Abstract
Background
Despite being a highly vascularized tumor, glioblastoma response to anti-vascular endothelial growth factor (VEGF) therapy is transient, possibly because of tumor co-option of preexisting blood vessels and infiltration into surrounding brain. Integrins, which are upregulated after VEGF inhibition, may play a critical role in this resistance mechanism. We designed a study of cediranib, a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor, combined with cilengitide, an integrin inhibitor.
Methods
This phase I study was conducted through the Adult Brain Tumor Consortium in patients with recurrent glioblastoma. Once the maximum tolerated dose was determined, 40 patients enrolled in a dose expansion cohort with 20 being exposed to anti-VEGF therapy and 20 being naive. The primary endpoint was safety. Secondary endpoints included overall survival, proportion of participants alive and progression free at 6 months, radiographic response, and exploratory analyses of physiological imaging and blood biomarkers.
Results
Forty-five patients enrolled, and no dose toxicities were observed at a dose of cediranib 30 mg daily and cilengitide 2000 mg twice weekly. Complete response was seen in 2 participants, partial response in 2, stable disease in 13, and progression in 21; 7 participants were not evaluable. Median overall survival was 6.5 months, median progression-free survival was 1.9 months, and progression-free survival at 6 months was 4.4%. Plasma-soluble VEGFR2 decreased with treatment and placental growth factor, carbonic anhydrase IX, and SDF1α, and cerebral blood flow increased.
Conclusions
The combination of cediranib with cilengitide was well tolerated and associated with changes in pharmacodynamic blood and imaging biomarkers. However, the survival and response rates do not warrant further development of this combination.
Keywords: angiogenesis, biomarker, glioblastoma, invasion, treatment
The anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab is the only US FDA-approved drug for recurrent glioblastoma (GBM), but the duration of response is short with most patients relapsing within 4–5 months.1 Two of the proposed mechanisms of bevacizumab resistance, based on preclinical studies, include upregulation of alternate proangiogenic pathways and co-option of native brain blood vessels in order to maintain adequate nutrient sources for the growing tumor.2,3 It is unclear if one or both of these mechanisms mediates resistance in recurrent GBM patients.
Preclinical data in orthotopic mouse models suggest that treatment with an anti-VEGF agent blocks angiogenesis and/or results in pruning of existing GBM vessels. In turn, this may lead to a shift in the mode of growth characterized by tumor cells tracking along or co-opting native brain blood vessels and infiltrating into the surrounding brain.4,5 This allows tumor cells to hide behind an intact blood-brain barrier, making them less susceptible to chemotherapies that do not penetrate the brain well. In support of this resistance mechanism in GBM patients, clinical trials using antiangiogenic agents suggested that there was a disproportionate increase in peritumoral fluid-attenuated inversion recovery (FLAIR) hyperintensity compared with contrast enhancement, which was thought to represent infiltrative tumor growth.6 Given this heightened concern about infiltrative relapse, radiographic guidelines for measuring brain tumor response were revised to include a significant increase in FLAIR hyperintensity as a possible criterion to determine tumor progression.7
Integrins are cell surface receptors mediating cell-cell and cell-extracellular matrix interactions and are key players in allowing tumor cells to migrate in the brain. Blocking integrins decreases tumor cell motility.8–10 Moreover, integrin inhibition is known to upregulate VEGFR2 activity and paradoxically stimulate tumor growth and angiogenesis when the alpha(v)beta(3) and alpha(v)beta(5) inhibitors reach low (nanomolar) concentrations in preclinical models.11 This further supports the rationale of combining anti-VEGFR2 therapy with cilengitide.
We designed a phase I trial of cediranib (an oral pan-VEGFR tyrosine kinase inhibitor) and cilengitide (an integrin inhibitor with anti-invasive and antiangiogenic properties) in patients with recurrent GBM to determine if we could safely block infiltrative tumor growth and enhance the efficacy of cediranib. The trial included MRI and blood biomarker studies to evaluate tumor infiltration and explore the association of biomarker candidates with GBM response or resistance to this regimen.
Materials and Methods
Study Design
This was an open-label phase I study conducted through the Adult Brain Tumor Consortium (ABTC) in patients with recurrent GBM (NCT00979862). The trial was approved by the institutional review boards at all participating sites. Prior to enrollment, all patients signed an informed consent document. The first part of the study consisted of a dose-finding phase to determine the maximal tolerated dose (MTD) of cediranib in combination with cilengitide. The starting dose for cediranib was 30 mg by mouth daily, and cilengitide was 2000 mg intravenously twice weekly. Based on prior studies with these drugs, either alone or in combination, this dosing schedule was anticipated to be the MTD; thus, dose de-escalation seemed reasonable.12,13 Once the MTD was determined, a dose cohort expansion group was enrolled in which there were 2 arms. Arm 1 included participants who had received prior anti-VEGF therapy, and arm 2 included participants who had never received prior anti-VEGF therapy. Treatment response was assessed every 8 weeks by the Response Assessment in Neuro-Oncology (RANO) criteria.7
Patient Eligibility
Patients with recurrent GBM (WHO grade IV) who met the following criteria were eligible: age ≥18 years, Karnofsky performance status score ≥60%, mini-mental score >15, at least 1 cm of residual enhancing disease, at least 3 months since radiation, ≤2 prior tumor relapses, stable steroid dose for 5 days prior to baseline MRI, and adequate bone marrow/organ function. Key exclusion criteria included concurrent therapeutic anticoagulation, pregnancy/breast feeding, significant intercurrent illness, concurrent malignancy, concurrent enzyme-inducing antiepileptic drug use, and significant intratumoral hemorrhage.
Correlative Imaging Studies
Fifteen of the participants enrolled on arm 2 (patients who had not received prior anti-VEGF therapy) underwent advanced MRI scans that included dynamic susceptibility contrast imaging as well as routine sequences (eg, post contrast, diffusion weighted, and FLAIR images). These MRIs were obtained at the following time points: within 5 days before starting therapy, 24–72 hours after starting therapy, and 1 month and 2 months after starting therapy. For these participants, tumor volumes were outlined on postcontrast images, and surrounding areas of abnormal hyperintensity were outlined on FLAIR images. Median apparent diffusion coefficient (ADC) and median cerebral blood flow (CBF)/cerebral blood volume (CBV) were calculated in these regions of interest. Both spin echo and gradient echo dynamic susceptibility contrast images were obtained to look at changes in small vessels (measured by spin echo sequences) and larger vessels (measured by gradient echo sequences).
Correlative Blood Biomarker Studies
Blood was obtained all patients participating in the dose-cohort expansion to assess circulating levels of plasma biomarkers of angiogenesis and inflammation. The blood was processed as previously described.14 In brief, blood samples were collected in EDTA-containing tubes. Plasma samples were separated by centrifugation and then aliquoted and stored at −80°C until being used for ELISA measurements. Measurements were carried out for circulating VEGF, placental growth factor (PlGF), soluble (s)VEGFR1, and basic fibroblast growth factor using the Human Angiogenesis Panel 1 Kit (K15190D). Interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α were analyzed using Human Proinflammatory-4 Kit (K15025A) from Meso-Scale Discovery. Stromal cell-derived factor (SDF1α), carbonic anhydrase IX (CAIX), sVEGFR2, sTie-2, and Ang-2 were measured using ELISA kits from R&D Systems. Collagen IV was measured using ELISA kits from Exocell, Inc. Every sample was run in duplicate.
Statistical Analysis
The primary endpoint of the study was to determine the safe dose of cediranib in combination with cilengitide in patients with recurrent GBM. Secondary endpoints included assessment of median overall survival (OS), 6-month progression-free survival (PFS-6), and radiographic response using RANO criteria as measured from treatment start date. Survival probability was estimated using the Kaplan-Meier method. The proportion of PFS-6 was estimated using binomial distribution along with 95% confidence interval. Exploratory analyses using Cox proportional hazard models were performed to explore potential associations of the impact imaging and blood biomarkers had on OS and PFS. Changes in blood biomarkers during treatment were expressed as median and interquartile range and were compared with baseline levels. Changes in imaging parameters were expressed as absolute difference from baseline. Given the exploratory nature of these analyses, no adjustment was made for multiple testing. All participants were included in the analyses for response since the dose of both drugs was the same. All P values were reported as 2 sided, and all analyses were conducted using SAS software (version 9.2, SAS Institute).
Results
Patient Characteristics
Forty-five patients with recurrent GBM were enrolled between March 2010 and December 2011. Five participants were enrolled in the dose-finding cohort, and 20 participants were enrolled in each of the 2 arms of the dose cohort expansion. Participant characteristics are outlined in Table 1.
Table 1.
Baseline participant characteristics
| Prior, Anti-VEGF (N = 20) | Anti-VEGF, Naïve (N = 20) | Total (N = 45) | |
|---|---|---|---|
| Age, y | |||
| Median | 54.18 | 54.35 | 54 |
| Range | 22.5–69.41 | 25.9–72.7 | 22.5–80.4 |
| Sex, n (%) | |||
| Male | 14 (70) | 14 (70) | 33 (73) |
| Female | 6 (30) | 6 (30) | 12 (27) |
| Karnofsky performance status | |||
| Median | 80% | 80% | 80% |
| Range | 60%–100% | 60%–100% | 60%–100% |
| Mini-mental score | |||
| Median | 27 | 29 | 29 |
| Range | 17–30 | 22–30 | 17–30 |
| Anticonvulsant, n (%) | |||
| Yes | 15 (75) | 13 (65) | 33 (73) |
| No | 5 (25) | 7 (35) | 12 (27) |
Toxicity
No dose-limiting toxicities were observed, so dose reductions were not required; the combined dose of cediranib 30 mg p.o. daily and cilengitide 2000 mg i.v. twice weekly was used for the dose cohort expansion phase of the study. All grade 3/4 toxicities possibly or likely related to treatment were expected (Table 2). Seven participants (16%) stopped treatment because of toxicity.
Table 2.
Grade III or IV toxicities possibly or likely related to treatment (N = 45)
| Toxicity | Cediranib | Cilengitide |
|---|---|---|
| Elevated ALT/AST | 5 (11%) | 2 (4%) |
| Cognitive disturbance/confusion | 1 (2%) | 1 (2%) |
| Diarrhea | 2 (4%) | 1 (2%) |
| Duodenal hemorrhage | 1 (2%) | 1 (2%) |
| Headache | 2 (4%) | 2 (4%) |
| Hypertension | 13 (29%) | 4 (9%) |
| Decreased lymphocyte | 1 (2%) | 1 (2%) |
| Hypophosphatemia | 1 (2%) | 1 (2%) |
| Thrombocytopenia | 0 | 3 (7%) |
| Seizure | 1 (2%) | 1 (2%) |
| Abdominal cramping | 1 (2%) | 0 |
| Fatigue | 1 (2%) | 1 (2%) |
Response
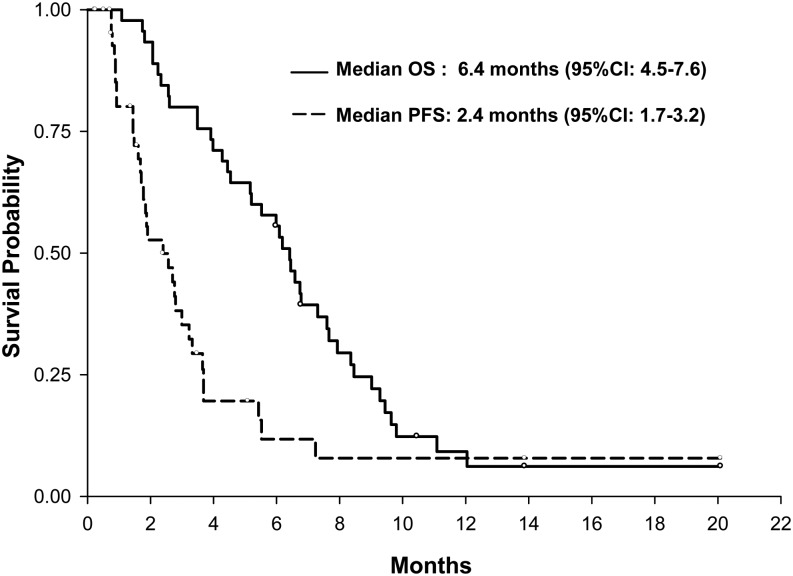
The best response was 2 participants with complete response (5%). Two participants achieved partial response (5%), 13 participants showed stable disease (30%), and 21 participants demonstrated progression (49%) (Table 3). The total response rate was 8.9% (95%CI, 2.5%–21.2%). Seven participants were not evaluable because they failed to undergo repeat imaging to assess response. Median OS for all participants was 6.5 months (95% CI, 5.2–7.6 mo), and median PFS was 1.9 months (95% CI, 1.5–2.8 mo) (Fig. 1). The PFS-6 was 4.4% (95%CI, 0.5%–15.2%). Although not designed as a 2-arm comparator study, there was no difference in PFS or OS between the anti-VEGF naïve and prior anti-VEGF therapy arms (Supplementary. Fig. 1). Median duration of response was 2 cycles. Thirty-one participants (69%) stopped the trial because of disease progression, and 6 (13%) participants elected to withdraw. As of October 2013, 42 participants had died, and 3 were alive. Ten participants progressed both clinically and radiographically, 22 progressed radiographically only, and 2 progressed clinically. Of the radiographic progressions, 5 were new distant lesions (>2 cm away from the original disease site), and 28 were local recurrences.
Table 3.
Clinical responses
| Prior Anti-VEGF (n = 20) | Anti-VEGF Naïve (n = 25) | Total (N = 45) | |
|---|---|---|---|
| Tumor response | |||
| CR (%) | 1 (5.0) | 1 (4.0) | 2 (4.4) |
| PR (%) | 0 | 2 (8.0) | 2 (4.4) |
| SD (%) | 6 | 7 | 13 |
| PD (%) | 9 | 12 | 21 |
| Not evaluable* | 4 | 3 | 7 |
| PFS-6 (%) | 2 (10.0) | 0 | 2 (4.4) |
| Median PFS (95%CI) | 1.9 (0.9–2.8) | 1.9 (1.0–3.0) | 1.9 (1.5–2.8) |
| Median OS (95%CI) | 6.3 (2.6–8.5) | 6.5 (4.6–8.4) | 6.5 (5.2–7.6) |
Abbreviations: CR, complete response; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response, SD, stable disease; VEGF, vascular endothelial growth factor.
*Failed to undergo follow-up imaging to assess response.
Fig. 1.
Overall survival (OS) and progression-free survival (PFS) Kaplan-Meier curves for all participants.
Imaging
Fifteen participants underwent additional imaging to study the impact of these drugs on tumor biology. Baseline values of enhancing tumor volume, FLAIR volume, median tumor ADC, and median tumor CBF/CBV did not correlate with either OS or PFS; neither did they change over time. However, the volume of enhancement, the volume of FLAIR hyperintensity, and the median ADC within FLAIR hyperintensity decreased significantly from baseline to each time point. (Table 4). In this subset, no participants progressed based on enlarging FLAIR hyperintensity prior to progression on contrast imaging. Perfusion imaging revealed a statistically significant increase in median tumor CBF prior to cycle 2 of treatment in both large and small tumor vessels.
Table 4.
Average change in imaging parameters during treatment in the 15 participants who underwent additional imaging. In bold are the significant changes (*P < .05)
| Imaging Parameter | Baseline (Mean) | Day 1−Baseline (95% CI) | Pre-Cycle 2−Baseline (95% CI) | Pre-Cycle 3−Baseline (95% CI) |
|---|---|---|---|---|
| T1 CE Volume | 25.07 | −9.3 (−18.62 to 0.01) | −6.75 (−15.8 to 2.29) | −15.35 (−38.77 to 8.07) |
| FLAIR Volume | 104.08 | −11.47 (−20.74 to −2.21)* | −19.96 (−37.39 to −2.54)* | −34.99 (−64.63 to −5.34)* |
| T1 CE Median ADC | 1.21E-03 | −9.71 E-05 (−17.16E-05 to −2.26E-05)* | −25.48E-05 (−34.68E-05 to −16.29E-05)* | −11.81E-05 (−44.12E-05 to 20.51E-05) |
| FLAIR Median ADC | 1.25E-03 | −2.9E-05 (−5.37E-05 to −0.44E-05)* | −11.07E-05 (−21.38E-05 to −0.76E-05)* | −6.89E-05 (−18.25E-05 to 4.46E-05) |
| CBF GE FLAIR | 0.86 | 0.05 (−0.24 to 0.33) | 0.35 (−0.08 to 0.79) | −0.01 (−0.24,0.22) |
| CBF GE T1 CE | 1.36 | −0.04 (−0.54 to 0.45) | 0.51 (0.03–1)* | 0.3 (−1.1 to 1.69) |
| CBF SE FLAIR | 0.63 | −0.02 (−0.08 to 0.03) | 0.12 (−0.01 to 0.24) | 0.05 (−0.003 to 0.11) |
| CBF SE T1 CE | 1.07 | −0.15 (−0.31 to 0.01) | 0.19 (0.01–0.37)* | −0.03 (−0.32 to 0.25) |
| CBV GE FLAIR | 0.9 | 0.06 (−0.24 to 0.36) | 0.38 (−0.07 to 0.83) | 0.03 (−0.19 to 0.25) |
| CBV GE T1 CE | 1.64 | −0.004 (−0.54 to 0.53) | 0.45 (−0.05 to 0.95) | 0.09 (−2.16 to 2.35) |
| CBV SE FLAIR | 0.68 | −0.03 (−0.09 to 0.03) | 0.1 (−0.03 to 0.23) | 0.05 (0.005–0.1)* |
| CBV SE T1 CE | 1.15 | −0.15 (−0.35 to 0.04) | 0.18 (−0.09 to 0.44) | −0.04 (−0.32 to 0.25) |
Abbreviations: ADC, apparent diffusion coefficient; CB volume SE T1 CE, cerebral blood volume within all vessels with in contrast enhancement; CBF GE FLAIR, cerebral blood flow within all vessels within FLAIR hyperintensity; CBF GE T1 CE, cerebral blood flow within all vessels within contrast enhancement; CBF SE FLAIR, cerebral blood flow within small vessels within FLAIR hyperintensity; CBF SE T1 CE, cerebral blood flow within all vessels within contrast enhancement; CBV GE FLAIR, cerebral blood volume within all vessels within FLAIR hyperintensity; CBV GE T1 CE, cerebral blood volume within all vessels within contrast enhancement; CBV SE FLAIR, cerebral blood volume within small vessels within FLAIR hyperintensity; T1CE, contrast enhanced T1 weighted image.
Blood Biomarkers
Thirty-eight participants had blood biomarkers assessed but given the high dropout rate, we report only the data from the baseline visit, day 1–3 of cycle 1, day 15 of cycle 1, and prior to cycle 2 (4 time points). Baseline values of any biomarkers did not correlate with either OS or PFS (Supplementary Table S1). Change in biomarkers during treatment is shown in Table 5, and fold change from baseline is shown in Supplementary Table S2. CAIX increased significantly from baseline to day 15 of cycle 1. Prior to cycle 2, PlGF and SDF1 α increased significantly from baseline to each time point, and Tie-2 decreased significantly from baseline to each time point. VEGFR2 decreased over time, but the change was statistically significant only at day 1–3 of cycle 1.
Table 5.
Change in blood biomarkers during therapy
| Biomarker | Baseline | Cycle1_Day2 | Cycle1_Day15 | Cycle2_Day1 |
|---|---|---|---|---|
| VEGF | 212 [122–525] (N = 34) | 314 [197–619] (N = 30) | 318 [227–535] (N = 31) | 297 [226–565] (N = 28) |
| P value | NA | .0006 | .0005 | .17 |
| PlGF | 31 [22–39] (N = 34) | 61 [53–89] (N = 30) | 82 [49–102] (N = 31) | 81 [68–111] (N = 28) |
| P value | NA | <.0001 | <.0001 | <.0001 |
| bFGF | 45 [19–63] (N = 34) | 32 [21–66] (N = 30) | 33 [19–77] (N = 31) | 39 [20–62] (N = 28) |
| P value | NA | .33 | .91 | .35 |
| sVEGFR1 | 116 [96–127] (N = 34) | 103 [85–126] (N = 30) | 114 [100–163] (N = 31) | 126 [92–192] (N = 28) |
| P value | NA | .04 | .89 | .79 |
| sVEGFR2 | 7946 [6873–9252] (N = 34) | 7927 [6774–9100] (N = 30) | 7001 [5916–7945] (N = 31) | 6062 [5453–7303] (N = 28) |
| P value | NA | .49 | <.0001 | <.0001 |
| Ang-2 | 2022 [1437–2631] (N = 34) | 2131 [1468–2757] (N = 30) | 2040 [1427–2672] (N = 31) | 1838 [1475–2325] (N = 28) |
| P value | NA | .86 | .12 | .24 |
| sTie-2 | 16.7 [14.9–19.2] (N = 34) | 16.4 [13.2–19.1] (N = 29) | 15.1 [13.7–18.4] (N = 31) | 14.9 [13.6–17.6] (N = 28) |
| P value | NA | .03 | .0002 | .13 |
| SDF1α | 1655 [1333–2021] (N = 34) | 1801 [1538–2119] (N = 30) | 2005 [1450–2335] (N = 31) | 1811 [1385–2295] (N = 28) |
| P value | NA | .009 | .0001 | .008 |
| CAIX | 34 [19–54] (N = 34) | 38 [25–67] (N = 30) | 52 [31–87] (N = 31) | 55 [36–90] (N = 28) |
| P value | NA | .44 | .001 | .002 |
| Collagen IV | 0.24 [0.19–0.35] (N = 34) | 0.25 [0.20–0.30] (N = 30) | 0.28 [0.21–0.37] (N = 31) | 0.26 [0.19–0.33] (N = 28) |
| P value | NA | .84 | .09 | .94 |
| IL-1β | 0.66 [0.50–1.19] (N = 34) | 0.81 [0.52–1.32] (N = 30) | 0.75 [0.52–1.18] (N = 31) | 0.67 [0.53–1.64] (N = 28) |
| P value | NA | 0.82 | 0.76 | 0.70 |
| IL-6 | 2.25 [1.59–3.65] (N = 34) | 2.69 [1.83–3.54] (N = 30) | 2.83 [1.56–4.71] (N = 31) | 2.77 [1.73–4.24] (N = 28) |
| P value | NA | .75 | .02 | .09 |
| IL-8 | 4.70 [3.21–5.62] (N = 34) | 5.63 [3.63–6.93] (N = 30) | 5.55 [3.79–7.6] (N = 31) | 5.95 [4.94–6.82] (N = 28) |
| P value | NA | .38 | .005 | .11 |
| TNF-α | 6.19 [5.07–6.77] (N = 34) | 5.7 [4.85–7.51] (N = 30) | 6.5 [5.11–7.635] (N = 31) | 5.99 [4.82–7.24] (N = 28) |
| P value | NA | .67 | .15 | .99 |
Data are shown as medians and interquartile ranges (in square brackets) and are compared with baseline levels. Changes: increase highlighted in bold with dark shading, decrease highlighted in italics with light shading. P values are from the paired Wilcoxon test.
Discussion
In participants with recurrent GBM, the combination of cediranib and cilengitide was well tolerated with no unexpected toxicities. However, the combination showed disappointing PFS-6, median PFS, and median OS rates when compared with data from prior studies using either drug as monotherapy where PFS-6 was 12%–26%.12,13 Therefore, despite promising early suggestions that combining an anti-VEGFR agent with an anti-invasive agent would be efficacious in inhibiting vascular permeability, angiogenesis, and the infiltrative tumor spread, we found no benefit. The median duration of response was a short 2 months. Although we did not design the study to specifically compare the participants who had previously received prior anti-VEGF therapy with the anti-VEGF-naïve participants, we saw no difference in the 2 cohorts in terms of toxicity, PFS, or OS.
There are several possible explanations for these results. One possible explanation is that the drug doses tested were not optimal. If we had designed our study with a dose escalation rather than dose de-escalation design, we might have seen a more robust response. A second possibility is that the actual number of participants who relapsed via increased infiltration was likely smaller than initially thought. The few studies that have quantified the percentage based on MRI studies of changes in FLAIR hyperintensity suggest that only 10%–20% of participants fail with this pattern and that the majority of patients who progress do so first on postcontrast imaging, suggesting different relapse mechanism(s) that remain unclear.3,15–18 There are also conflicting reports about the impact of a shift towards infiltrative tumor growth on survival, with some studies reporting no impact on survival and others suggesting that the pattern of change on FLAIR sequences is more important than the magnitude of change.15–17 Thus, it remains unclear if targeting infiltrative tumor growth will have a substantial beneficial impact on outcome since a shift to infiltrative growth may reflect a slowing of tumor growth rather than a complete halt of tumor growth.
With only 40 participants, our ability to detect a meaningful impact on blocking infiltration was limited because the number who relapsed via infiltration in our study was likely to be small. Part of the challenge in identifying this relapse pattern is the lack of a radiographic gold standard to correctly identify infiltrating tumor cells. Peritumoral T2/FLAIR changes on MRI represent a combination of edema, tumor cells, and gliosis from prior radiation as well as possible ischemia or a seizure-related phenomenon. Current imaging techniques preclude distinguishing these conclusively. We examined diffusion imaging as a tool to measure tumor cell density and physiological MRI parameters including tumor perfusion, but we were unable to find a consistent predictor of response that was in part due to the small number of individuals who participated in the imaging substudy. Moreover, there are likely to be regional responses within a tumor, so looking at median tumor values on MRI may be too blunt a tool in some cases. Most participants who underwent the advanced imaging experienced stable or decreased volume of FLAIR hyperintensity and decreased median ADC values within the FLAIR hyperintensity, likely reflecting the antipermeability effects of cediranib. A decrease in permeability may have also influenced the penetration of cilengitide and its potential impact on infiltrating tumor cells. Thus, we were unable to identify any participants who clearly relapsed via increased tumor infiltration.
Blood biomarker data indicated a pronounced antivascular effect of combination cediranib and cilengitide with increases in hypoxia-inducible (VEGF, SDF1α, and CAIX) and proinflammatory molecules (IL-6 and IL-8). If these effects were due to excessive pruning because of strong antivascular effects of the combination, then the increased tumor hypoxia and lack of cilengitide penetration may be another explanation for the lack of efficacy. There was an increase in CBF, but this may have reflected improved blood flow from decreased interstitial fluid pressure resulting from vessel pruning. These changes support the notion that there may be an optimal biological dose of antiangiogenic agents that allows for vascular remodeling but not excessive pruning of blood vessels.19
Similar to prior reports on the effects of anti-VEGF agents, sVEGFR1, sVEGFR2, and sTie-2 decreased after treatment.14,20–22 Some of these biomarkers may have pharmacodynamic value in tracking response to antiangiogenic therapy (especially sVEGFR2 and PlGF).20,23 Interestingly, while anti-VEGF therapy is known to decrease circulating Ang2 levels (including cediranib in recurrent and newly diagnosed GBM patients), this effect was not seen after combining cilengitide with cediranib therapy.13,22 This may indicate that cilengitide and cediranib together have an antagonistic effect on Ang-2 expression, which is considered a potential resistance mechanism for anti-VEGF therapies. While none of the baseline biomarkers was associated with PFS or OS, an early (day 2) increase in PlGF and a more delayed (cycle 2) decrease in sTie-2 were associated with longer PFS. Further study is warranted since these biomarkers may shed light on salvage therapies and what pathways need to be targeted at relapse.
Both cediranib and cilengitide failed to show clinical benefit in recent, randomized phase III clinical trials for GBM.24,25 Our study further showed no clinical benefit for the combination of cediranib and cilengitide therapy despite modulation of pharmacodynamic biomarkers. These results provide strong motivation for further trials, including correlative studies, to improve our understanding of anti-VEGF/VEGFR2 treatment resistance mechanisms in recurrent GBM, to tailor this salvage therapy, and to identify new potential targets for more efficacious combination therapies.
Supplementary Material
Funding
This trial was funded by the Adult Brain Tumor Consortium and an American Recovery and Reinvestment Act of 2009 (ARRA) grant.
Conflict of interest statement. D.G.D. has served as a consultant for Hexal/Sandoz.
R.K.J. has received research grants from MedImmune and Roche for research unrelated to this study; received consultant fees from Enlight, Ophthotech, SPARC, and SynDevRx; owns equity in Enlight, Ophthotech, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, and Tekla Healthcare Opportunities Fund.
P.Y.W. has received research support from Angiochem, Astra Zeneca, Genentech/Roche, GlaxoSmith Kline, Merck, Novartis, Sanofi-Aventis, and Vascular Biogenics; serves on the advisory board of Abbvie, Celldex, Foundation Medicine, Genentech/Roche, Novartis, SigmaTau, Vascular Biogenics, Midatech, and Momenta; and been a speaker for Merck.
J.J.O has received research funds from Millenium and Genentech; received research pharmaceuticals from Merck; and serves as an editorial consultant for the American Cancer Society.
T.T.B. has consulted for Merck & Co., Inc., Kirin Pharmaceuticals, Proximagen/Upsher, and Foundation Medicine; provided CME lectures/material for Up to Date, Inc., Research to Practice, and Oakstone Medical Publishing; and received research support from Pfizer, Astra Zeneca, and Millenium.
M.S.A. has received research support from Novartis, Tracon Pharmaceuticals, Novocure, and Spectrum pharmaceuticals unrelated to this study; served as a consultant for Monteris Medical; served on the advisory board of Caris Lifesciences, Genentech/Roche, Elekta, and Novocure; and been a speaker for Merck, Sigma Tau, and Elekta.
Supplementary Material
References
- 1.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 2.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–6628. [PubMed] [Google Scholar]
- 5.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2(4):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 7.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 8.Bello L, Francolini M, Marthyn P, et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–389, discussion 390. [DOI] [PubMed] [Google Scholar]
- 9.Schnell O, Krebs B, Wagner E, et al. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008;18(3):378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stupp R, Hegi ME, Neyns B, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed Clin Oncol. 2010;28(16):2712–2718. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds AR, Hart IR, Watson AR, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15(4):392–400. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert MR, Kuhn J, Lamborn KR, et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03–02, a phase II trial with measures of treatment delivery. J Neurooncol. 2012;106(1):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radbruch A, Lutz K, Wiestler B, et al. Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the Response Assessment in Neurooncology criteria. Neuro-Oncol. 2012;14(2):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowosielski M, Wiestler B, Goebel G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014;82(19):1684–1692. [DOI] [PubMed] [Google Scholar]
- 17.Schaub C, Greschus S, Seifert M, et al. FLAIR-only progression in bevacizumab-treated relapsing glioblastoma does not predict short survival. Oncology. 2013;85(3):191–195. [DOI] [PubMed] [Google Scholar]
- 18.Gerstner ER, Chen PJ, Wen PY, et al. Infiltrative patterns of glioblastoma spread detected via diffusion MRI after treatment with cediranib. Neuro Oncol. 2010;12(5):466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Groot JF. High-dose antiangiogenic therapy for glioblastoma: less may be more?. Clin Cancer Res. 2011;17(19):6109–6111. [DOI] [PubMed] [Google Scholar]
- 20.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raut CP, Boucher Y, Duda DG, et al. Effects of sorafenib on intra-tumoral interstitial fluid pressure and circulating biomarkers in patients with refractory sarcomas (NCI protocol 6948). PLoS One. 2012;7(2):e26331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batchelor TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110(47):19059–19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duda DG, Willett CG, Ancukiewicz M, et al. Plasma Soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet. Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 25.Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.