Abstract
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that degrade the extracellular matrix (ECM) and regulate the extracellular microenvironment. Despite the significant role that MMP activity plays in cell-cell and cell-ECM interactions, migration, and differentiation, analyses of MMPs in vitro and in vivo have relied upon their abundance using conventional immunoassays, rather than their enzymatic activities. To resolve this issue, diverse nanoprobes have emerged and proven useful as effective activity-based detection tools. Here, we review the recent advances in luminescent nanoprobes and their applications in in vitro diagnosis and in vivo imaging of MMP activity. Nanoprobes with the purpose of sensing MMP activity consist of recognition and detection units, which include MMP-specific substrates and luminescent (fluorescent or bioluminescent) nanoparticles, respectively. With further research into improvement of the optical performance, it is anticipated that luminescent nanoprobes will have great potential for the study of the functional roles of proteases in cancer biology and nanomedicine. [BMB Reports 2015; 48(6): 313-318]
Keywords: ECM, Enzyme activity, Imaging, Matrix metalloproteinase, Nanoprobe
INTRODUCTION
The ability to degrade extracellular matrix (ECM) components is a prerequisite for any individual cell to interact with its surroundings during cell proliferation and differentiation. Among extracellular proteinases, matrix metalloproteinases (MMPs) have been recognized as a principal mediator in the alteration of microenvironments, especially in association with cancer progression. MMPs belong to a zinc-dependent family of endopeptidases, and in humans 24 MMP genes (23 MMP proteins) have been characterized (1). Recent studies have revealed that MMPs not only degrade ECM components, but also cleave cell surface molecules and other pericellular nonmatrix proteins. Thus they participate in many physiological processes, such as inflammation, embryogenesis, tissue remodeling, wound healing, and angiogenesis (2-6). On the basis of substrate specificity, endogenous inhibitor sensitivity, and domain organization (a pro-domain, a catalytic domain, a hinge region and a hemopexin domain), the multiplicity of MMPs are regulated with distinct but somewhat overlapping functions. The increasing attention, therefore, has been paid to how the activities of different MMPs are dissected in vitro and in vivo, in terms of MMP activity rather than MMP abundance.
To date, many attempts to validate their activity have relied upon in vitro zymography, which are limited to only a few MMPs without kinetic information on the catalytic activity. With the advent of proteomics and degradomics over the past decade, a great number of substrates for MMPs have been identified with high specificity (7), which makes it possible to design highly sensitive smart probes with dissecting MMP specificity, based on short peptide substrates. Although many different types of molecular probes have been reported to target MMP activity (8-11), they are primarily based on genetically encoded fluorescent (or bioluminescent) proteins through the incorporation of the annotated peptide substrates, where detection sensitivity and in vivo application of these probes were still unsatisfied. To this end, much attention has been paid to nanoscaled probes with physiochemically unprecedented properties, due to higher surface-to-volume ratio than that in bulk probes (typically, nanoprobes occupy the targeting nanomaterials with 1-100 nanometers in size). These nanoprobes present several advantages over other molecular probes, including i) improved activatable performance with a multivalent format, which can amplify the distinguishable signal between activated and inactive latent enzymes, ii) improved sensitivity, which is accomplished by using luminescent nanomaterials with high quantum yield, high extinction coefficients, and a long lifetime (12), iii) multi-functionality, which includes affinity-based targeting, activity-based detection, and therapy by decorating the nanomaterials with various functional molecules, and iv) improved delivery to the target site in vivo. For example, nano-sized probes are capable of penetrating the blood vessel via the enhanced permeability and retention (EPR) effect in tumors (13-15). Indeed, in order to unveil the complexity of MMP activity, it is necessary that MMP-related research in the field of biomedicine or molecular biology is coordinated with different panels of nanoprobes. This will help in the understanding of the physiological roles of MMPs.
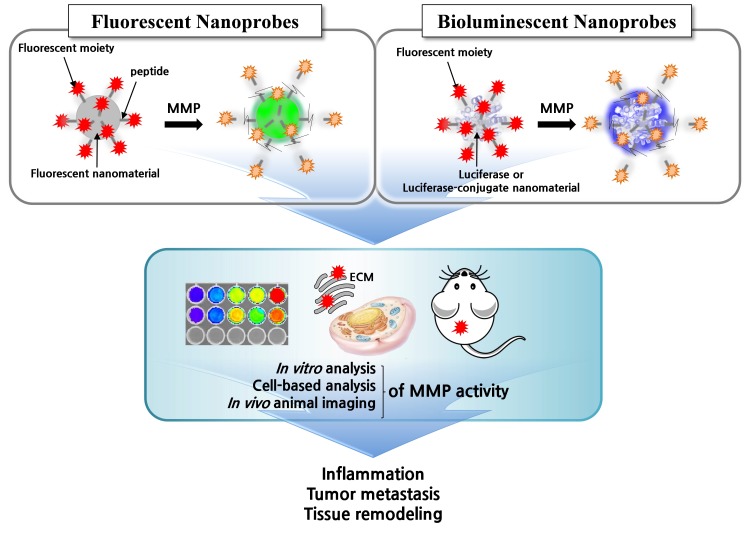
Despite the recent review articles on MMPs (16) or protease-targeting nanoprobes (17), there is a need to highlight nanoprobes in terms of in vitro and in vivo diagnosis of MMPs. Here, we review recent advances in nanoprobes targeting MMP activity. To avoid redundancy in a myriad of nanoprobes with different modalities, such as magnetic resonance imaging, computed tomography, and surface-enhanced Raman scattering, we will focus on two types of luminescent nanoprobes (fluorescence and bioluminescence) for in vitro and in vivo detection of MMP activity (Fig. 1). We will also discuss recent progress in in vivo imaging of MMP activity which has shed light on the functional relevance of these enzymes to physiological and pathological processes.
Fig. 1. Schematic of fluorescent or bioluminescent nanoprobes to analyze MMP activity in vitro and in vivo. The activatable nanoprobes can be designed in different manners in order to study MMP functions in relation to cellular pathology and physiology.
FLUORESCENT NANOPROBES
Fluorescence has long been used as a key tool for diagnosis and imaging in biology, because it simply distinguishes a particular target labeled with a fluorophore within a complex background. Fluorescent nanoprobes allow for higher intensity, higher signal-to-background ratio, and longer monitoring ability, compared with conventional bulk fluorescent materials or single fluorescent dye. Most significantly, multiple binding of the targeting ligands can be achieved on the nanoparticle surface, enabling it to react with a number of targets simultaneously. When choosing a fluorescent moiety, it is important to consider physical properties including excitation maximum, emission maximum, Stokes shift (the difference between excitation and emission), and fluorescence emission especially when applied to complex sample in vitro and in vivo analysis. In addition to fluorescent nanoparticles (e.g. quantum dot), the appropriate fluorescence labeling of nanomaterials and/or polymeric nanoparticles can produce different types of nanoprobes: dye-doped nanoprobe, core-shell nanoprobes, where a core includes a high concentration of dyes, externally labeled nanoprobe, and liposome or polymersome loaded with dyes. We will introduce representative examples of these fluorescent nanoprobes becoming “switched on” by the enzyme activity when they are attached to the target MMPs (18).
Quantum dot-based nanoprobes
Quantum dots (QDs) are fluorescent semiconductor nanocrystals with unique optical properties, including improved signal brightness, high quantum yield, size tunable light emission and high photostability (12, 19-21). In particular, the long-term stability and multiple colors at a single wavelength enables the QDs to be preferable for multiplexed detection of simultaneous signals, compared with traditional organic fluorescent dyes (22). Initial design of QD nanoprobes on MMP activity was reported by Zhang et al., where a peptide-conjugated QD nanoprobe acquired cell-permeability by the activities of MMP-2 and MMP-7 in HT-1080 (human fibrosarcoma cells) (23). Since the gelatinase MMP-2 and matrilysin MMP-7 were well known to be secreted into the extracellular matrix, R4XPLGVRGE4 and R3XGRPLALWRSGE5, where X denotes 6-aminohexanoyl as a spacer inserted to increase the enzyme accessibility, were used as the substrates for MMP-2 and MMP-7, respectively. The nanoprobe was prepared by appending biotinylated peptide ligands to streptavidin-coated QD, affording QD@bio-peptide. The QD-based nanoprobes encompassed three components: i) transporting groups with positive arginine sequences that transport QDs into cells, ii) intracellular transport blockers presenting negatively charged groups (i.e. glutamic acid residues), and iii) sensing groups that were cleaved by MMPs and sandwiched between the transporter and the blocker. Once MMP-2 or MMP-7, present in extracellular regions, cleaved their substrate sequence, the transport blocker sequences were removed from the transporting groups. As a result, the QD nanoprobes were able to penetrate the cell membrane. They confirmed that a sequence of 4 arginine residues (RRRR) was sufficient for intracellular transport of QDs, where the activities of MMP-2 and MMP-7 were proven by transportation of the QDs into the cytoplasm as shown by an increased fluorescence intensity.
Similar work related to MMP-2 activity was demonstrated by Li et al., using QD-based fluorescence resonance energy transfer (FRET) (24), where the peptide substrate (GPLGVRGKGG) linked with a fluorescent dye (energy acceptor) was appended to the surface of the QD (energy donor) via a carboxyl-amine coupling reaction. In general, the FRET could be accomplished by the energy donor and acceptor being within a proximity of 10-nm, leading to acceptor emission upon donor excitation. They designed two different coupling probes (585 nm-emitting Rhodamine B with 535-nm-emitting QD and 720-nm-emitting near infrared (NIR) dye (indocyanine green, ICG) with 540-nm-emitting QD) for use in vitro and in vivo, respectively. Upon MMP-2 activation in the cultured media of MDA-MB-231 cells, the decreased fluorescence ratio of donor-to-acceptor was confirmed, as a result of intermittent energy transfer by peptide cleavage. They showed that nude mice bearing MDA-MB-231 generated a strong NIR fluorescence at a tumor site. Furthermore, there was no toxicity according to the biochemical parameters at a low dosage of QD (less than 10 mg kg-1). However, despite the improved imaging resolution of the QD-based MMP activity in vivo, the toxicity of CdTeS still remains a challenge for in vivo application.
For the rapid in vitro analysis of MMP-7 activity, Kim et al., (25) demonstrated the chip-based version of QD-FRET. While the photoluminescence (PL) of the donor streptavidin-QD525 immobilized on a surface was quenched by the acceptor red dye fused with biotinylated peptide substrate, the protease activity caused modulation in the efficiency of the energy transfer between the acceptor and donor, thereby enabling highly sensitive detection of MMP-7 activity. In contrast to solutionbased analysis, chip-based format allowed for more reliable analysis with no aggregation of QDs and with a much smaller reaction volume. In this regard, the chip-based format of QD-FRET is likely to have potential in the screening of the activity of disease-associated MMPs for the development of therapeutics and diagnostics in a high-throughput manner.
Other fluorescent nanoprobes
In addition to QDs, silica nanoparticles (SiNP) have drawn much attention, due to their excellent biocompatibility without toxicity. Achatz et al. reported SiNP-based FRET for the detection of MMP activity (26). Initially, the peptide substrate (GPLGVR) for MMP-2 was synthesized with a cyclooctyne (CyOCO) and a propagylglycine (PrGly) at both ends. To construct the fluorescent core-shell SiNPs bearing fluorescently labeled substrates, CyOCO was conjugated with azide-modified fluorescent SiNPs (emission at 470-nm) by strain-promoted 1,3 dipolar cycloaddition without copper(I), and subsequently PrGly (alkyne) of the peptide was conjugated with azide-linked dye (emission at 630 nm) via copper(I)-mediated click chemistry. The emission ratio of 470 nm (donor emission) to 630 nm (acceptor emission) at an excitation of 410 nm increased as a function of MMP-2 concentration, which resulted from the cleavage of the peptide substrate. Independent of nanoparticles, a membrane-targeting FRET reporter to visualize MMP-12 activity was developed by the Shultz group (27). Although MMP-12 has been implicated in multiple sclerosis and atherosclerosis, little is known about MMP-12 activity, due to a lack of appropriate research methods. To overcome this issue, and to image MMP-12 activity in individual cells, this group synthesized a membrane-fixed probe (not a free-floating type), using a membrane-bound moiety and FRET pair with the human MMP-12-specific peptide sequence (PLGLEEA). The peptide was flanked by two fluorophores (coumarin343 and TAMRA) and palmitic acid (hydrophobic group). This probe allowed for the monitoring of MMP-12 activity in RAW macrophages activated by lipopolysaccharide, as well as in bronchoalveolar lavage from a mouse model of pulmonary inflammation. Compared to nanomaterial-based probes, a relatively higher concentration of lipidated chemical probes was needed to image the target MMP, therefore the nanomaterial-hybrid probe will have great potential in the monitoring of MMP activity in individual cells.
Gold nanoparticles (AuNPs) have also been utilized in FRETbased MMP detection as an optical quencher. Kim’s group reported an AuNP-based fluorescence quenching probe via Ni(II) coordination for the detection of MMP-7 activity (28). While the substrate peptide (GPLGMRGL), bearing His6 at the N-terminus and TAMRA dye at the C-terminus, was mixed with the carboxyl AuNP, the addition of Ni(II) caused a strong coordination between the histidine group of the peptide and the carboxyl group of the AuNPs, leading to a strong reduction of TAMRA as a result of the quenching effect. Conversely, the presence of MMP-7 increased the fluorescence intensity, where the detection sensitivity was found to be as low as 10 ng ml-1. Although it is not feasible for in vivo analysis, this method would be preferable to multiplexed analysis of diverse MMP activities because the AuNP can be used as a common quencher for different fluorophores. Lee et al., initially demonstrated that the fluorescence-quenched AuNPs made it possible to apply MMP activity imaging in vivo (29), however imaging life time was very limited, due to the labile breakage of gold-thiol linkage. To overcome this issue, Chen’s group made progress by using an AuNP-Fe3O4 composite optical nanosensor, which detected MMP-13 activity in vivo (30). Together with the superior quenching capability of AuNPs in vivo, a strong biding of iron oxide on the AuNP was alternatively suggested for in vivo MMP imaging. Cy5.5-GPLGVRG was appended to the hybrid AuNP-Fe3O4 nanoprobes by introducing tridihydroxylphenylalanine (TDOPA) at the N-terminus of peptide to increase the anchoring ability on the surface of iron oxide nanoparticles. They confirmed the MMP-13 activity using a mouse model bearing SCC-7 (head and neck squamous cell carcinoma) cells. Although this system has many advantages including the use of AuNPs and iron nanoparticles that are recognized to be biologically safe, and the quenching effect between NIR Cy5.5 dye and AuNP which is favorable in in vivo imaging, such a free-floating nanoprobe is sought to be still limited to trafficking MMP activity in a localized area.
In contrast to QDs and other metallic or non-metallic nanomaterials, protein-type nanoprobes were employed using human serum albumin (HSA) to detect MMP-2 and MMP-9 activity in vivo (31). Since HSA is abundant in blood plasma and has excellent biocompatibility and biodegradability as well as in vivo stability as a delivery reagent, this nanoprobe can be harnessed for in vivo imaging in a non-invasive manner. They designated HSA-based fluorogenic nanoprobes with an MMP peptide substrate bearing an NIR dye (Cy5.5) and a dark quencher (BHQ-3), which specifically imaged MMP-2 and MMP-9 activity in vivo in the mouse hindlimb ischemia model.
BIOLUMINESCENT NANOPROBES
Bioluminescent nanoprobes are generally comprised of a nanomaterial and a bioluminescent protein (usually luciferase), which produces a flash of light by a catalytic reaction in the presence of a light-emitting pigment (chemical substrate). Fluorescent probes often encounter fast photobleaching, light-induced cytotoxicity and high autofluorescence, due to the long-time use of an external light excitation (32, 33). In contrast, bioluminescent nanoprobes offer greater sensitivity with a low background noise, thereby enabling effective in vitro analysis and in vivo imaging by the avoidance of autofluorescence or light scattering in complex media or tissues.
For effective in vitro analysis of MMP activity, a bioluminescent nanosensor comprising AuNPs and luciferases was proposed by Kim et al. (33). By conjugating the expressed Renilla luciferase proteins fused with the MMP-2 substrate (IPVSLRSG) to AuNPs via click chemistry and an intein-mediated ligation, they demonstrated that the AuNPs efficiently quenched the bioluminescent emission from luciferase, and the bioluminescent nanoprobe could be utilized to detect the proteolytic activity of MMP-2. This is a new strategy for targeting MMPs based on the site-specific conjugation and bioluminescence-quenching phenomenon, however copper ions affected the luciferase activity during click chemistry, leading to a reduction in the detection sensitivity of MMP activity. Therefore, in order to further bioluminescent gold nanoprobes, alternative conjugation methods, such as copper-free click chemistry may be required.
It is noteworthy that QDs can be combined with bioluminescent proteins. The Rao group developed a simple and sensitive nanoprobe for in vitro detection of MMP activity based on bioluminescence resonance energy transfer (BRET) between luciferase (energy donor) and QDs (energy acceptor) (34). The MMP-2 peptide substrate (GGPLGVRGGHHHHHH) bearing a hexahistidine tag was genetically fused to the mutant of Renilla luciferase (RLuc), resulting in RLuc-pep-His6. In the presence of Ni(II), the RLuc-pep-His6 was associated with carboxyl QDs via Ni(II) coordination, leading to a strong BRET signal that was caused by the BRET acceptor QD emission (at 655-nm) from the BRET donor RLuc emission (at 480 nm). Once the peptide substrate was digested by MMP-2, the BRET signal decreased with the relative increase of RLuc emission in the presence of coelenterazine-h (luciferin derivative). This system showed high sensitivity, enough to distinguish 2 ng ml-1 (30 pM) of MMP-2. To investigate the multiplexed capability of QD-BRET for probing MMP activity, the same research group created three types of QD-BRET nanoprobes by incorporating MMP-2, MMP-7, and urokinase substrates in a further study (32). Instead of Ni(II)-mediated association, intein-mediated conjugation helped to strongly conjugate RLuc including different peptides with differently emitting QDs, which enabled traceless ligation of the QD-BRET couple. Using cultured media from MMP-2-overexpressing HT1080 and MMP-2-deficient HT29 cells, they showed that QD-BRET nanoprobes were able to realize multiplexed detection of different MMP activities in a single run from a complex sample. Unlike QD-FRET nanoprobes, these QD-BRET nanoprobes exerted improved energy transfer efficiency by both the removal of autofluorescence from external illumination and the avoidance of cross-talk found in the FRET system. As a result, they generated high detection sensitivity of MMP activity. However, it is likely that luciferase needs to be made more stable in order to maintain its activity for long-term monitoring of MMP activity. Furthermore, this method is possibly limited only to cultured cells, and not suitable for in vivo animal models, due to QD-related toxicity and the instability of QD-luciferase conjugate.
For in vivo detection of MMP activity, Xia et al. reported an in vivo bioluminescent probe for the detection of MMP-2 and MMP-9 activity (35). Notably, they designed a reporter immobilized onto the collagen in the extracellular microenvironment through collagen binding protein (CNA35) derived from the surface component of Staphylococcus aureus. CNA35-fused luciferase protein was randomly conjugated with dabcyl (quencher)-tethered peptide (dabcyl-GPLGVRGC) via cysteinemediated cross-linking. These luciferase-quenched protein nanoprobes bound to collagen prevented them from both being free-floating and being internalized into cells, thereby retaining them in the extracellular microenvironment for effective MMP sensing. Once this probe was injected via the tail-vein into a nude mouse with a xenograft HT 1080 tumor, the activity of MMP-2 and MMP-9 in vivo enabled this probe to generate a strong bioluminescence following injection of the substrate, coelenterazine-h. Significantly, total intensity of the bioluminescence emission throughout the mouse’s body was maintained without excretion due to the immobilized effect, showing only a 5% signal loss during the first 4 hours. Therefore, this system could fulfill long-term and serial real-time imaging of MMP activity under the microenvironmental condition with the improved detection sensitivity of the tumor site, compared with that of fluorescent probes.
Besides the diagnostics of MMP activity, luminescent nanoprobes are being evolved into a multifunctional format for clinical purposes. For example, loading chemotherapeutic drugs into nanoprobes can be combined with the detection of MMPs (31), which will open a new route for the application of cancer therapy based on MMP activity.
CONCLUSIONS AND FUTURE PERSPECTIVES
In this review, we have demonstrated recent advances in fluorescent and bioluminescent nanoprobes for the detection of MMP activity. When MMPs are either secreted by cancerous and stromal cells in ECMs or localized at the surface of cells, it has been revealed by proteomic analysis, identifying a diverse range of MMP substrates, that only 15% of MMP substrates belong to the ECM (36). This suggests that MMPs are involved in a wide range of functions and thus, they become significant drug targets in several diseases and tissue remodeling. Nonetheless, activity-based MMP research is far from being successfully carried out in vivo in order to scrutinize the functional roles of MMPs, due to a lack of competent detection methods. In this regard, fluorescent and bioluminescent nanoprobes with innate high sensitivity and stability hold promise to dissect different MMP activities in vitro and in vivo, which is otherwise difficult using conventional methods. Despite the successful role of nanoprobes, one issue related to in vivo toxicity of nanomaterials is still not clearly understood, especially due to the long residence in vivo with less degradability that may be accumulated in any local areas. Therefore, the development of alternative nanoprobes based on self-degradable capability is likely to be the most demanded. Another matter is accomplishment of real specificity among different MMPs, because the developed nanoprobes are based on a short peptide substrate, which may overlap with different MMP activities. For example, the activity of MMP-2 and MMP-9 shares similar substrate sequences. With the furthering of studies on MMP specificity and biocompatible nanoprobes, one anticipates that future work using nanoprobes will find applications to dissect the physiological roles of MMPs.
Acknowledgments
This work was supported by the research fund of Hanyang University (HY-2013).
References
- 1.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. (2006);69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. (1999);274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 3.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. (2001);17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. (2001);89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 5.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. (2002);90:251–262. [PubMed] [Google Scholar]
- 6.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. (2005);9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler GS, Overall CM. Updated biological roles for matrix metalloproteinases and new "intracellular" substrates revealed by degradomics. Biochemistry. (2009);48:10830–10845. doi: 10.1021/bi901656f. [DOI] [PubMed] [Google Scholar]
- 8.Bremer C, Bredow S, Mahmood U, Weissleder R, Tung CH. Optical imaging of matrix metalloproteinase-2 activity in tumors: feasibility study in a mouse model. Radiology. (2001);221:523–529. doi: 10.1148/radiol.2212010368. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. (2005);111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Zhang Z, Lin J, et al. Detection of MMP activity in living cells by a genetically encoded surface-displayed FRET sensor. Biochim Biophys Acta. (2007);1773:400–407. doi: 10.1016/j.bbamcr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Stawarski M, Rutkowska-Wlodarczyk I, Zeug A, et al. Genetically encoded FRET-based biosensor for imaging MMP-9 activity. Biomaterials. (2014);35:1402–1410. doi: 10.1016/j.biomaterials.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Weng J, Ren J. Luminescent quantum dots: a very attractive and promising tool in biomedicine. Curr Med Chem. (2006);13:897–909. doi: 10.2174/092986706776361076. [DOI] [PubMed] [Google Scholar]
- 13.Rosi NL, Mirkin CA. Nanostructures in biodiagnostics. Chem Rev. (2005);105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 14.Katz E, Willner I. Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications. Angew Chem Int Ed. (2004);43:6042–6108. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 15.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. (2009);9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 16.Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. FEBS J. Vol. 278. 28: (2011). Regulation of matrix metalloproteinase activity in health and disease. pp. 28–45. [DOI] [PubMed] [Google Scholar]
- 17.Serim S, Haedke U, Verhelst SH. Activity-based probes for the study of proteases: recent advances and developments. Chem Med Chem. (2012);7:1146–1159. doi: 10.1002/cmdc.201200057. [DOI] [PubMed] [Google Scholar]
- 18.Terai T, Nagano T. Fluorescent probes for bioimaging applications. Curr Opin Chem Biol. (2008);12:515–521. doi: 10.1016/j.cbpa.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Bruchez M Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. (1998);281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 20.Alivisatos P. The use of nanocrystals in biological detection. Nat Biotechnol. (2004);22:47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 21.Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol. (2002);13:40–46. doi: 10.1016/S0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 22.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. (2005);4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, So MK, Rao J. Protease-modulated cellular uptake of quantum dots. Nano Lett. (2006);6:1988–1992. doi: 10.1021/nl0611586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Deng D, Xue J, Qu L, Achilefu S, Gu Y. Quantum dots based molecular beacons for in vitro and in vivo detection of MMP-2 on tumor. Biosens Bioelectron. (2014);61:512–518. doi: 10.1016/j.bios.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Kim YP, Oh YH, Oh E, Kim HS. Chip-based protease assay using fluorescence resonance energy transfer between quantum dots and fluorophores. Biochip J. (2007);1:228–233. [Google Scholar]
- 26.Achatz DE, Mezo G, Kele P, Wolfbeis OS. Probing the activity of matrix metalloproteinase II with a sequentially click-labeled silica nanoparticle FRET probe. Chem Biochem. (2009);10:2316–2320. doi: 10.1002/cbic.200900261. [DOI] [PubMed] [Google Scholar]
- 27.Cobos-Correa A, Trojanek JB, Diemer S, Mall MA, Schultz C. Membrane-bound FRET probe visualizes MMP12 activity in pulmonary inflammation. Nat Chem Biol. (2009);5:628–630. doi: 10.1038/nchembio.196. [DOI] [PubMed] [Google Scholar]
- 28.Park SY, Lee SM, Kim GB, Kim YP. Gold nanoparticle-based fluorescence quenching via metal coordination for assaying protease activity. Gold Bulletin. (2012);45:213–219. doi: 10.1007/s13404-012-0070-9. [DOI] [Google Scholar]
- 29.Lee S, Cha EJ, Park K, et al. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew Chem Int Ed. (2008);47:2804–2807. doi: 10.1002/anie.200705240. [DOI] [PubMed] [Google Scholar]
- 30.Xie J, Zhang F, Aronova M, et al. Manipulating the power of an additional phase: a flower-like Au-Fe3O4 optical nanosensor for imaging protease expressions in vivo. ACS Nano. (2011);5:3043–3051. doi: 10.1021/nn200161v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu JH, Shin JY, Kim SA, et al. Non-invasive optical imaging of matrix metalloproteinase activity with albumin-based fluorogenic nanoprobes during angiogenesis in a mouse hindlimb ischemia model. Biomaterials. (2013);34:6871–6881. doi: 10.1016/j.biomaterials.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 32.Xia Z, Xing Y, So MK, Koh AL, Sinclair R, Rao J. Multiplex detection of protease activity with quantum dot nanosensors prepared by intein-mediated specific bioconjugation. Anal Chem. (2008);80:8649–8655. doi: 10.1021/ac801562f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YP, Daniel WL, Xia Z, Xie H, Mirkin CA, Rao J. Bioluminescent nanosensors for protease detection based upon gold nanoparticle-luciferase conjugates. Chem Commun. (2010);46:76–78. doi: 10.1039/B915612G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao H, Zhang Y, Xiao F, Xia Z, Rao J. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew Chem Int Ed. (2007);46:4346–4349. doi: 10.1002/anie.200700280. [DOI] [PubMed] [Google Scholar]
- 35.Xia Z, Xing Y, Jeon J, et al. Immobilizing reporters for molecular imaging of the extracellular microenvironment in living animals. ACS Chem Biol. (2011);6:1117–1126. doi: 10.1021/cb200135e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison CJ, Butler GS, Rodriguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol. (2009);21:645–653. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]