Abstract
OBJECTIVE
To measure effectiveness of liraglutide in reducing glycated hemoglobin (HbA1C), weight, and systolic blood pressure (SBP) in Emirati patients.
DESIGN
A retrospective cohort study.
SETTING
Endocrinology clinic in a 300-bed military hospital.
PATIENTS
A total of 152 patients who qualified for liraglutide between September 21, 2012, (first patient visit) and May 5, 2014 (last patient visit).
METHODS
Team collected demographic and clinical data using a standard form. Data keeper performed univariate analyses to measure the effect of liraglutide in reducing the three outcomes of interest; namely, HbA1C, weight, and SBP.
RESULTS
One hundred patients had at least the first visit in the clinic and 98 patients came for a second follow-up visit while on the medication. Adherence of clinicians to the internal criteria for prescribing liraglutide was 92%. Patients’ ages were 47.9 ± 11.7 years. Male-to-female ratio was almost 1:1. Overall, in the paired analyses, HbA1C decreased from first to second visits (8.7 ± 1.9 vs. 7.6 ± 1.8, P > 0.0001) and remained unchanged in subsequent visits (eg, in visit 3, HbA1C was 7.4 ± 1.8). Patients lost an average of 1.3 kg between the first and second visits (99.3 ± 19.3 vs. 98.0 ± 19.5, P = 0.0003). The reduction in SBP between visits 1 and 2 was less (130.9 ± 15.8 vs. 129.9 ± 16.5, P = 0.5896). ANOVA yielded a significant reduction in HbA1C at 4 months and 6 months (P values < 0.05). SBP dropped by about 3.6 mmHg and weight by about 2.3 kg (P values > 0.05).
CONCLUSIONS
Liraglutide is effective in reducing HbA1C, weight, and to a lesser extent, SBP in Emirati patients.
Keywords: liraglutide, diabetes mellitus, type II, Emirati
Introduction
Liraglutide, a glucagon-like peptide 1 (GLP 1) receptor agonist, reduced glycated hemoglobin (HbA1C) when combined with metformin in a dose-dependent fashion (ie, 0.7% for the 0.6 mg dose and 1% for doses ≥1.2 mg) in the Liraglutide Effect and Action in Diabetes (LEAD) study.1 In this trial, liraglutide further decreased patients’ weights by 1.8–2.8 kg and systolic blood pressure (SBP) by about 2–3 mmHg. Multiple studies have replicated these findings in real-world practice and in other regions of patients with type II diabetes mellitus.2,3 On the other hand, the Middle East and North Africa (MENA) and Gulf Corporation Council (GCC) regions lack data on liraglutide effectiveness in its residents, and especially local populations. Members of these two regions have been in the top 20 lists of highest incidence and prevalence of diabetes for several years.4 Nevertheless, authors fail to find primary literature on the effectiveness of liraglutide and other new diabetes medications in the United Arab Emirates (UAE) or nearby countries. Therefore, this study measures the effect of liraglutide on HbA1C, weight, and SBP in Emirati patients followed at our military hospital.
Patients and Methods
Inclusion and exclusion criteria
Pharmacy and therapeutics committee approved the internal prescribing criteria for liraglutide, which included all of the following: type II diabetes mellitus, body mass index (BMI) >30 kg/m2, and metformin failure. On the other hand, study team assessed adherence to these criteria during the first visit. Pharmacy department recorded names, identifiers, and dispensing history of patients who qualified and received liraglutide. First patient visit dated September 21, 2012, and the last follow-up visit coincided with May 5, 2014. Patients had scheduled follow-up visits every 2 months. However, patients had different number of visits because they can choose to follow up at other governmental hospitals. Patients received liraglutide as an add-on to their treatments. In addition, patients had counseling about increasing the liraglutide to the maintenance dose of 1.8 mg daily. Subgroups of patients whose baseline treatment included insulin, sulfonylureas, metformin, sitagliptin (DPP4 inhibitors), or metformin + sitagliptin underwent analyses for changes in HbA1C. Patients entered the paired analyses if they met these criteria: followed up at our hospital, are ≥18-year old, and had the pertinent visits data (eg, HbA1C). In contrast, for the unpaired analyses, the team set the same criteria mentioned above; however, they included all available data for the visits. In addition, study team excluded patients who discontinued treatment before the second follow-up visit (within 2 months of the start of liraglutide) or failed to follow up at our hospital from the paired comparisons of HbA1C, weight, and SBP. Other variables, including alanine aminotransferase (ALT), low-density lipoprotein (LDL), blood urea nitrogen (BUN), and serum creatinine (SCr) underwent similar analyses.
Definitions
Metformin failure means an individualized HbA1C that remains outside a range of 6.5–8.5% within 3 months of treatment. Overweight translates to a BMI in the range of 25–30 kg/m2. On the other hand, obesity corresponds to a BMI of more than 30 kg/m2. Additionally, hypertension or high blood pressure (HTN) matches a SBP of more than 140 mmHg, a diastolic blood pressure (DBP) of more than 90 mmHg, or a patient receiving antihypertensive treatment. In contrast, team diagnosed dyslipidemia (DLP) in accordance with the American College of Cardiology and American Heart Association prevention guidelines.5 Moreover, primary physician decided whether a patient has Ischemic Heart Disease (IHD).6 Chronic Kidney Disease (CKD) refers to the term per the National Kidney Foundation Kidney Disease Outcome Quality Initiative (KDOQI) guidelines.7,8
Setting
Study site comprises the main facility that provides health-care services to the UAE military and their family members. It amounts to approximately 300 beds with about 50% occupancy and 8,000 admissions per year. We have an annual outpatient clinics census of about 270,000. In addition to endocrinology, medical and surgical specialties provided include cardiology, neurology, pulmonology, gastroenterology, infectious diseases, nephrology, pediatrics, and critical care, as well as general, specialty, and orthopedic surgeries.
Team
Study team consisted of two endocrinology consultants and one specialist. One clinical pharmacist rounded daily with the team. In addition, two dedicated pharmacists recorded the names, identifiers, and liraglutide, as well as other medications dispensing history. Moreover, pharmacists counseled patients to start liraglutide at a dose of 0.6 mg daily and increase weekly by 0.6 mg to the maintenance dose of 1.8 mg daily. Furthermore, clinical pharmacy supervisor served as the data keeper and analyst.
Procedure
Team developed a standardized data collection tool and definitions. Both endocrinologists3 and clinical pharmacists2 retrospectively filled these forms anonymously. Another staff validated first five cases of each investigator. Data included patient and staff codes, demographics, age and gender, visit number and date, follow-up site, height and weight, HbA1C, SBP and DBP, BUN, SCr, ALT, LDL, comorbidities, and medications used. Laboratory performed data measurements with standardized procedures and kits and reported them with common units and reference ranges. Patients consented to participation and the medical services corp ethics committee approved the study. Our research complied with the principles of the Declaration of Helsinki.
Statistical analyses
Authors present continuous data with average ± standard deviation and categorical data with ratios or percentages. They performed no simulation or replacement of missing data in both cases. Data keeper analyzed the data in two different ways. First, he performed a paired comparison of visits when variables were available for both visits using the paired t-test. Second, he ran ANOVA test (unpaired analysis) for more than two visits to compare the HbA1C, weight, and SBP and then calculated unpaired t-test P values as needed. In addition, subgroup analyses were carried out to determine the effectiveness of liraglutide in patients on or off other antidiabetic treatments (eg, on insulin vs. those off insulin). Team used StatTools, version 6.3.0 (Palisades Corp). In all univariate analyses, the team a priori considered a P value of less than 0.05 as statistically significant. However, the data analyst determined that multivariate analyses were not possible.
Results
A total of 152 patients received liraglutide from Zayed Military Hospital, according to the pharmacy-maintained list. However, only 100, 98, 62, 32, 8, and 5 had at least one, two, three, four, five, or six follow-up visits at our clinics, respectively. Table 1 shows the baseline characteristics of these patients. Our patients were middle age and were equally divided between genders and in terms of insulin therapy. All patients were either overweight, obese, or morbidly obese. Comorbidities with highest prevalence in the cohort were DLP and HTN occurring in 95 and 73 patients, respectively. CKD and IHD presented in around 10% of our cohort. Adherence to internal criteria of use of liraglutide was high at 92%.
Table 1.
Cohort baseline characteristics.
| VARIABLE | CATEGORIES (N) OR MEAN ± STANDARD DEVIATION |
|---|---|
| Age | 48.3 ± 11.6 years |
| Age 35–65 years | No: 22 |
| Yes: 78 | |
| Gender | M: Male (52) |
| F: Female (48) | |
| BMI | 25–30 kg/m2 (6) |
| 30–35 kg/m2 (28) | |
| 35–40 kg/m2 (21) | |
| 40–50 kg/m2 (17) | |
| Above 50 kg/m2 (3) | |
| Insulin | No: 54 |
| Yes: 46 | |
| Comorbidities | HTN (73) |
| CKD (11) | |
| IHD (9) | |
| DLP (95) |
Abbreviations: HTN, Hypertension; CKD, Chronic Kidney Disease; IHD, Ischemic Heart Disease; DLP, Dyslipidemia.
Glycated hemoglobin
First, liraglutide decreased HbA1C from first to second visit in all patients with available paired data regardless of their other treatments (8.7 ± 1.9 vs. 7.6 ± 1.8, paired t-test P < 0.0001, number [N] of patients who met inclusion and exclusion criteria in this comparison is 53), and this reduction was maintained in subsequent visits (visit 2: 7.6 ± 1.8 vs. visit 3: 7.4 ± 1.8, P = 0.1198, N = 36). Patients on insulin at baseline had large decline in HbA1C between the first and second follow-up visits (9.3 ± 2.0 vs. 8.1 ± 1.8, paired t-test P = 0.002, N = 26) and further dipped between second and third visits (8.3 ± 2.1 vs. 7.6 ± 2.2, P = 0.0131). Patients not on insulin had smaller but still statistically significant reductions in HbA1C between first and second visits (8.0 ± 1.8 vs. 7.2 ± 1.7, P = 0.016, N = 27), and this reduction was maintained in subsequent visits (visit 2: 6.9 ± 1.6 vs. 7.0 ± 1.6, P = 0.5363). All other patient subgroups including those with or without sulfonylureas, metformin, or combination of DPP4 inhibitor (sitagliptin) and metformin at baseline had significant reductions in their HbA1C between visits 1 and 2, and these comparisons are shown in Table 2. In the unpaired analysis of the data, HbA1C was reduced from first to second visit, although it did not reach significance. However, when we compared the reduction at third (fourth month) and fourth visits (sixth month) to the first visit, HbA1C reduction was significant (P value < 0.05). Overall absolute HbA1C reduction from first to fourth visit was 1.5% (Fig. 1).
Table 2.
Subgroup analyses using two-tailed paired t-test for HbA1C between 1st and 2nd visits (0 and 2 months).
| SUBGROUP (N) | HbA1C ON 1st VISIT | HbA1C ON 2nd VISIT | P VALUE |
|---|---|---|---|
| All (53) | 8.7 ± 1.9 | 7.6 ± 1.8 | <0.0001 |
| No insulin (27) | 8.0 ± 1.8 | 7.2 ± 1.7 | 0.016 |
| Insulin (26) | 9.3 ± 2.0 | 8.1 ± 1.8 | 0.002 |
| No metformin (11) | 8.6 ± 2.1 | 7.2 ± 0.8 | 0.0247 |
| Metformin (42) | 8.7 ± 2.0 | 7.7 ± 1.9 | 0.0012 |
| No DPP4 I + Met (37) | 8.7 ± 2.0 | 7.7 ± 1.7 | 0.0064 |
| DPP4 I + Met (16) | 8.7 ± 1.9 | 7.4 ± 1.9 | 0.0023 |
| No sulfonyurea (31) | 8.8 ± 1.5 | 7.9 ± 1.8 | 0.0063 |
| Sulfonylurea (22) | 8.5 ± 2.6 | 7.2 ± 1.6 | 0.0051 |
Note: P value < 0.05 is significant.
Abbreviations: N, Number of patients; DPP4 I, Dipeptidyl peptidase-4 Inhibitor (i.e. Sitagliptin in this study).
Figure 1.

Unpaired comparison of HbA1C in % mean ± 95% confidence interval error bounds. Months of follow-up (visits) on X axis. ANOVA P value < 0.05. All P values between visits were non-significant except for comparisons between visits on months 0 with months 4 or 6.
Weight
For all patients with available data, weight decreased between first and second follow-up visits (99.3 ± 19.3 vs. 98.0 ± 19.5, paired t-test, CI (−2.02, −0.64), P = 0.0003, N = 89). It seemed that this weight reduction was maintained between the second and third visits for the available weight data (100.4 ± 20.1 vs. 100.3 ± 20.3, CI (-0.70, 0.49), P = 0.8284). In the ANOVA and unpaired t-tests, an overall weight reduction of about 2.6 kg from visit 1 to visit 5 was not statistically significant (Fig. 2). However, unavailable data may have resulted in the analysis being underpowered to detect an intracohort reduction in weight between visits.
Figure 2.

Unpaired comparison of weights in kg mean ± 95% confidence interval error bounds. Months of follow-up (visits) on X axis. ANOVA P value > 0.05. All P values between visits were non-significant.
Blood pressure
Blood pressure decreased by about 1 mmHg between paired visits 1 and 2 (130.9 ± 15.8 vs. 129.9 ± 16.5, CI (−4.80, 2.72), P = 0.5896). It was further reduced to about 2 mmHg between visits 2 and 3 (130.3 ± 16.1 vs. 128.4 ± 17.2, CI [−6.35, 2.62], P = 0.4232). Moreover, no other paired comparisons yielded statistically significant results. Furthermore, in ANOVA and unpaired t-tests, a blood pressure reduction from visit 1 to visit 5 was by a mean of about 3.6 mmHg, and all comparisons were statistically nonsignificant as well (Fig. 3).
Figure 3.
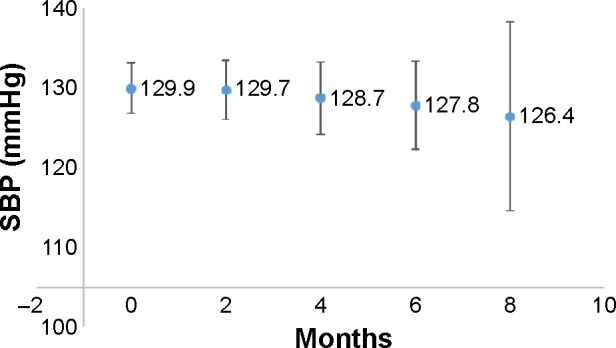
Unpaired comparison of SBP in mmHg mean ± 95% confidence interval error bounds. Months of follow-up (visits) on X axis. ANOVA P value > 0.05. All P values between visits were non-significant.
Other findings
Liraglutide has little and nonsignificant effect on ALT, LDL, BUN, and SCr. For example, ALT increased negligibly by about 2.5 U/L from visit 1–2 (45.2 ± 13.1 vs. 47.7 ± 17.5, not significant). In terms of tolerability, three patients discontinued liraglutide due to nausea and vomiting and only one had acute pancreatitis, which required hospitalization. Liraglutide was generally well tolerated.
Discussion
Liraglutide has significant paired reductions in HbA1C in all patients and across all subgroups of baseline therapies in our study. HbA1C average reductions from first to second followup visits ranged from 0.8% to 1.4%, depending on the background diabetes treatment. In the unpaired analysis, overall HbA1C decreased by an average of 1.5% from first to fourth follow-up visit. It was largely evident by the first 2–4 months (ie, between second and third follow-up visits). In addition, liraglutide maintained this effect in subsequent visits. These findings are the first reported in our region and are comparable with that seen in the LEAD program1 and other international literture.2,3 It further lends itself as evidence on the effectiveness of liraglutide in Emirati patients and possibly Arab patients.
Patients in our study have been titrated up to the maximum dose of 1.8 mg daily. Therefore, the effects on HbA1C (about 1.5% reduction overall) in our patients seem to be the maximum that could be achieved in our real-world scenario in Emirati patients. Although these effects are greater than those encountered with the lower dose of 0.6 mg daily, higher doses of up to 3 mg were used by others in weight reduction and to produce more HbA1C control.9,10 Hence, our team could not assess the effects that these higher doses may have.
Patients on insulin had greater reductions between first and second as well as second and third visits that were significant compared with patients who were not on insulin at baseline. This finding may be due to the fact that patients using insulin had greater baseline HbA1C. Additionally, other studies have shown concordant observations that adding liraglutide to insulin would result in greater effects.11,12 Moreover, we have shown that reductions in HbA1C are significant between first and second visits across all baseline diabetes treatment medication subgroups. On the other hand, Haraguchi et al13 showed that multiple oral antidiabetic medications, especially sulfonylureas, are predictors of a poor response to liraglutide. Interestingly, in our study, despite continuing their sulfonylureas and other antidiabetic medications, patients show significant reductions in HbA1C regardless of baseline treatments. This finding is very important because it indicates that Emirati patients taking sulfonylureas or other treatments can still benefit from liraglutide. Another important implication is that patients on insulin seem to be better responders to the additional control of HbA1C that liraglutide offers.
Our team studied HbA1C as a surrogate marker without evaluating the end outcomes such as micro- and macro-vascular complications. However, previous studies14,15 have shown that HbA1C reductions lead to significant decrease in diabetes complications. For example, in the United Kingdom Prospective Diabetes Study (UKPDS),14 it has been shown that a reduction of 1% in HbA1C is associated with a 25% risk reduction in diabetes micro-vascular complications. Although our region lacks similar data, authors expect that our patients will have similar benefits from the reduction in glycated hemoglobin.
Several studies demonstrated that liraglutide produces significant weight reduction in patients with diabetes.2,16,17 For example, Nauck et al16 found that adding liraglutide to metformin and titrating to a maintenance dose of 2 mg for 5 weeks resulted in about 3 kg weight reduction in patients with diabetes type II, which was significantly larger than that observed in patients who received metformin plus glimepride. Another research group explained that this weight loss in the liraglutide-treated patients might be due to diminishing fat tissue.18 Astrup et al10 published data that it was significantly a superior effect compared with the known medication orlistat. These findings lead to the use of liraglutide in female patients with polycystic ovary syndrome (PCOS) where it has been associated with more weight reduction than metformin and may as a result have a role in managing infertility in such patients.19 Potts et al20 has shown that both exenatide and liraglutide caused reduction in weight and that liraglutide 1.8 mg has an additional reduction compared with placebo of about 1.5 kg over a 5-week period. At the same time, our team region member countries have the highest prevalence of diabetes, PCOS, and infertility in the world.4 Therefore, liraglutide may offer our Emirati patients a new hope. Nevertheless, in our real-life evaluation, the weight reduction was not as pronounced as it has been shown in the other studies. Our maintenance dose of 1.8 mg significantly reduced weight but to a lesser extent of about 1.3 kg in the first 2 months of treatment. Multiple explanations may help understand these differences. One could be that efficacy trials are likely to produce higher estimates of effect. Another explanation is that our study recruits no control group, which, if present, may reveal a greater effect on weight in our patients. Additionally, it seems that there is a range of weight reductions in different studies with ours being on the lowest end and that of Nauck et al being at the other extreme. Potts et al gives a more modest and closer weight reduction estimate to the one we have in this study. Of note, Rizzo et al21,22 has summarized the effects of GLP1 agonists, including liraglutide, on weight stating that it extends from signaling satiety and reducing food intake to positive effects on myocardial cells, lipid profiles, blood pressure, and endothelial dysfunction. These agents may therefore be promising in even more dangerous situations such as metabolic syndrome and atherosclerosis.
Wang et al23 have carried out a meta-analysis of clinical trials to study the effect of liraglutide on blood pressure. They showed that liraglutide lowered both systolic and DBP by 1–5 mmHg when compared with placebo and other anti-diabetic medications. These reductions in blood pressure were statistically significant. For example, they have showed that liraglutide 1.8 mg resulted in a SBP reduction of about 4.49 mmHg compared with placebo. Fonseca et al24 has also shown in their pooled analysis of six randomized trials that a dose of 1.8 mg of liraglutide resulted in a significant SBP reduction compared with placebo at 26 weeks and evident by week 2 (2.9 ± 0.7 mmHg vs. 0.5 ± 0.9 mmHg). In our study, overall SBP reduction was not statistically significant in the paired analyses, but when considering all available data into account, there was about 3.6 mmHg reduction in SBP between visits 1 and 5, yet insignificant. We could reconcile these findings by noting that our study did not have a placebo group. Therefore, it is impossible to replicate the previous findings. Although we have seen SBP reductions in our group, which in the unpaired analysis could reach a 3.6 mmHg in 8 months, our intragroup comparisons between the months or visits were not statistically significant. Finally, measuring compliance, if it was at all possible, could have partially elucidated the reasons of these differences for both weight and SBP.
Liraglutide has a relatively good safety and tolerability profile in our study. Although pancreatitis has been reported,25,26 it only occurred in one patient (~1%) in our cohort. Patient was morbidly obese, and the case severity was mild–to-moderate. Although, we admitted the patient to the hospital and discontinued the liraglutide, the patient improved immediately on fluids, the pancreatic enzymes normalized, and patient had a good, short, and uncomplicated course. Also only three patients discontinued the medication due to nausea and vomiting. A previous study27 has shown that liraglutide can actually decrease liver enzymes through its effect on weight and glucose. In our study, however, there were no significant effects on liver enzymes. Lastly, liraglutide had very little effect on all the other variables including ALT and LDL.
Conclusion
This is the first study on liraglutide in the MENA and GCC regions to the best knowledge of the authors. It confirms that liraglutide in a real-world practice setting significantly reduced glycated hemoglobin and, to a lesser degree, weight as well as blood pressure in Emirati patients with type II diabetes with a relatively good tolerability profile. More studies are needed in our region to confirm these findings and to further elaborate on the effects of liraglutide on weight and blood pressure. For example, we would be interested to find out if the higher dose of 3 mg daily would have more significant effects on our female patients with diabetes, PCOS, and infertility. We would also love to see new data on the effects that liraglutide has on SBP, which may bridge the gap between our findings and those of international research groups.
Acknowledgments
The authors thank Dr. Ayesha Al Qasemi, Head of Pharmacy Department, at Zayed Military Hospital for her continuous support on doing this research project.
Footnotes
ACADEMIC EDITOR: Nigel Irwin, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,408 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: NKG, MN, DS, MA. Analyzed the data: NKG, LMS, SIAM. Wrote the 1st manuscript: NKG, LMS. Contributed to writing the manuscript: NKG, LMS, MN, DS, SIAM, MA. Agree with manuscript results and conclusions: NKG, LMS, MN, DS, SIAM, MA. Jointly developed the structure and arguments for the paper: NKG, LMS, MN, DS, SIAM, MA. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Nauck M, Frid A, Hermansen K, et al. Lead-2 Study Group Efficacy and safety comparison of liraglutide, glimperide, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fadini GP, Simioni N, Frison V, et al. Independent glucose and weight-reducing effect of liraglutide in a real-world population of type 2 diabetic outpatients. Acta Diabetol. 2013;50(6):943–949. doi: 10.1007/s00592-013-0489-3. [DOI] [PubMed] [Google Scholar]
- 3.Inoue K, Maeda N, Fujishima Y, et al. Long-term impact of liraglutide, glucagon-like peptide-1 (GLP-1) analogue, on body weight and glycemic control in Japanese type 2 diabetes: an observational study. Diabetol Metab Syndr. 2014;6(1):95–103. doi: 10.1186/1758-5996-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation World Atlas. 6th ed. 2013. [Accessed October 23, 2014]. Available at: http://www.idf.org/diabetesatlas/download-book.
- 5.Stone NJ, Robinson J, Liechtenstein HA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 6.Qaseem A, Fihn SD, Williams S, et al. Clinical Guidelines Committee of the American College of Physicians Diagnosis of stable ischmic heart disease: summary of a clinical practice guideline from the American College of Physicians/American Gollege of Caridology Foundation/American Heart Association/American Assosciatin for Thoracic Surgery/Preventive Cardiovascular Nurses Association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157(10):729–734. doi: 10.7326/0003-4819-157-10-201211200-00010. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 8.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 10.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2011;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827–832. doi: 10.1111/dom.12286. [DOI] [PubMed] [Google Scholar]
- 12.Gough SC, Bode B, Woo V, et al. NN9068-3697 (DUAL-I) Trial Investigators Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocinol. 2014;2(11):885–893. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- 13.Haraguchi A, Fujishima K, Ando T, et al. Multiple drug combination of antidiabetic agents as predictor of poor clinical response to liraglutide. Minerva Endocrinol. 2014;39(4):289–297. [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study (UKPDS) Group Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications with type 2 diabetes. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 15.Maple-Brown LJ, Ye C, Retnakaran R. Area-under-the-HbA1c-curve above the normal range and the prediction of microvascular outcomes: an analysis of data from the diabetes control and complications trial. Diabet Med. 2013;30(1):95–99. doi: 10.1111/dme.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nauck MA, Hompesch M, Filipczak R, et al. NN2211-1499 Study Group Five weeks of treatment with the GLP-1 analogue liraglutide improves glycaemic control and lowers body weight in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2006;114(8):417–423. doi: 10.1055/s-2006-924230. [DOI] [PubMed] [Google Scholar]
- 17.Niswender K, Pi-Sunyer X, Buse J, et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab. 2013;15(1):42–54. doi: 10.1111/j.1463-1326.2012.01673.x. [DOI] [PubMed] [Google Scholar]
- 18.Jendle J, Nauck MA, Matthews DR, et al. LEAD-2 and LEAD-3 Study Groups Weight_loss with_liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11(12):1163–1172. doi: 10.1111/j.1463-1326.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 19.Jensterle Sever M, Kocjan T, Pfeifer M, Kravos NA, Janez A. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol. 2014;170(3):451–459. doi: 10.1530/EJE-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The effect of glucagon-like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta-analysis. PLoS One. 2015;10(6):e0126769. doi: 10.1371/journal.pone.0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzo M, Nikolic D, Banach M, Patti AM, Montalto G, Rizvi AA. Incretin-based therapies, glucometabolic health and endovascular inflammation. Curr Pharm Des. 2014;20(31):4953–4960. doi: 10.2174/1381612819666131206102255. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo M, Chandalia M, Patti AM, et al. Liraglutide decreases carotid intimamedia thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc Diabetol. 2014;22(13):49. doi: 10.1186/1475-2840-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B, Zhong J, Lin H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013;15(8):737–749. doi: 10.1111/dom.12085. [DOI] [PubMed] [Google Scholar]
- 24.Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complications. 2014;28(3):399–405. doi: 10.1016/j.jdiacomp.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeyaraj S, Shetty AS, Kumar CR, et al. Liraglutide-induced acute pancreatitis. J Assoc Physicains India. 2014;62(1):64–66. [PubMed] [Google Scholar]
- 26.Chamler T, Almdal TP, Vilsbøll T, Knop FK. Adverse drug reactions associated with the use of liraglutide in patients with type 2 diabetes-focus on pancreatitis and pancreas cancer. Expert Opin Drug Saf. 2014;3:1–10. doi: 10.1517/14740338.2015.975205. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong MJ, Houlihan DD, Rowe IA, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Ailment Pharmacol Ther. 2013;37(2):234–242. doi: 10.1111/apt.12149. [DOI] [PubMed] [Google Scholar]


