Abstract
We found that resveratrol enhances interferon (IFN)-γ-induced tryptophanyl-tRNA-synthetase (TTS) expression in bone marrow-derived dendritic cells (BMDCs). Resveratrol-induced TTS expression is associated with glycogen synthase kinase-3β (GSK-3β) activity. In addition, we found that resveratrol regulates naïve CD8+ T-cell polarization by modulating GSK-3β activity in IFN-γ-stimulated BMDCs, and that resveratol induces upregulation of TTS in CD8+ T-cells in the in vivo tumor environment. Taken together, resveratrol upregulates IFN-γ-induced TTS expression in a GSK-3β-dependent manner, and this TTS modulation is crucial for DC-mediated T-cell modulation. [BMB Reports 2015; 48(5): 283-288]
Keywords: Bone marrow-derived dendritic cells, CD8+ T cell, Glycogen synthase kinase 3, Resveratrol, Tryptophanyl-tRNA synthetase
INTRODUCTION
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) involved in capture, processing, and presentation of antigens to T-cells (1). Mature stimulated DCs initiate immune and inflammatory responses, but immature DCs in the absence of appropriate stimulation lead to tolerance. Thus, DCs determine the balance between immunity and tolerance. (2). A representative mechanism that contributes to this tolerance is expression of the immunoregulatory enzyme indoleamine 2, 3-dioxygenase (IDO), which is essential for degrading the amino acid tryptophan (Trp) via the kynurenine pathway (2, 3). Tryptophanyl-tRNA synthetase (TTS) is a protector of IDO-induced immune tolerance by blocking IDO-mediated Trp catabolism. TTS is an IFN-γ-inducible enzyme and is constitutively expressed in various cell types (4). TTS is involved in the host-cell protective mechanism against Trp self-starvation (5). TTS catalyzes attachment of Trp to its cognate tRNA molecule, and the resulting Trp-tRNA complex is used for protein synthesis (6, 7).
Glycogen synthase kinase-3β (GSK-3β) is a multifunctional serine/threonine kinase found in all eukaryotes and was initially identified as a key regulator of insulin-dependent glycogen synthesis (8). In addition, GSK-3β is involved in diverse cellular processes, including proliferation, differentiation, motility, and survival (9). GSK-3β is a key integrator protecting mice against endotoxemia (10, 11). Furthermore, dysregulation of GSK-3β has been implicated in tumorigenesis and cancer progression.
Resveratrol (3,5,4’-trihydroxystilbene) is a phytochemical that has a variety of biological and pharmacological activities, such as anti-cancer, anti-inflammatory, and antioxidant effects in various cell types (12, 13). We reported previously that resveratrol downregulates lipopolysaccharide (LPS)-induced expression of interleukin (IL)-12 in LPS or IFN-γ-stimulated bone marrow-derived dendritic cells (BMDCs) (14, 15). The physiological functions of resveratrol under various conditions are well defined, but the detailed molecular mechanism mediated by resveratrol has not been addressed.
In this study, we showed that resveratrol significantly upregulated TTS as a protective mechanism against IDO-mediated Trp depletion in IFN-γ-stimulated BMDCs. In addition, we showed that resveratrol upregulates TTS by enhancing GSK-3β activity. Furthermore, we found that this GSK-3β-dependent TTS modulation is crucial for naïve CD8+ T-cell polarization.
RESULTS
Resveratrol upregulates IFN-γ-induced TTS expression in BMDCs
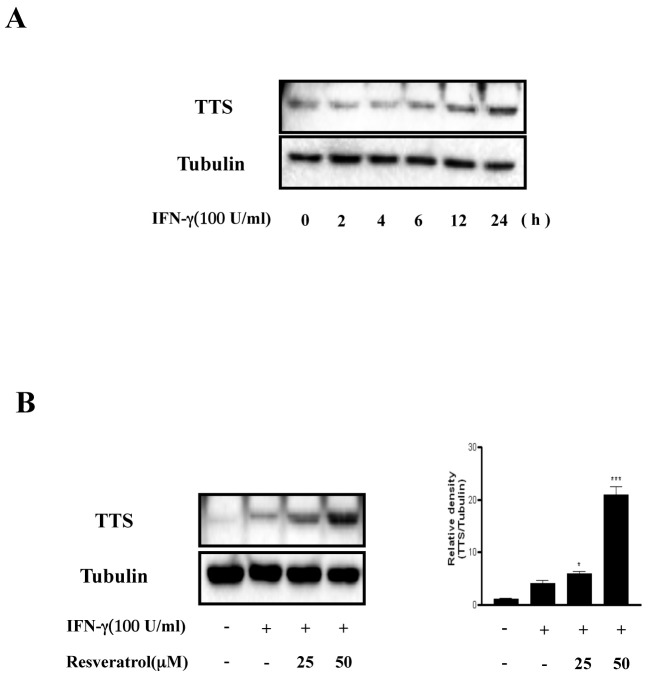
In a previous study, we found that resveratrol suppresses tumor progression by inhibiting IDO expression (15). TTS mainly functions as a protector against IDO-mediated Trp depletion. TTS catalyzes the attachment of Trp to a specific tRNA, resulting in the formation of a Trp-tRNA complex used for protein synthesis. This series of events protects cells from IDO-mediated Trp depletion (5). Based on the finding that resveratrol inhibits IFN-γ-induced IDO expression, we investigated whether resveratrol affects IFN-γ-mediated TTS expression. TTS was expressed 12-24 h after IFN-γ treatment (Fig. 1A). In addition, TTS expression was significantly higher in resveratrol- and IFN-γ-treated cells than that in IFN-γ-treated cells (Fig. 1B). We also observed IFN-γ-induced hyper-expression of TTS by resveratrol in splenocytes (Supplementary Fig. S1). These results indicate that IFN-γ-induced TTS expression is upregulated by resveratrol.
Fig. 1. Resveratrol upregulates TTS expression in BMDCs. (A), After IFN-γ (100 U/ml) treatment, the cells were harvested at the indicated times. (B), BMDCs were pretreated for 1 h in the absence or presence of resveratrol (25-50 μm) and incubated with IFN-γ (100 U/ml) for 18 h. The protein levels in the cell lysates were analyzed by Western blot analysis using the indicated antibodies. The mean and standard error values shown represent three independent experiments. * and *** represent significant differences at P < 0.05 and P < 0.001, respectively, compared with unstimulated cultures.
GSK-3β plays a key role regulating TTS
TTS is closely related to IDO and participates in the host-cell protective mechanism against Trp depletion (5). Thus, we investigated the direct regulation between IDO and TTS. IFN-γ- induced TTS expression remained unchanged with or without IDO (Supplementary Fig. S2). Thus, upregulation of TTS by resveratrol appears to be independent of the existence of IDO.
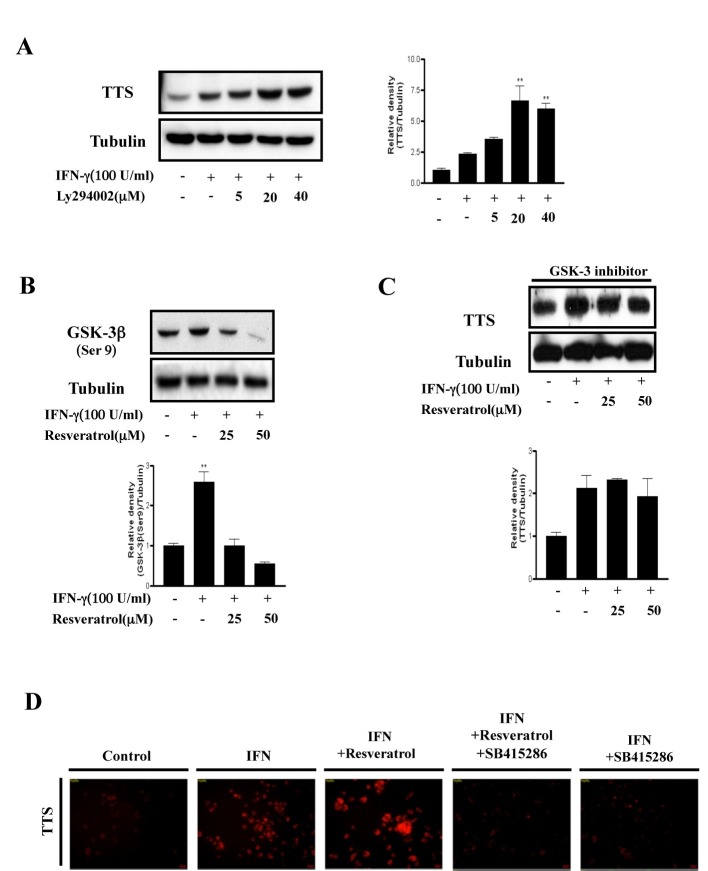
Phosphatidylinositol 3-kinase (PI3K) is crucial for IFN-γ-induced differential regulation of IDO and TTS in mouse microglia cells (4). In that study, inhibiting PI3K reduced IDO expression but enhanced that of TTS in the presence of IFN-γ. Consistent with those findings, we found previously that IFN-γ-induced IDO expression is downregulated by inhibiting PI3K (15). Here, we found that IFN-γ-induced TTS expression was upregulated by inhibiting PI3K using a PI3K-specific inhibitor (LY294002) (Fig. 2A). Next, we investigated whether GSK-3β, a downstream molecule of PI3K, plays an important role upregulating TTS expression. Resveratrol significantly reduced GSK-3β phosphorylation at Ser9, which is the GSK-3β inhibitory phosphorylation site (Fig. 2B). This result indicates that resveratrol potently activates GSK-3β. Next, we investigated the effect of GSK-3β on IFN-γ-induced TTS expression. As shown in Fig. 2C, IFN-γ-induced TTS expression did not increase in response to resveratrol in the presence of a GSK-3 inhibitor (SB415286). We also confirmed this result by immunofluorescence assay (IFA) (Fig. 2D). These results show that GSK-3β activity is crucial for resveratrol-mediated IFN-γ-induced TTS hyper-expression in BMDCs.
Fig. 2. GSK-3β plays a key role regulating TTS. (A), BMDCs were pretreated for 30 min in the absence or presence of a specific PI3K inhibitor (LY294002; 25-50 μm) and incubated with IFN-γ (100 U/ml) for 18 h. The mean and standard error values shown represent two independent experiments. * and ** represent significant differences at P < 0.05 and P < 0.01, respectively, compared with unstimulated cultures. (B), BMDCs were preincubated for 1 h in the absence or presence of resveratrol (25-50 μm) and stimulated with IFN-γ (100 U/mL) for 30 min. ** significant difference at P < 0.01, compared with unstimulated cells. The results are representative of two independent experiments. (C), BMDCs were pretreated for 30 min in the presence of a GSK-3 specific inhibitor (SB415286), treated with resveratrol (25-50 μm) for 1 h, and incubated with IFN-γ (100 U/ml) for 18 h. The protein levels in the cell lysates were analyzed by Western blot using the indicated antibodies. The mean and standard error values shown represent two independent experiments. (D), BMDCs were pretreated with or without a GSK-3 inhibitor (SB415286) for 30 min, treated with resveratrol (25-50 μm) for 1 h, and then incubated with IFN-γ (100 U/ml) for 18 h. The cells were fixed in 4% paraformaldehyde for 10 min, stained with mouse anti-TTS antibodies overnight at 4℃, and then stained with Alexa568-conjugated anti-mouse antibodies for 1 h at room temperature. Cell morphology and fluorescence intensity were analyzed using a Zeiss LSM510 Meta confocal laser scanning microscope. Results are representative of three independent experiments.
Resveratrol modulates DC-mediated naïve CD 8+ T-cell proliferation
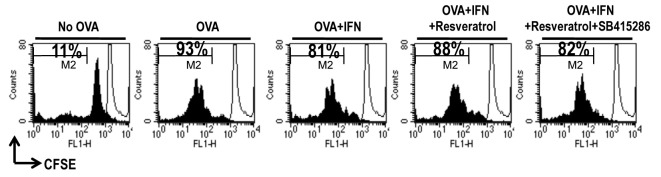
We performed a mixed lymphocyte reaction (MLR) assay using CD8+ T-cells from OT-1 TCR transgenic mice, which express a TCR specific for the MHC class I-restricted OVA peptide 257-264 antigen (OVA257-264) in DCs, to determine whether resveratrol-mediated TTS hyper-expression under an IFN-γ-stimulated condition affects T-cell proliferation (16). Proliferation of CFSE-labeled OVA-specific CD8+ T-cells co-cultured with OVA257-264-pulsed DCs was significantly higher than that of T-cells co-cultured with unpulsed DCs (Fig. 3). OVA-pulsed DC-induced naïve CD8+ T-cell polarization was inhibited by IFN-γ, and resveratrol restored the IFN-γ-mediated suppression of CD8+ T-cell proliferation (Fig. 3). Furthermore, naïve CD8+ T-cell proliferation due to resveratrol was not restored under the GSK-3 inhibitor-treated condition (Fig. 3). In addition, we obtained data consistent with previous naïve CD8+ T-cell proliferation data in an OVA-specific CTL assay (Supplementary Fig. S3). Thus, IFN-γ-induced activation of GSK-3β in DCs is crucial for naïve CD8+ T-cell proliferation.
Fig. 3. Resveratrol modulates DC-mediated naïve CD 8+ T-cell proliferation. Immature DCs, OVA-pulsed DCs, OVA-pulsed IFN-γ-treated DCs, OVA-pulsed IFN-γ + resveratrol-treated DCs, or OVA-pulsed IFN-γ + resveratrol + GSK-3 inhibitor-treated DCs were cultured with CFSE-labeled splenocytes from OT-1 T-cell receptor transgenic mice (1 × 106 per well) for 96 h. The cells were harvested after 4 days, stained with Cy5-labeled anti-CD8 monoclonal Ab, and analyzed by flow cytometry. Histograms showing CD8+ T-cell proliferation as assessed by flow cytometry. Results are representative of three independent experiments.
Administering resveratrol suppresses tumor growth by regulating TTS expression in E.G7 thymoma tumors
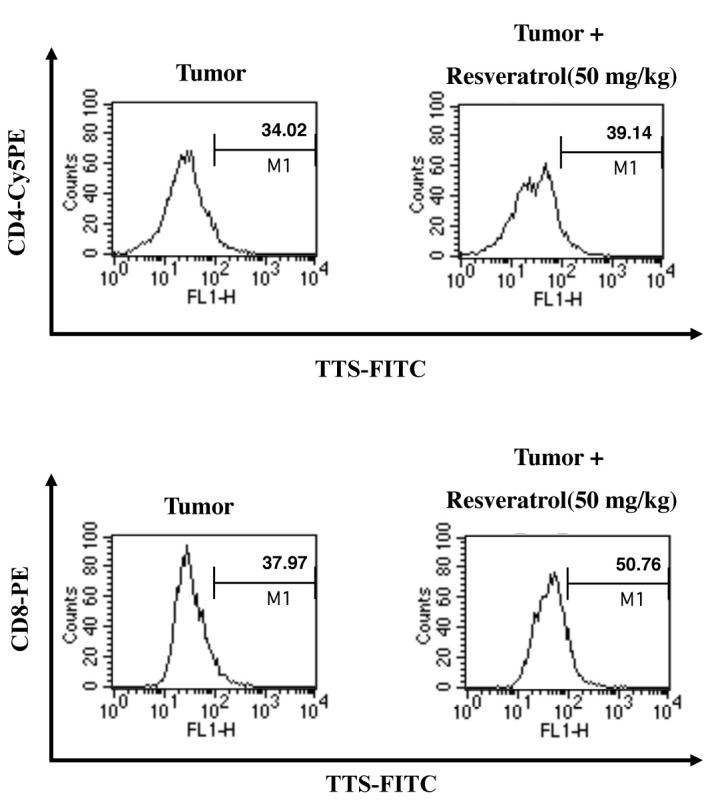
Resveratrol modulates the TTS immunoregulatory protein; thus, we investigated the anti-tumor activity of resveratrol in vivo by determining its effect on regulation of TTS. In a previous report, we found that tumor growth in resveratrol-treated mice is suppressed (15) and examined TTS levels. We confirmed whether retarding tumor growth by administering resveratrol was caused by enhanced TTS expression in CD4+ and CD8+ T cells using intracellular staining and fluorescence- activated cell sorting (FACS) analysis. Resveratrol increased the population of TTS-positive CD4+ and CD8+ T-cells in tumor- draining lymph nodes (Fig. 4). TTS expression was significantly higher in CD8+ T-cells than that in CD4+ T-cells. These data suggest that resveratrol enhances the CD8+ T cell-mediated anti-tumor response by upregulating TTS in the tumor environment.
Fig. 4. Resveratrol increases the TTS-positive CD4+ and CD8+ T cell populations in vivo. C57BL/6 mice were implanted s.c. with 3 × 105 E.G7 thymoma cells in 0.1 ml PBS. The mice were injected i.p. with resveratrol (50 mg/kg) every 2 days for 3 weeks, and tumor masses were obtained. Disrupted tumor cells were stained with Cy5 anti-mouse CD4, phycoerythrin (PE)-conjugated anti-CD8, and anti-mouse TTS monoclonal Abs (as primary Abs) and FITC-conjugated rabbit-anti-mouse IgG (as a secondary antibody). The cells were analyzed by flow cytometry. Results are representative of three independent experiments.
DISCUSSION
Tumors induce immune tolerance by modulating the host immune system. Therefore, they evade local immune destruction, despite the systemic presence of tumor-reactive T- cells (2). It is important to understand that immunoregulatory proteins contribute to the immune response to understand tumor-mediated immune tolerance. The molecular mechanisms underlying tumor-induced tolerance are poorly understood. DCs play a pivotal role initiating the immune response, as APCs present tumor antigens to T-cells. The presentation of tumor-derived antigens by host APCs is a key step by which naïve T-cells first become sensitized to tumor antigens (17). IDO is expressed in various cells, including DCs, macrophages, and tumors. Enhanced IDO expression in tumor-associated APCs inhibits the T-cell response to tumor antigens by suppressing T-cell priming in tumor-draining lymph nodes (18).
IDO regulates the immune response by inhibiting T-cell function, depleting the essential amino acid Trp, and generating Trp catabolites (2). Trp starvation caused by IDO can be prevented by TTS, a host cell-expressed enzyme, as a protective mechanism (5). This enzyme ensures that Trp is directly available for protein synthesis and protects cells from IDO-induced Trp depletion by allowing them to use limited amounts of Trp during protein synthesis (5). Paradoxically, several studies have emphasized the importance of downregulating TTS in several autoimmune diseases, such as rheumatoid arthritis and Graves’ disease (19). This is because TTS overexpression contributes to T-cell hyperactivity and results in the destruction of normal cells.
Thus, IDO and TTS play important roles in immune suppression and activation (5, 20). However, the detailed molecular mechanisms underlying the differential expression of these enzymes in immune regulation are unknown. Thus, we investigated regulation of these immunomodulatory enzymes using resveratrol in DCs and the tumor environment. In a previous study, we revealed that resveratrol suppresses IDO transcriptional and functional activities (15). In addition, resveratrol dramatically inhibits interferon regulatory factor-1 expression and IFN-γ-induced activation of the JAK/STAT1- and PKC-δ-dependent signaling pathways, which are essential for IDO expression (15). A recent study showed that STAT-1 involvement is essential for modulating IDO and TTS in microglia, and that expression of either enzyme is modulated by a different mechanism (4). Consistent with this result, we found that resveratrol induced differential expression of IDO and TTS via the JAK/STAT1-, PKC-δ-, and GSK-3β-dependent signaling pathways here and in a previous report (15). Several in vitro and animal model studies have reported that resveratrol has anti-cancer properties (21-23). In particular, resveratrol suppresses the development and progression of various cancers by regulating multiple pathways, including apoptosis, cell cycle arrest, and activation of transcription factors, such as nuclear factor-kappa B and activator protein-1 (24). Thus, we inferred that differential regulation of IDO and TTS by resveratrol could be a crucial mechanism of immunogenicity and tumor-mediated immunological escape by cancer. Consistent with previous studies and our hypothesis, we found that resveratrol suppressed tumor growth by regulating the immune response via modulation of two distinct enzymes, such as IDO and TTS, in a GSK-3β-dependent manner in immune cells and the tumor environment.
Interestingly, the resveratrol-mediated increase in the population of TTS-positive cells was more pronounced in CD8+ T-cells than that in CD4+ T-cells in the in vivo tumor environment (Fig. 4). Based on these data, we concluded that the resveratrol-induced anti-tumor effect occurs via TTS-mediated polarization to CD8+ T-cells.
Taken together, our results suggest that resveratrol regulates the DC-mediated immune response via GSK-3β-dependent-TTS expression. In addition, resveratrol enhances the T cell-mediated anti-tumor response by upregulating of TTS in the tumor environment.
MATERIALS AND METHODS
Mice
Eight- to ten-week-old male C57BL/6 (H-2Kb and I-Ab) mice were purchased from the Korean Institute of Chemistry Technology (Daejeon, Korea). C57BL/6 OT-1 T-cell receptor transgenic mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The animals were housed in a specific pathogen-free environment within our animal facility and handled in accordance with the institutional guidelines for animal care.
Cells and cell culture
The E.G7 cell line, an OVA-expressing EL4 variant, was purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM L-glutamine (all from Invitrogen, Carlsbad, CA, USA) at 37℃ in a 5% CO2 atmosphere.
Reagents and antibodies
Recombinant mouse (rm) granulocyte macrophage colony- stimulating factor (GM-CSF), rm IL-4, and rm IFN-γ were purchased from R&D Systems (Minneapolis, MN, USA). Resveratrol (>99% purity) was obtained from Sigma-Aldrich (St. Louis, MO, USA). SB415286, a GSK-3 inhibitor, was obtained from Tocris Bioscience (Bristol, UK). Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated monoclonal antibodies (Abs) used to detect expression of CD11c (HL3), CD4 (L3T4), and CD8 (Lyt-2) were purchased from BD Pharmingen (San Diego, CA, USA). To detect protein levels by Western blotting, anti-phosphoserine-GSK-3β (Ser9) was purchased from Cell Signaling Technology (Beverly, MA, USA). Polyclonal rabbit anti-mouse Abs against TTS and α-tubulin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Generation of murine BMDCs
BMDCs were isolated and cultured as described previously (15, 25, 26). BM was flushed from the tibiae and femurs of C57BL/6 mice and depleted of red blood cells with ammonium chloride. The cells were plated in 6-well culture plates (106 cells/ml; 3ml/well) in OptiMEM (Invitrogen) supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μm 2-mercaptoethanol, 10 mM HEPES (pH 7.4), 20 ng/ml rm GM-CSF, and 10 ng/ml rm IL-4 at 37℃ in a 5% CO2 atmosphere. On culture day 3, floating cells were gently removed, and fresh medium was added. Nonadherent cells and loosely adherent proliferating DC aggregates were harvested for analysis or stimulation on culture day 6. On day 6, ≥ 80% of the nonadherent cells expressed CD11c. The DCs were labeled with a bead-conjugated anti-CD11c mAb (Miltenyi Biotec, Bergisch Gladbach, Germany) to obtain highly purified CD11c-expressing populations for subsequent analyses, which were subjected to positive selection on paramagnetic columns (LS columns; Miltenyi Biotec), according to the manufacturer’s instructions. The purity of the selected cell fraction was > 90%.
Western blot analysis
Cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk in washing buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.1% Tween 20) and incubated with the indicated antibodies for 1 h at room temperature. The membranes were washed and incubated for 1 h at room temperature with the appropriate secondary antibodies conjugated with horseradish peroxidase (Amersham Pharmacia Biotech, Uppsala, Sweden). Protein bands were visualized using an enhanced chemiluminescence system (Amersham Pharmacia Biotech).
IFA
Cells were fixed in 4% paraformaldehyde for 10 min, and incubated with the indicated antibodies. The cells were incubated with Alexa 488- and Alexa 568-conjugated secondary antibodies (Invitrogen) for 1 h at room temperature. All images were captured with a fluorescence microscope (D80i; Nikon, Tokyo, Japan). Results are representative of three independent experiments.
Mixed lymphocyte reaction
The MLR was performed as described previously (27). Transgenic OVA-specific CD8+ T-cells were purified from bulk splenocytes by negative selection using a mouse CD8+ T-cell kit (Miltenyi Biotec). The purity of the cell population obtained was >93% by flow cytometry (Becton Dickinson, San Jose, CA, USA) after staining with a Cy5-conjugated anti-CD8 Ab. Briefly, the cells were resuspended in 5 μM carboxyfluorscein diacetate succinimidyl ester (CFSE) in PBS and shaken for 10 min at room temperature. Next, the cells were washed once in pure FBS and twice in PBS with 10% FBS. Immature DCs, OVA-pulsed DCs, OVA-pulsed IFN-γ-treated DCs, OVA-pulsed IFN-γ + resveratrol-treated DCs, or OVA-pulsed IFN-γ + resveratrol + GSK-3 inhibitor-treated DCs (1 × 105) were subsequently co-cultured with 1 × 106 allogeneic CFSE-labeled T lymphocytes in 96-well U-bottom plates. The cells were harvested after 4 days, stained with a Cy5-labeled anti-CD8 monoclonal Ab (to gate OT-1 T-cells), and assessed by flow cytometry.
Analysis of intracellular TTS expression in E.G7 thymoma tumor-bearing mice by flow cytometry in vivo
Tumors were excised, disrupted, and the cells were harvested and washed twice with PBS containing 2% FBS and 0.1% sodium azide to detect intracellular levels of TTS in tumor-infiltrated CD4+ and CD8+ T-cells. We used the BD Cytofix/Cytoperm Kit (BD Pharmingen) for intracellular TTS staining, following the manufacturer’s instructions. The intracellular TTS protein was detected with a mouse anti-TTS mAb (Santa Cruz Biotechnology) and FITC conjugated-anti-mouse IgG (Invitrogen). The cells were stained with Cy5 anti-mouse CD4 and PE-conjugated anti-CD8 for the surface molecule analysis. The stained cells were analyzed by flow cytometry.
Acknowledgments
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (NRF-2012R1A1A2042924 and NRF-2012R1A2A1A03008433).
References
- 1.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. (2000);18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. (2004);4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 3.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. (2009);9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 4.Yadav MC, Burudi EM, Alirezaei M, et al. IFN-gamma-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia. (2007);55:1385–1396. doi: 10.1002/glia.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. (2005);105:1574–1581. doi: 10.1182/blood-2004-06-2089. [DOI] [PubMed] [Google Scholar]
- 6.Fleckner J, Martensen PM, Tolstrup AB, Kjeldgaard NO, Justesen J. Differential regulation of the human, interferon inducible tryptophanyl-tRNA synthetase by various cytokines in cell lines. Cytokine. (1995);7:70–77. doi: 10.1006/cyto.1995.1009. [DOI] [PubMed] [Google Scholar]
- 7.Rubin BY, Anderson SL, Xing L, Powell RJ, Tate WP. Interferon induces tryptophanyl-tRNA synthetase expression in human fibroblasts. J Biol Chem. (1991);266:24245–24248. [PubMed] [Google Scholar]
- 8.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. (2003);116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. (2009);273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. (2005);6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noh KT, Park YM, Cho SG, Choi EJ. GSK-3beta-induced ASK1 stabilization is crucial in LPS-induced endotoxin shock. Exp Cell Res. (2011);317:1663–1668. doi: 10.1016/j.yexcr.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. (2006);5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 13.Subramanian L, Youssef S, Bhattacharya S, Kenealey J, Polans AS, van Ginkel PR. Resveratrol: challenges in translation to the clinic--a critical discussion. Clin Cancer Res. (2010);16:5942–5948. doi: 10.1158/1078-0432.CCR-10-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.253. Kim GY, Cho H, Ahn SC, Oh YH, Lee CM, Park YM. Resveratrol inhibits phenotypic and functional maturation of murine bone marrow-derived dendritic cells. Int Immunopharmacol. (2004);4:245–253. doi: 10.1016/j.intimp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Noh KT, Chae SH, Chun SH, Jung ID, Kang HK, Park YM. Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. (2013);431:348–353. doi: 10.1016/j.bbrc.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 16.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. (1994);76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 17.Yu Z, Theoret MR, Touloukian CE, et al. Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance. J Clin Invest. (2004);114:551–559. doi: 10.1172/JCI200421695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. (2005);338:20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Ji F, Wang Y, et al. Synovial autoreactive T cells in rheumatoid arthritis resist IDO-mediated inhibition. J Immunol. (2006);177:8226–8233. doi: 10.4049/jimmunol.177.11.8226. [DOI] [PubMed] [Google Scholar]
- 20.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. (2008);27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 21.Dorrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. (2001);61:4731–4739. [PubMed] [Google Scholar]
- 22.Fukui M, Yamabe N, Zhu BT. Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur J Cancer. (2010);46:1882–1891. doi: 10.1016/j.ejca.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun W, Wang W, Kim J, et al. Anti-cancer effect of resveratrol is associated with induction of apoptosis via a mitochondrial pathway alignment. Adv Exp Med Biol. (2008);614:179–186. doi: 10.1007/978-0-387-74911-2_21. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. (2004);24:2783–2840. [PubMed] [Google Scholar]
- 25.Lee SJ, Noh KT, Kang TH, et al. The Mycobacterium avium subsp. Paratuberculosis protein MAP1305 modulates dendritic cell-mediated T cell proliferation through Toll-like receptor-4. BMB Rep. (2014);47:115–120. doi: 10.5483/BMBRep.2014.47.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, Shin SJ, Lee SJ, et al. Mycobacterium abscessus MAB2560 induces maturation of dendritic cells via Toll-like receptor 4 and drives Th1 immune response. BMB Rep. (2014);47:512–517. doi: 10.5483/BMBRep.2014.47.9.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung ID, Jeong SK, Lee CM, et al. Enhanced efficacy of therapeutic cancer vaccines produced by co-treatment with Mycobacterium tuberculosis heparin-binding hemagglutinin, a novel TLR4 agonist. Cancer Res. (2011);71:2858–2870. doi: 10.1158/0008-5472.CAN-10-3487. [DOI] [PubMed] [Google Scholar]