomic expression indicates that they are closer to fibroblasts than osteoblasts.20 Although they are widely used in anticancer research, they do not play a large role in biomaterial testing. In contrast, (3) Saos2 are often used for this type of research because of their high mineralisation and proliferation capacity.21-23 They also have been shown to be osteoinductive in vivo.24
In this study, proliferating cells (i.e., cultured without factors promoting osteogenic differentiation) were used. Primary human osteoblasts, as well as 3 human osteosarcoma-derived cell lines (MG63, SaoS2 and U2OS), were evaluated for their reaction to enhanced extracellular magnesium concentrations. The aim of our study was to examine whether we can find evidence of an osteoconductive effect of high extracellular magnesium concentrations on bone cells. Because this potential might be different for cell lines and primary cells, we performed a comparative study to determine which type of cell is the most reliable for use in future in vitro investigations of degrading magnesium materials.
Results
Osmolality of the media
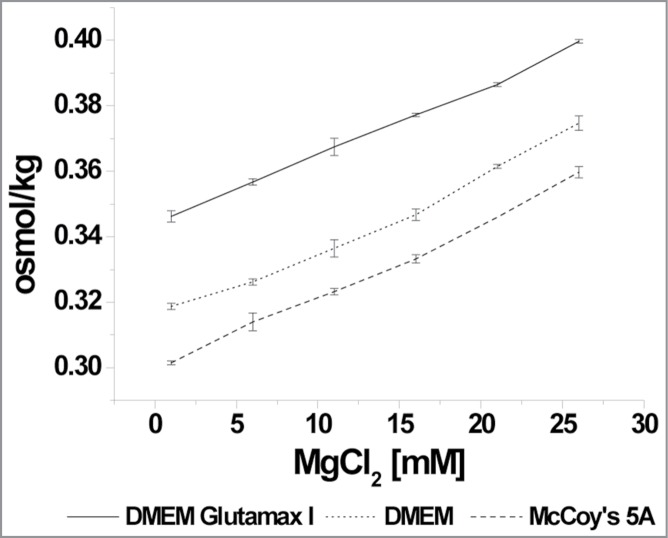
To ensure that the cells were not dying of osmotic shock or pH changes due to high MgCl2 concentrations, the osmolality and pH were measured (Fig. 1). The osmolality increased in DMEM GlutaMAX I from 0.35 osmol/kg ± 0.002 (control) to 0.4 osmol/kg ± 0.001 (25 mM MgCl2). In DMEM, the osmolality increased from 0.32 osmol/kg ± 0.001 (control) to 0.37 osmol/kg ± 0.002 (25 mM MgCl2). The osmolality in McCoy's 5A medium from 0.3 osmol/kg ± 0.001 (control) to 0.36 osmol/kg ± 0.002 (25 mM MgCl2). As expected, the slopes were linear in each case. The pH values were not influenced by the addition of MgCl2.
Figure 1.
Media osmolality. Osmolality due to increased MgCl2 concentrations in media used in this study. DMEM GlutaMAX I is indicated by diamonds, DMEM is indicated by squares and McCoy's 5A is indicated by triangles.
Cell proliferation and cell viability
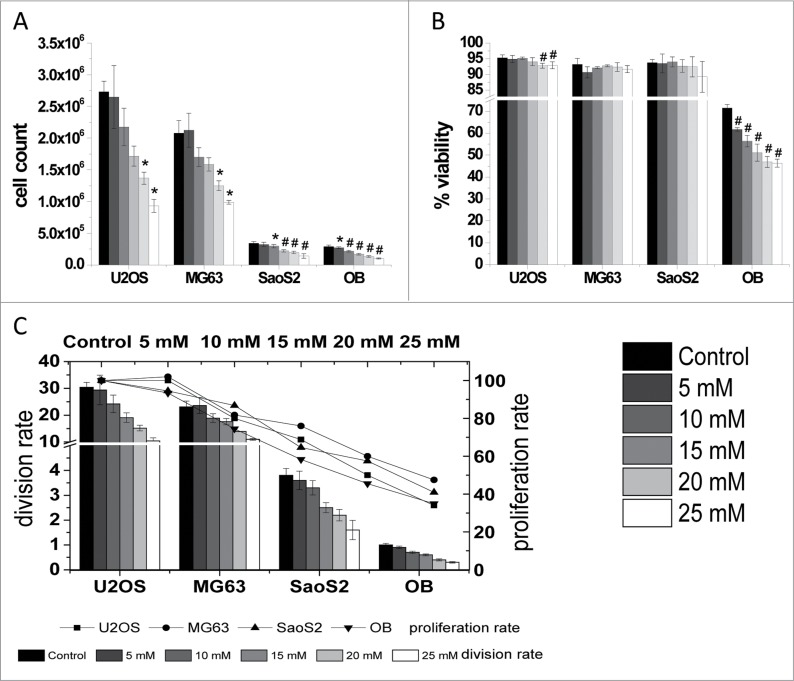
The effects of enhanced extracellular MgCl2 were first examined by measuring the cell count (i.e., proliferation rate) and viability of normal proliferating cells. Proliferation is generally reduced by the addition of MgCl2 (Fig. 2A). Though the proliferation rate of SaoS2 cells is significantly reduced by the addition of 10 mM MgCl2, the growth of osteoblasts is already significantly affected at a concentration of 5 mM. In contrast, cell count is significantly reduced for U2OS and MG63 with the addition of 20 mM MgCl2. The reduction of the cell growth induced by the addition of 25 mM MgCl2 can reach 60% in U2OS and osteoblasts compared with the control and can reach at least 50% in MG63 and SaoS2.
Figure 2.
Cell proliferation. Cell count (A), viability (B) and division and proliferation rate (C) of the osteosarcoma derived cell lines U2OS, MG63 and SaoS2 and osteoblasts (OB) after incubation with increasing concentrations of MgCl2 (0-25 mM). Significant differences between control and indicated conditions are presented by asterisks or hash marks (P < 0.05 = *, P < 0.001 = #). The viability is normalized to the total amount of cells measured in (A).
Further regarding cell viability, the cell lines show different behavior compared with primary human osteoblasts. Whereas the cell lines are mostly unaffected, the viability of osteoblasts decreases significantly if more extracellular MgCl2 is available (Fig 2B). Only U2OS cells are significantly influenced by 20 mM MgCl2, although their viability remains above 90% (Fig. 2B).
Cell size
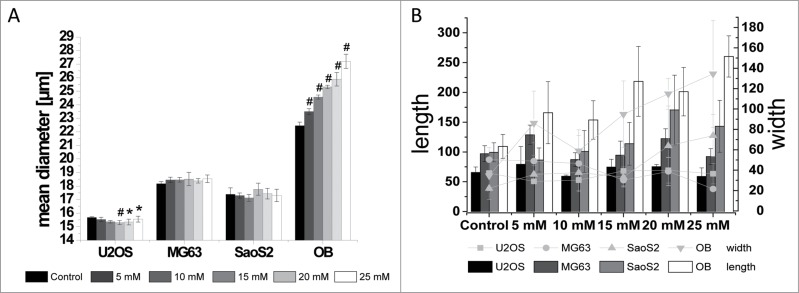
During the determination of proliferation, it was observed that the cells increased in size after MgCl2 addition. The cell spreading area is an important factor for the entry into the differentiation phase.8 Therefore, the cell size was determined for 1) trypsinised cells in suspension and 2) adherent cells.
The sizes of the cell lines MG63 and SaoS2 were unaffected measured with trypsinised cells. U2OS cells were significantly influenced by the addition of 10 to 20 mM MgCl2, and the size of trypsinised osteoblasts was significantly increased by the addition of MgCl2 (Fig. 3A). Whereas these observations were determined for spherical shaped cells, the adhered cells exhibit a different behavior.
Figure 3.
Cell size of trypsinised cells in suspension (A) and adherent cells on fibronectin coated glass slides (B) of the osteosarcoma derived cell lines U2OS, MG63 and SaoS2 and osteoblasts (OB) after incubation with increasing extracellular MgCl2 concentrations (0-25 mM). Significant differences between the control and indicated conditions are presented by asterisks or hash marks (P < 0.05 = *, P < 0.001 = #).
To observe whether incubation with MgCl2 has an influence on the shape of the cells, cytoskeletal staining (i.e., actin filaments (green) and the nuclei (blue)) was performed. For MG63 and U2OS, no differences in appearance could be detected (Fig. 4A-D). Moreover, U2OS cells grow in “islands,” which makes it difficult to detect single cells.
SaoS2 cells showed an increase in cell size in the adhered state when 25 mM MgCl2 was added (Fig. 4E and F). They exhibited a more laminar shape compared with the control. This effect was even more pronounced for osteoblasts. The adherent cells were extremely large if enhanced MgCl2 concentrations were present. This phenomenon can be observed for extracellular concentrations above 10 mM MgCl2. Osteoblasts can expand up to twice in length and 4-fold in width if adhered (Fig. 4G and H). In relation to the size, the nucleus was also enlarged.
Figure 4.
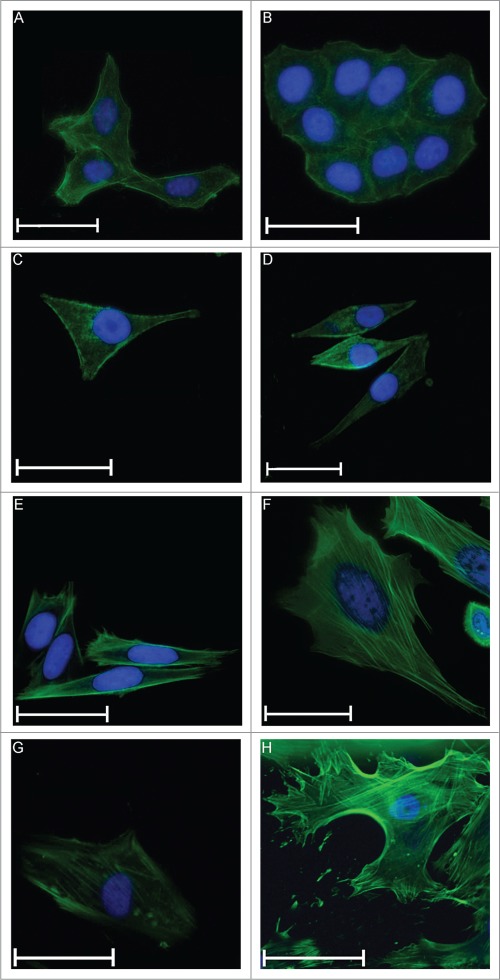
Cell size of adherent cells on fibronectin coated glass slides. Fluorescent microscopy of U2OS cells under cell culture conditions (A) and after addition of 25 mM MgCl2 (B), MG63 (C and D), SaoS2 (E and F) and osteoblasts (G and H), respectively. Actin filaments were stained green, and the nuclei were stained blue. The scales show a length of 50 μm, except the scale in H, where the length is 100 μm.
Gene expression
RT-PCR
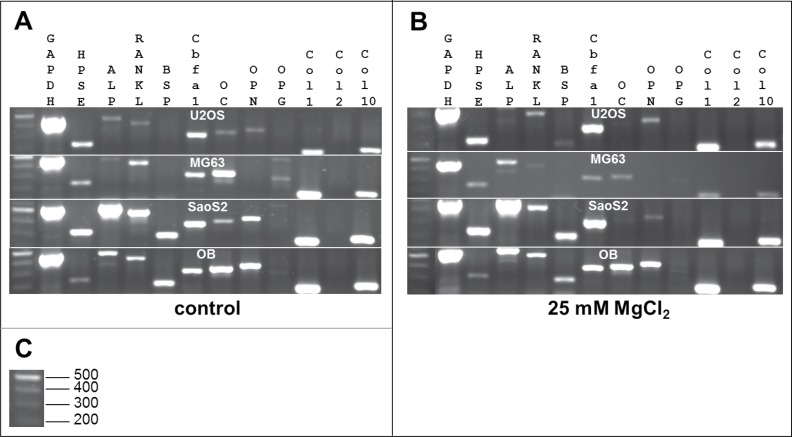
To determine whether MgCl2 has an influence on the gene expression of proliferating cells, a semi-quantitative analysis of bone-specific genes was performed using RT-PCR. Though 25 mM of MgCl2 exhibited a slightly toxic effect in observing measurable effects, control cells and cells with the highest exposition to MgCl2 (25 mM) were analyzed.
As expected, the gene pattern of the different cell types was different. Qualitatively, compared with osteoblasts, SaoS2 cells exhibited the most similar gene expression pattern (Fig. 5A). The gene expression of MG63 cells revealed a pattern that is strikingly different from that of osteoblasts. Here, no osteopontin (OPN) or bone sialoprotein (BSP) expression could be detected. Additionally, the alkaline phosphatase (ALP) expression was very weak (Fig. 5A).
Figure 5.
Qualitative gene expression. Comparison of gene expression of various genes involved in bone formation from human osteoblasts (OB) and osteosarcoma cell lines MG63, SaoS2 and U2OS under cell culture conditions (A) and after incubation with 25 mM MgCl2 (B). The sizes of the DNA ladder are indicated in (C). GAPDH: glyceraldehyde 3-phosphate dehydrogenase; HPSE: Heparanase; ALP: alkalinephosphatase; RANKL: RANK ligand; BSP: bone sialoprotein; Cbfa1: runt-related transcription factor 2; OC: osteocalcin, OPN: osteopontin; OPG: osteoprotegerin; Col: Collagen.
The addition of 25 mM MgCl2 had no crucial influence on the gene expression pattern regarding osteoblasts and SaoS2. In U2OS cells, the expression of BSP seemed to be enhanced, whereas no osteocalcin (OC) expression could be determined after the addition of 25 mM MgCl2 to the environment. Moreover, an addition of 25 mM MgCl2 led to increased ALP expression in MG63, but no osteoprotegerin (OPG) expression could be determined (Fig. 5B).
By analyzing the relative densities (Table 1) of the gene expression pattern, some general statements can be made: 1. The gene expression of MG63 is extremely changed by the addition of MgCl2. All markers are downregulated except heparanase (HPSE), which is the only marker comparable to primary osteoblasts. 2. In primary osteoblasts, the only genes affected are ALP (upregulated) and BSP (downregulated). In SaoS2 cells, the affected genes are HPSE, ALP, runt-related transcription factor 2 (cbfa1) and collagen type 1 (Col 1) (upregulated), whereas OC and OPG are downregulated. In U2OS cells HPSE, OC and collagen type 10 (Col 10) are upregulated, whereas cbfa1 and Col 1 are downregulated.
Table 1.
Change in relative density (% of control)
| U2OS | MG63 | SaoS2 | osteoblasts | |
|---|---|---|---|---|
| GAPDH | 100 | 100 | 100 | 100 |
| HPSE | 169.71 | 111.45 | 140.14 | 82.56 |
| ALP | 69.87 | 29.63 | 127.97 | 152.46 |
| RANKL | 304.56 | 0.01 | 89.23 | 108.45 |
| BSP | 0.011 | 49.93 | 115.67 | 55.29 |
| Cbfa1 | 957.25 | 29.17 | 139.36 | 111.50 |
| OC | 0.015 | 0.019 | 0.01 | 87.63 |
| OPN | 33.32 | 37.64 | 0.02 | 84.23 |
| OPG | 210.18 | 46.22 | 128.98 | 29.27 |
| Col 1 | 169.71 | 111.45 | 101.19 | 98.92 |
| Col 10 | 69.87 | 29.63 | 140.14 | 92.15 |
qPCR
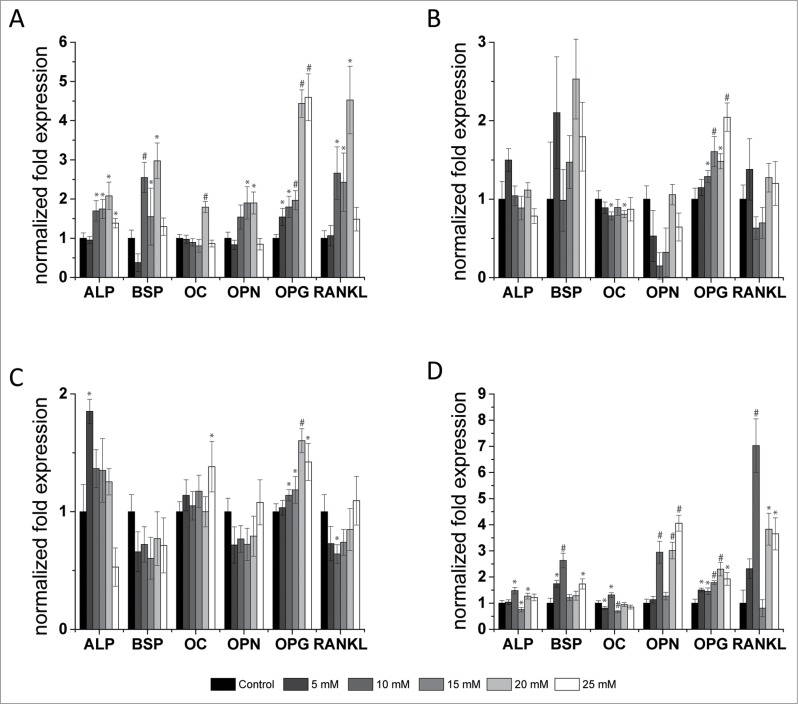
Using real-time polymerase chain reaction (qPCR), gene expression was determined semi-quantitatively and in a concentration dependent manner for the most interesting genes. Contrary to the observations for proliferation and size, where a linear positive or negative correlation for increasing concentrations was found, the picture is more diverse in terms of gene expression. For the cell lines, U2OS gene expression was considerably different compared with those for MG63 and SaoS2. Increasing MgCl2 concentrations led, in nearly all genes, to an increase of expression, whereas at 25 mM, it approaches the control level. The prominent exception here is OPG (Fig. 6A). MG63 cells showed little differences in ALP and OC, whereas BSP and OPG expression was increased. The OPN and receptor activator of nuclear factor κ B ligand (RANKL) showed a negative bell-shaped expression (Fig. 6B) that was comparable to SaoS2 (Fig. 6C). In this cell type, the general gene expression was not greatly influenced (compare the scales in Fig. 6C).
Figure 6.
Semi-quantitative gene expression. Gene expression of genes involved in bone metabolism from osteosarcoma-derived cell lines U2OS (A), MG63 (B), SaoS2 (C) and osteoblasts (D) after incubation with various MgCl2 concentrations (0-25 mM). Gene expression was normalized to the expression of GAPDH and ß-Actin. Expression of 0 mM MgCl2 was set as the control (= 1). GAPDH: glyceraldehyde 3-phosphate dehydrogenase; ALP: alkaline phosphatase; BSP: bone sialoprotein; OPN: osteopontin; OPG: osteoprotegerin; RANKL: RANK ligand. Significant differences between the control and indicated conditions are presented by asterisks or hash marks (P < 0.05 = *, P < 0.001 = #).
For all cell types, OC was not dramatically influenced by increasing extracellular MgCl2 concentrations (Fig. 6), but the gene expression of OPG was increased. The highest rise was observed in U2OS for OPG and RANKL, where the expression increased by 4.5 times. In MG63, the OPG/RANKL quotient increased 2.3-fold following the addition of 15 mM MgCl2 (Table 2). The OPG/RANKL ratio showed only slight alterations in SaoS2, whereas it was very inconsistent in U2OS. With the addition of 5 mM MgCl2, the quotient was 1.5 and decreased with the addition of 10-20 mM MgCl2, though it increased up to 3.1 if 25 mM MgCl2 was added to the environment.
Table 2.
Osteoprotegerin/RANK ligand gene expression ratios examined by qPCR
| U2OS | MG63 | SaoS2 | osteoblasts | |
|---|---|---|---|---|
| 5 mM MgCl2 | 1.5 | 0.8 | 1.4 | 0.7 |
| 10 mM MgCl2 | 0.7 | 2.0 | 1.8 | 0.2 |
| 15 mM MgCl2 | 0.8 | 2.3 | 1.6 | 2.2 |
| 20 mM MgCl2 | 1.0 | 1.2 | 1.8 | 0.6 |
| 25 mM MgCl2 | 3.1 | 1.7 | 1.3 | 0.5 |
The strongest influence of MgCl2 was observable in primary osteoblasts, where BSP, OPG, OPN and RANKL were upregulated more than 2-fold (Fig. 6D). In addition, the OPG/RANKL ratio was increased 2.2-fold if 15 mM MgCl2 was added. With the other concentrations, however, this ratio was below 1. This can be counted as the first direct indication that magnesium can also have osteoconductive properties for proliferating primary osteoblasts.
Discussion
Magnesium implants are considered to have a functional aspect that might lead to osteoconductive properties. Therefore, as a first step, we studied the influence of a magnesium salt on several cellular parameters that are involved in osteoconductivity in proliferating cells.
MgCl2 was used as a model system to eliminate other environmental factors, such as pH alterations, which can arise due to magnesium degradation.
First, the medium osmolality and pH values were measured after the addition of MgCl2 to ensure that the cells were not dying because of osmotic shock or high toxicity. As expected, the pH did not change, both with and without the presence of cells (data not shown). The osmolality increased slightly with the addition of up to 25 mM MgCl2, which resulted in a 25-fold higher magnesium concentration than that observed in a normal medium. All osmolality values are in a regime that is not toxic for the cells.25 Therefore, we could only ensure that the influence of elevated extracellular magnesium concentrations was measured.
The analysis of cell proliferation and viability showed that only an inhibiting effect of magnesium can be observed. The cell size, however, indicates that with increased MgCl2 concentration, an osteoconductive effect could be present for primary osteoblasts. It was reported that the size and shape of the cells is crucial to the fate of the cell, i.e., if the cell exhibits a large spreading area combined with an abundant cytoskeleton, the cell is inclined to enter the differentiation phase faster.8 This could be an explanation for the reduced viability in combination with an enlarged cell size for primary human osteoblasts.
In addition to cell number, viability and cell size, the expression of genes involved in bone and matrix formation can indicate whether high magnesium concentrations may be involved in the step from proliferation to differentiation. qPCR has indeed shown an increase in gene expression for bone-relevant genes in primary osteoblasts. Highly significant changes (more than 3-fold) were only observed in U2OS and primary osteoblasts. Regarding primary osteoblasts, the addition of 10 mM magnesium salts induced the highest alterations in BSP and OPN, which are involved in bone formation. OPN was even further upregulated with increasing magnesium concentrations.
After the enlarged cell size, we can count the gene expression as the second osteoconductive effect of magnesium in primary osteoblasts.
Our investigations of possible indications of osteoconductivity were paralleled by a detailed comparison of cell types that are usually used as bone models. Here, we must state that depending on which parameter is studied, only primary osteoblasts are suitable to provide a realistic picture about the influence of magnesium because the cell lines were reacting in all aspects in a controversial and quite heterogeneous way.
From the literature, it is known that different cell lines exhibit various characteristics.17-24 Our results indicate a stronger influence of MgCl2 on osteoblast proliferation and viability compared with the osteosarcoma-derived cell lines. Regarding the cell lines, it seems that the addition of magnesium leads to reduced proliferation activity as the cell count is reduced from 10 mM MgCl2, but the viability is not influenced dramatically. The viability of the cell lines is at the highest concentration above 90%. In terms of viability, primary cells seem to be more sensitive; a severe decrease in osteoblast viability due to enhanced extracellular MgCl2 concentrations is observed. This decrease is not caused by osmolality values that are too high but is instead directly related to the presence of magnesium. Here, it should be noted that in our experiment, the viability of the osteoblasts in general was already quite low under the control conditions without the addition of MgCl2. However, for mammary epithelial cells, it was shown that proliferation is stimulated with up to 40 mM magnesium.26
It has been proven that the presence of divalent cations, such as magnesium, stimulates cell adhesion.27 It could be observed that with augmented extracellular MgCl2 concentrations, SaoS2 cells and osteoblasts adhere stronger to tissue culture plastic. From a concentration of 10 mM MgCl2, the use of cell scrapers was necessary to detach the cells from the tissue culture plastic, whereas MG63 and U2OS easily detached from the surface by trypsinisation. This observation can also be associated with both cell types becoming very large when magnesium was added to the environment. Moreover, it was reported that Mg2+ regulates the organization of integrins in focal adhesions,28 which are important for cell adhesion to implant materials.29
The genes involved in different stages of osteoblast maturation were tested by PCR. The transcription factor cbfa1, which initiates the maturation of osteoblasts, is expressed in all cells and does not show great alteration upon the addition of magnesium. We concluded that its gene expression is independent of enhanced magnesium concentrations and thus did not consider it for qPCR.
ALP was used as an early marker because it is already present in pre-osteoblasts.30 The presence of increased expression of this gene indicates that osteoblast maturation is initiated. It is only weakly expressed in MG63, though it can be found in all other cells.
BSP, OC and OPN were used as markers for mature osteoblasts.31-33 Here, we can observe that these genes are expressed in osteoblasts, whereas there are some alterations regarding the cell lines. Magnesium does not lead to a genotype compared with the mature osteoblasts in MG63 and U2OS. Differentiation does not occur in those cells.
Col 1 and Col 10 were chosen as components of the matrix, and collagen type 2 (Col 2) was chosen as a negative control because it should not be present in osteoblasts.34 The gene expression is similar, as expected, for all cells and did not change for MgCl2. Therefore, these genes were not further investigated by qPCR.
HPSE provokes cell adhesion to the extracellular matrix.35 Because the expression does not change, it was not further investigated in this study. The increased adhesion strength observed for osteoblasts and SaoS2 is presumably not due to an amplified expression of this gene but to an augmented adhesion area that occurs because of the increased size of adherent cells.
Finally, the antagonists in the bone remodelling process, OPG and RANKL,36 were analyzed. Overall RT-PCR showed a high similarity of the gene profile for osteoblasts and SaoS2.
The genes whose expression promised to be most interesting because of enhanced MgCl2 concentrations in the environment were chosen for qPCR, which revealed more detailed changes in gene expression.
The results of qPCR are heterogenic for all cell types; it is very complicated to determine a marker for osteoblastic maturation under enhanced extracellular magnesium conditions. The expression pattern of MG63 is most different compared with that of osteoblasts, whereas similarities between U2OS and, to some extent, SaoS2 were observed. The consequence is that no cell line is a completely suitable model for studying the influence of magnesium on bone cells. On the one hand, magnesium availability is important for bone formation, and it was found that magnesium deficiency causes OPG/RANKL ratios that promotes osteoclastogenesis (in osteoblasts, up to 16-fold after a 50% magnesium nutrition diet for 6 months compared with the control).37 On the other hand, magnesium supplementation increases serum OPG and leads to variations in the OPG and RANKL presence, which seems to favor bone formation.38 In our case, the OPG/RANKL ratios are heterogenic (Table 2). Nevertheless, to appraise cellular osteogenesis because of enhanced magnesium concentrations, further experiments such as histological staining and biochemical assays should be performed.
Distinct differences in gene expression can be examined by comparing RT-PCR and qPCR. These differences can be explained by the use of the 2 different methods. RT-PCR was only used as a qualitative method to examine whether a gene is expressed. However, the method sensitivity can be discussed. qPCR is a more sensitive method, and in it, gene expression is normalized to 2 housekeeping genes (Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and ß-Actin). Those genes were chosen because they are constitutively expressed in a wide range of cell types and are involved in important mechanisms of the cell, e.g., glycolysis (GAPDH) or cell structure (ß-Actin).
The results obtained from the diverse experiments performed for this study showed that different characteristics and behaviors exist when comparing primary human osteoblasts and osteosarcoma-derived cell lines. Though osteosarcoma-derived cell lines are commonly used as osteoblast-like models to circumvent the disadvantages of osteoblasts, such as their slow proliferation rate or patient-dependent variability, in this special case, they are only useful to a limited extent. The commonly used MG63 cell line exhibits characteristics that differ most from osteoblasts. The SaoS2 cell line is comparable to osteoblasts in their proliferation and adherent cell size, whereas the gene expression of the U2OS cell line is similar to that of osteoblasts. However, the results must be carefully evaluated compared with osteoblasts. Regardless, for investigating magnesium in vitro, MG63 is not an applicable model for bone cells.
Conclusion
We can conclude that MgCl2 has a measurable effect on bone cells irrespective their origin. An increased cell size can be determined for primary cells, which would be the second osteoconductive clue, following a gene expression that favors bone formation.
Additionally, we can note that neither MG63 nor U2OS is an appropriate model for bone cells when magnesium is investigated. If necessary, we recommend SaoS2, the only one of the 3 cell lines that is able to mineralise.22,23
Further experiments on differentiated cells, including ones using extracts of degrading magnesium materials, will be performed to determine whether the effect of magnesium on bone formation is stronger.
Materials and Methods
Osmolality
All of the media used already contained magnesium sulfate (MgSO4 – 7H2O) at a concentration of 200 mg/L which correlates to 0.813 mM. To ensure that enhanced MgCl2 concentrations in the medium did not dramatically affect its osmolality, which would affected cell homeostasis and viability, osmolality and pH were measured. Solutions of the special medium necessary for the different cell types supplemented with 10% (v/v) foetal bovine serum (FBS) were prepared with various MgCl2 concentrations (MgCl2 – 6H2O (Merck, Darmstadt, Germany); 0-25 mM in 5 mM steps). Osmolality was then determined after 2 to 3 days before the medium change with a freezing point osmometer (Osmomat 030-D; Gonotec, Berlin, Germany). Then, 60 μL of each sample were analyzed. Each condition was performed in quadruplicate.
Cell culture
The osteosarcoma derived cell lines MG63, SaoS2 and U2OS were purchased from the European Collection of Cell Cultures (ECACC, Salisbury, United Kingdom). U2OS were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Life Technologies, Darmstadt, Germany) containing 10% (v/v) FBS (PAA Cell Culture Company, Linz, Austria). SaoS2 cells were cultured in McCoy's 5A (Life Technologies, Darmstadt, Germany) containing 10% (v/v) FBS, and MG63, as well as primary human osteoblasts, were cultured in DMEM-GlutaMAX I (Life Technologies, Darmstadt, Germany) containing 10% (v/v) FBS. Isolation and culture of primary human osteoblasts was approved by the local ethics committee and performed according to the Declaration of Helsinki as described before.25 Briefly, the osteoblasts used in this study were either from artificial hip joint (proliferation, viability and cell size measurements) or cruciate ligament operations (gene expression); the latter were healthy osteoblasts without an osteoporotic background. Cancellous bone material was removed from bone pieces and transferred to a cell culture flask. Cancellous particles were covered with cell culture medium and incubated in the incubator until approximately 90% confluential growth and then divided.
Osteoblasts were used up to the 3rd passage, and the cell lines were used between the 4th and 10th passages. The medium was changed every 2 to 3 days. Each experiment was performed using 5 independent cultures (n = 5).
Cell count, viability and cell size
To examine the impact of magnesium on proliferation, 10,000 cells were incubated with various concentrations of MgCl2 in cell culture flasks. No addition of MgCl2 was set as the control. Then, 5 to 25 mM MgCl2 were added in 5 mM steps. Cell lines were incubated for one week and osteoblasts for 4 weeks due to their lower growth rate. The cells were trypsinised (0.05% Trypsin-EDTA (1X), Phenol Red, Life Technologies, Darmstadt, Germany), and the cell number, viability and mean diameter were determined using CASY Model TT 150 μm (Roche, Mannheim, Germany). Briefly, CASY uses the pulse area analysis technique. Hundreds of readings per cell are possible because the frequency is set at a million measurements per second for each object. CASY quantifies viable and dead cells with individual adjustments for each cell type concurrently. More precisely, below the size of 7.25 μm, CASY measures cell debris for U2OS; between the range of 7.25 μm – 11.5 μm, dead cells are detected, and particles above the size of 11.5 μm are living cells and cell aggregates. For MG63, the values were below 7.75 μm (cell debris), 7.75 μm – 12.75 μm (dead cells) and above 12.75 μm (living cells and aggregates); for SaoS2, the values were below 7.0 μm (cell debris), 7.0 μm – 11.25 μm (dead cells) and above 11.25 μm (living cells and aggregates); and for primary human osteoblasts, the values were below 7.63 μm (cell debris), 7.63 μm – 14.88 μm (dead cells) and above 14.88 (living cells and aggregates) μm, respectively. Sample volumes from 25 μL (MG63 and U2OS), 50 μL (SaoS2) and 200 μL (OB) were applied.
Fluorescence microscopy
The shape of adhered cells was visualised by fluorescence microscopy. Cells were grown on sterile glass slides that had previously been coated with fibronectin (10 ng/mL; Sigma-Aldrich, Taufkirchen, Germany). Fixation of the cells was performed with 3.7% (v/v) formaldehyde (Sigma-Aldrich, Taufkirchen, Germany). Actin filaments were stained with Alexa Fluor Phalloidin (Life Technologies, Darmstadt, Germany). Then, 1% (v/v) BSA (Life Technologies, Darmstadt, Germany) and 1 unit of Alexa Fluor Phalloidin were added to the cells. 4,6-Diamidino-2-Phenylindole Dihydrochloride (DAPI; 5 μg/mL; Sigma-Aldrich Chemie, Taufkirchen, Germany) was used to stain the nuclei.
The analysis was performed using an Eclipse Ti-S microscope and NIS-Elements Microscope Imaging Software (Nikon GmbH, Düsseldorf, Germany). The applied filters were DAPI (Ex: 325-375 nm; Em: 435-495 nm; Mirror at 400 nm) and FITC (Ex: 460-500 nm; Em: 510-560 nm; Mirror at 505 nm). The length and width of 3 adherent cells were measured.
RNA isolation and cDNA synthesis
Total cellular ribonucleic acids (RNA) were extracted using an RNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's manual. Up to 4 million cells were used and washed twice with PBS (phosphate-buffered saline; 0.137 M NaCl, 0.0027 M KCl, 0.01 M Na2HPO4 x 2 H2O, 0.00176 M KH2PO4, pH 7.4) before lysis by adding a guanidine isothiocyanate containing buffer with 1% (v/v) β-Mercaptoethanol (Merck, Darmstadt, Germany). The lysate was homogenized using the QIAshredder spin column (Qiagen, Hilden, Germany). Subsequently, 70% (v/v) ethanol was added to the flow through. The mixture was then applied to an RNeasy spin column (a silica-gel membrane) and washed twice. Total RNA was eluted with RNase free water. RNA integrity and concentration were measured photometrically at 260 nm and 280 nm using a NanoDrop 2000c Spectrophotometer (PEQLAB Biotechnologie GmbH, Erlangen, Germany) and on a 1% (w/v) agarose gel containing 0.01% (v/v) GelRed (Biotium, Hayward, California, USA). Subsequently, 2 μg RNA was used for reverse transcription. Reverse transcription was performed as recommended by the supplier (Omniscript RT Kit, Qiagen, Hilden, Germany) using the corresponding buffer, 0.5 mM of each dATP, dGTP, dTTP and dCTP, 1 μM of Oligo-dT primer, 4 units Omniscript Reverse Transcriptase and 10 untits of RNase Inhibitor (Qiagen, Hilden, Germany). The incubation was performed at 37°C for 60 min.
Primer
To determine whether magnesium has an influence on bone metabolism, several genes that are involved in bone and matrix formation were analyzed. Primer sequences were selected either from publications or designed using the Primer3 Input software (version 0.4.039).
Primers were purchased from Eurofins MWG Operon (Ebersberg, Germany), and their sequences are listed in Tables 2 and 3. The annealing temperatures (Ta) are specified for RT-PCR primers (Table 3), and it was 60°C for all qPCR primers (Table 4).
Table 3.
Reverse transcription-PCR (RT-PCR) primer sequences
| Gene | abbreviation | RT-PCR-Primer (5’-3’) | Ta [°C] | product size |
|---|---|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase40 | GAPDH | ACCACAGTCCATGCCATCAC | 55 | 452 |
| TTCACCACCCTGTTGCTGTA | ||||
| Heparanase41 | HPSE | TGGACCTGGACTTCTTCACC | 53 | 216 |
| TTGATTCCTTCTTGGGATCG | ||||
| Alkaline phosphatase40 | ALP | ACGTGGCTAAGAATGTCATC | 53 | 475 |
| CTGGTAGGCGATGTCCTTA | ||||
| Receptor Activator of NF-κB Ligand42 | RANKL | CAGGAGACCTAGCTACAGA | 49 | 418 |
| CAAGGTCAAGAGCATGGA | ||||
| Bone sialoprotein43 | BSP | AAGCAATCACCAAAATGAAGACT | 55 | 188 |
| TGGAAATCGTTTTAAATGAGGATA | ||||
| Runt-related transcription factor 244 | Cbfa1 | CCCCACGACAACCGCACC | 55 | 289 |
| CACTCCGGCCCACAAATCTC | ||||
| Osteocalcin40 | OC | CATGAGAGCCCTCACA | 48 | 315 |
| AGAGCGACACCCTAGAC | ||||
| Osteopontin 40 | OPN | CCAAGTAAGTCCAACGAAAG | 55 | 347 |
| GGTGATGTCCTCGTCTGTA | ||||
| Osteoprotegerin42 | OPG | AGACTTTCCAGCTGCTGA | 49 | 469 |
| GGATCTCGCCAATTGTGA | ||||
| Collagen type 1 | Col1 | AAAGGCAATGCTCAAACACC | 49 | 159 |
| TCAAAAACGAAGGGGAGATG | ||||
| Collagen type 245 | Col2 | GTGGAGCAGCAAGAGCAAGGA | 61 | 334 |
| CTTGCCCCACTTACCAGTGTG | ||||
| Collagen type 10 | Col10 | TCCAAAAGGTGATCCTGGAG | 55 | 181 |
| CCCTTTAGACCCAGGGAATC |
Table 4.
Quantitative real-time PCR (qPCR) primer sequences
| Gene | abbreviation | qPCR-Primer (5’-3’) | product size |
|---|---|---|---|
| ß-Actin | ß-Actin | CTTCCTGGGCATGGAGTC | 134 |
| TGATCTTCATTGTGCTGGGT | |||
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | GTCGGAGTCAACGGATTTG | 143 |
| TGGGTGGAATCATATTGGAA | |||
| alkaline phosphatase | ALP | CACCCACGTCGATTGCATCT | 211 |
| TAGCCACGTTGGTGTTGAGC | |||
| Bone sialoprotein | BSP | TGGATGAAAACGAACAAGGCA | 200 |
| AAACCCACCATTTGGAGAGGT | |||
| Osteocalcin | OC | GGCAGCGAGGTAGTGAAGAG CTGGAGAGGAGCAGAACTGG | 230 |
| Osteopontin | OPN | CTCCATTGACTCGAACGACTC | 230 |
| CAGGTCTGCGAAACTTCTTAGAT | |||
| Osteoprotegerin | OPG | CGCTCGTGTTTCTGGACAT | 112 |
| GGACATTTGTCACACAACAGC | |||
| Receptor Activator of NF-κB Ligand46 | RANKL | ATACCCTGATGAAAGGAGGA | 202 |
| GGGGCTCAATCTATATCTCG |
Reverse-transcription polymerase chain reaction (RT-PCR) analysis
cDNA was used as the template for RT-PCR. All enzymes, nucleotides, buffers and other chemicals for RT-PCR were purchased from Qiagen (Taq PCR Core Kit, Hilden, Germany). Here, 2.5 units of Taq DNA Polymerase and 800 μM of each dNTP, 20 pmol of each primer and a total concentration of 2.5 mM MgCl2 were used for each reaction. In the case of osteocalcin (OC), 4% (v/v) glycerol (Merck, Darmstadt, Germany) was added. The PCR program was performed as follows: initial denaturation for 5 min at 95°C; 35 cycles with denaturation for 30 s at 95°C; primer-annealing for 30 s at the correspondent temperature; elongation for 45 s at 72°C; and additional elongation for 7 min at 72°C. The PCR products were then visualised on a 1% (w/v) agarose gel stained with 0.01% (v/v) GelRed. Glyceraldehyde 3- phosphate dehydrogenase (GAPDH) was used as evidence that cDNA synthesis was successful.
Real-time PCR analysis
Real-time PCR (qPCR) was used to semi-quantify the expression rate of the chosen genes. qPCR was performed using SsoFast EvaGreen Supermix (Bio-Rad Laboratories GmbH, München, Germany), 10 pmol of each primer and cDNA. The PCR program was performed as follows: initial denaturation for 30 s at 95°C; 60 cycles with denaturation for 5 s at 95°C; primer-annealing for 15 s at the correspondent temperature; elongation for 30 s at 72°C; and melt curve: 65°C to 95°C and 0.5°C per step. The results were analyzed using the CFX Manager Software 1.1 (Bio-Rad Laboratories GmbH, München, Germany). As internal controls (housekeeping genes), glyceraldehyde 3- phosphate dehydrogenase (GAPDH) and ß-Actin were used. Moreover, the homogeneity of the housekeeping genes was surveyed by analyzing the target stability values.
Statistical analysis
Statistics were performed using the SigmaStat package (Systat software GmbH, Erkrath, Germany). Standard analysis comparing more than 2 treatments was conducted using a one-way ANOVA. Depending on the data distribution, either a one-way ANOVA or an ANOVA on ranks was performed. The post-hoc tests were Holm-Sidak or Dunn's versus the control group. Statistics for qPCR were performed using CFX Manager Software 1.1 (regulation threshold 2.00 and P-Value threshold 0.001). Statistical values are indicated at the relevant experiments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed. The authors have no proprietary or commercial interest in any materials discussed in this article.
Acknowledgments
We wish to thank Gabriele Salamon and Helmholtz-Zentrum Geesthacht for their expert technical assistance regarding osteoblast cell culture.
References
- 1. Witte F. The history of biodegradable magnesium implants: A review. Acta Biomater 2010; 6:1680-92; PMID:20172057; http://dx.doi.org/ 10.1016/j.actbio.2010.02.028 [DOI] [PubMed] [Google Scholar]
- 2. Seuss F, Seuss S, Turhan MC, Fabry B, Virtanen S. Corrosion of Mg alloy AZ91D in the presence of living cells. J Biomed Mater Res Part B-App Biomater 2011; 99B:276-81; http://dx.doi.org/ 10.1002/jbm.b.31896 [DOI] [PubMed] [Google Scholar]
- 3. Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth CJ, Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomater 2005; 26:3557-63; PMID:15621246; http://dx.doi.org/ 10.1016/j.biomaterials.2004.09.049 [DOI] [PubMed] [Google Scholar]
- 4. Kraus T, Fischerauer SF, Hänzi AC, Uggowitzer PJ, Löffler JF, Weinberg AM. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater 2012; 8:1230-8; PMID:22107870; http://dx.doi.org/ 10.1016/j.actbio.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 5. Wolf FI, Cittadini A. Magnesium in cell proliferation and differentiation. Front Biosci 1999; 4:607-17; http://dx.doi.org/ 10.2741/Wolf [DOI] [PubMed] [Google Scholar]
- 6. Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev 2003; 24:47-66; PMID:18568054 [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin H. Magnesium: the missing element in molecular views of cell proliferation control. Bioessays 2005; 27:311-20; PMID:15714553; http://dx.doi.org/ 10.1002/bies.20183 [DOI] [PubMed] [Google Scholar]
- 8. Bacakova L, Filova E, Rypacek F, Svorcik V, Stary V. Cell adhesion on artificial materials for tissue engineering. Physiol Res 2004; 53:S35-S45; PMID:15119934 [PubMed] [Google Scholar]
- 9. Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279:349-52; PMID:9454332; http://dx.doi.org/ 10.1126/science.279.5349.349 [DOI] [PubMed] [Google Scholar]
- 10. Billiau A, Edy VG, Heremans H, Vandamme J, Desmyter J, Georgiades JA, Desomer P. Human interferon-mass-production in a newly established cell line, MG63. Antimicrob Agents Chemother 1977; 12:11-5; PMID:883813; http://dx.doi.org/ 10.1128/AAC.12.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dedhar S, Mitchell MD, Pierschbacher MD. The osteoblast-like differentiated phenotype of a variant of MG-63 osteosarcoma cell line correlated with altered adhesive properties. Connect Tissue Res 1989; 20:49-61; PMID:2533054; http://dx.doi.org/ 10.3109/03008208909023874 [DOI] [PubMed] [Google Scholar]
- 12. Pangalos M, Bintig W, Schlingmann B, Feyerabend F, Witte F, Begandt D, Heisterkamp A, Ngezahayo A. Action potentials in primary osteoblasts and in the MG-63 osteoblast-like cell line. J Bioenerg Biomembr 2011; 43:311-22; PMID:21523406; http://dx.doi.org/ 10.1007/s10863-011-9354-7 [DOI] [PubMed] [Google Scholar]
- 13. Torricelli P, Fini M, Borsari V, Lenger H, Bernauer J, Tschon M, Bonazzi V, Giardino R. Biomaterials in orthopedic surgery: Effects of a nickel-reduced stainless steel on in vitro proliferation and activation of human osteoblasts. Int J Artif Organs 2003; 26:952-7; PMID:14636013 [DOI] [PubMed] [Google Scholar]
- 14. Wang YB, Xie XH, Li HF, Wang XL, Zhao MZ, Zhang EW, Bai YJ, Zheng YF, Qin L. Biodegradable CaMgZn bulk metallic glass for potential skeletal application. Acta Biomater 2011; 7:3196-208; PMID:21571105; http://dx.doi.org/ 10.1016/j.actbio.2011.04.027 [DOI] [PubMed] [Google Scholar]
- 15. Zomorodian A, Garcia MP, Moura e Silva T, Fernandes JCS, Fernandes MH, Montemor MF. Corrosion resistance of a composite polymeric coating applied on biodegradable AZ31 magnesium alloy. Acta Biomater 2013; 9:8660-70; PMID:23454214; http://dx.doi.org/ 10.1016/j.actbio.2013.02.036 [DOI] [PubMed] [Google Scholar]
- 16. Krueger R, Seitz J-M, Ewald A, Bach F-W, Groll J. Strong and tough magnesium wire reinforced phosphate cement composites for load-bearing bone replacement. J Mech Behav Biomed Mater 2013; 20:36-44; PMID:23455162; http://dx.doi.org/ 10.1016/j.jmbbm.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 17. Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2OS in comparison to human osteoblasts. Anticancer Res 2004; 24:3743-8; PMID:15736406 [PubMed] [Google Scholar]
- 18. Isfort RJ, Cody DB, Lovell G, Doersen CJ. Analysis of oncogenes, tumor suppressor genes, autocrine growth-factor production, and differentiation state of human osteosarcoma cell-lines. Mol Carcinogen 1995; 14:170-8; http://dx.doi.org/ 10.1002/mc.2940140306 [DOI] [PubMed] [Google Scholar]
- 19. Orimo H, Goseki-Sone M, Hosoi T, Shimada T. Functional assay of the mutant tissue-nonspecific alkaline phosphatase gene using U2OS osteoblast-like cells. Mol Genet Metab 2008; 94:375-81; PMID:18455459; http://dx.doi.org/ 10.1016/j.ymgme.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 20. Niforou KM, Anagnostopoulos AK, Vougas K, Kittas C, Gorgoulis VG, Tsangaris GT. The proteome profile of the human osteosarcoma U2OS cell line. Cancer Genomics Proteomics 2008; 5:63-78; PMID:18359981 [PubMed] [Google Scholar]
- 21. Bilbe G, Roberts E, Birch M, Evans DB. PCR phenotyping of cytokines, growth factors and their receptors and bone matrix proteins in human osteoblast-like cell lines. Bone 1996; 19:437-45; PMID:8922641; http://dx.doi.org/ 10.1016/S8756-3282(96)00254-2 [DOI] [PubMed] [Google Scholar]
- 22. Czekanska EM, Stoddart MJ, Richards RG, Hayes JS. In search of an osteoblast cell model for in vitro research. Eur Cells Mater 2012; 24:1-17. [DOI] [PubMed] [Google Scholar]
- 23. Czekanska EM, Stoddart MJ, Ralphs JR, Richards RG, Hayes JS. A phenotypic comparison of osteoblast cell lines vs. human primary osteoblasts for biomaterials testing. J Biomed Mater Res 2013; 102:2636-43; http://dx.doi.org/ 10.1002/jbm.a.34937 [DOI] [PubMed] [Google Scholar]
- 24. Yu Y, Harris RI, Yang JL, Anderson HC, Walsh WR. Differential expression of osteogenic factors associated with osteoinductivity of human osteosarcoma cell lines. J Biomed Mater Res 2004; 70A:122-8; http://dx.doi.org/ 10.1002/jbm.a.30072 [DOI] [PubMed] [Google Scholar]
- 25. Fischer J, Pröfrock D, Hort N, Willumeit R, Feyerabend F. Improved cytotoxicity testing of magnesium materials. Mat Sci Eng B-Solid 2011; 176:830-4; http://dx.doi.org/ 10.1016/j.mseb.2011.04.008 [DOI] [Google Scholar]
- 26. Sgambato A, Faraglia B, Ardito R, Torsello A, Boninsegna A, Cittadini A, Wolf FI. Isolation of Normal Epithelial Cells Adapted to Grow at Nonphysiological Concentration of Magnesium. Biochem Biophys Res Commun 2001; 286:752-7; PMID:11520061; http://dx.doi.org/ 10.1006/bbrc.2001.5465 [DOI] [PubMed] [Google Scholar]
- 27. Paul W, Sharma CP. Effect of calcium, zinc and magnesium on the attachment and spreading of osteoblast like cells onto ceramic matrices. J Mater Sci Mater Med 2007; 18:699-703; PMID:17136605; http://dx.doi.org/ 10.1007/s10856-006-0005-1 [DOI] [PubMed] [Google Scholar]
- 28. Stuiver I, Ruggeri Z, Smith JW. Divalent cations regulate the organization of integrins α v β 3 and α v β 5 on the cell surface. J Cell Physiol 1996; 168:521-31; PMID:8816906; http://dx.doi.org/ 10.1002/(SICI)1097-4652(199609)168:3%3c521::AID-JCP4%3e3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 29. Goto T, Yoshinari M, Kobayashi S, Tanaka T. The initial attachment and subsequent behavior of osteoblastic cells and oral epithelial cells on titanium. Bio-Med Mater Eng 2004; 14:537-44. [PubMed] [Google Scholar]
- 30. Weinreb M, Shinar D, Rodan GA. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J Biomed Mater Res 1990; 5:831-42. [DOI] [PubMed] [Google Scholar]
- 31. Bianco P, Fisher L, Young M, Termine J, Robey P. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int 1991; 49:421-6; PMID:1818768; http://dx.doi.org/ 10.1007/BF02555854 [DOI] [PubMed] [Google Scholar]
- 32. Hauschka PV, Lian JB, Cole DEC, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev 1989; 69:990-1047; PMID:2664828 [DOI] [PubMed] [Google Scholar]
- 33. Sodek J, Chen J, Nagata T, Kasugai S, Todescan R, Li IWS, Kim RH. Regulation of osteopontin expression in osteoblasts. Ann N Y Acad Sci 1995; 760:223-41; PMID:7785896; http://dx.doi.org/ 10.1111/j.1749-6632.1995.tb44633.x [DOI] [PubMed] [Google Scholar]
- 34. van der Rest M, Garrone R. Collagen family of proteins. Faseb J 1991; 5:2814-23; PMID:1916105 [PubMed] [Google Scholar]
- 35. Goldschmidt O, Zcharia E, Cohen M, Aingorn H, Cohen I, Nadav L, Katz BZ, Geiger B, Vlodavsky I. Heparanase mediates cell adhesion independent of its enzymatic activity. Faseb J 2003; 17:1015-25; PMID:12773484; http://dx.doi.org/ 10.1096/fj.02-0773com [DOI] [PubMed] [Google Scholar]
- 36. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998; 93:165-76; PMID:9568710; http://dx.doi.org/ 10.1016/S0092-8674(00)81569-X [DOI] [PubMed] [Google Scholar]
- 37. Rude RK, Gruber HE, Wei LY, Frausto A. Immunolocalization of RANKL is increased and OPG decreased during dietary magnesium deficiency in the rat. Nutr Metab (Lond) 2005; 2:24; PMID:16162295; http://dx.doi.org/ 10.1186/1743-7075-2-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bae Y, Kim M-H. Calcium and magnesium supplementation improves serum OPG/RANKL in calcium-deficient ovariectomized rats. Calcif Tissue Int 2010; 87:365-72; PMID:20811796; http://dx.doi.org/ 10.1007/s00223-010-9410-z [DOI] [PubMed] [Google Scholar]
- 39. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 2000; 132:365-86; PMID:10547847 [DOI] [PubMed] [Google Scholar]
- 40. Kamata N, Fujimoto R, Tomonari M, Taki M, Nagayama M, Yasumoto S. Immortalization of human dental papilla, dental pulp, periodontal ligament cells and gingival fibroblasts by telomerase reverse transcriptase. J Oral Pathol Med 2004; 33:417-23; PMID:15250834; http://dx.doi.org/ 10.1111/j.1600-0714.2004.00228.x [DOI] [PubMed] [Google Scholar]
- 41. Smith PN, Freeman C, Yu D, Chen M, Gatenby PA, Parish CR, Li RW. Heparanase in primary human osteoblasts. J Orthop Res 2010; 28:1315-22; PMID:20309870; http://dx.doi.org/ 10.1002/jor.21138 [DOI] [PubMed] [Google Scholar]
- 42. Maddi A, Hai H, Ong S-T, Sharp L, Harris M, Meghji S. Long wave ultrasound may enhance bone regeneration by altering OPG/RANKL ratio in human osteoblast-like cells. Bone 2006; 39:283-8; PMID:16567138; http://dx.doi.org/ 10.1016/j.bone.2006.01.162 [DOI] [PubMed] [Google Scholar]
- 43. Mauney JR, Blumberg J, Pirun M, Volloch V, Vunjak-Novakovic G, Kaplan DL. Osteogenic differentiation of human bone marrow stromal cells on partially demineralized bone scaffolds in vitro. Tissue Eng 2004; 10:81-92; PMID:15009933; http://dx.doi.org/ 10.1089/107632704322791727 [DOI] [PubMed] [Google Scholar]
- 44. Gallagher JA. Human osteoblast culture. Methods Mol Med 2003; 80:3-18; PMID:12728706 [DOI] [PubMed] [Google Scholar]
- 45. Kim TK, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, Elisseeff JH. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthr Cartilage 2003; 11:653-64; http://dx.doi.org/ 10.1016/S1063-4584(03)00120-1 [DOI] [PubMed] [Google Scholar]
- 46. Koch FP, Merkel C, Ziebart T, Smeets R, Walter C, Al-Nawas B. Influence of bisphosphonates on the osteoblast RANKL and OPG gene expression in vitro. Clin Oral Investig 2012; 16:79-86; PMID:20938793; http://dx.doi.org/ 10.1007/s00784-010-0477-8 [DOI] [PubMed] [Google Scholar]