Abstract
Purpose of review
Hypertension, which is present in about one quarter of the world’s population, is responsible for about 41% of the number one cause of death, cardiovascular disease. Not included in these statistics is the effect of sodium intake on blood pressure, even though an increase or a marked decrease in sodium intake can increase blood pressure. This review deals with the interaction of gut microbiota and the kidney with genetics and epigenetics in the regulation of blood pressure and salt sensitivity.
Recent findings
The abundance of the gut microbes, Firmicutes and Bacteroidetes, is associated with increased blood pressure in several models of hypertension, including the spontaneously hypertensive and Dahl salt-sensitive rats. Decreasing gut microbiota by antibiotics can increase or decrease blood pressure that is influenced by genotype. The biological function of probiotics may also be a consequence of epigenetic modification, related, in part, to microRNA. Products of the fermentation of nutrients by gut microbiota can influence blood pressure by regulating expenditure of energy, intestinal metabolism of catecholamines, and gastrointestinal and renal ion transport, and thus, salt sensitivity.
Summary
The beneficial or deleterious effects of gut microbiota on blood pressure is a consequence of several variables, including genetics, epigenetics, lifestyle, and intake of antibiotics. These variables may influence the ultimate level of blood pressure and control of hypertension.
Keywords: microbiota, salt sensitivity, brain gut microbiome axis, gastro-renal axis
Introduction
Blood pressure is distributed continuously from low to high values, but the distribution is skewed to the higher end of the curve (1). There is a direct and quantitative relationship between high blood pressure values and mortality. Hypertension is a major contributor to the number one cause of death, cardiovascular disease (2). The Gaussian distribution and the lack of a definable bimodal distribution of blood pressure suggest that blood pressure is regulated by a complex group of interacting genes. The variation of blood pressure is further influenced by the interaction of these genes with epigenetic and environmental factors (3–8). This review deals with the interaction of gut microbiota with genetics and epigenetics in the regulation of blood pressure and salt sensitivity.
Salt sensitivity, defined as >5–10% change in blood pressure in response to a change in NaCl intake, is associated with increased cardiovascular risk, even if the blood pressure does not reach hypertensive levels (9). Mortality and morbidity are both higher in hypertensive subjects and in salt-sensitive normotensive subjects than in salt-resistant normotensive subjects (10–12). About 118 million Americans are afflicted with hypertension and/or salt sensitivity. Fifty to 60 million (≥18 years old) are hypertensive and 58 million are salt-sensitive; 26 million are both salt-sensitive and hypertensive (10, 13). It is recognized that a high sodium diet is deleterious and a low sodium diet has been advocated as part of a healthy life style and treatment of hypertension (2, 12). However, low sodium diet can actually increase blood pressure, i.e., inverse salt sensitivity (13–15), with other adverse consequences (16–18). The mechanisms leading to such adverse consequences and their relationship to “salt-resistant” and “salt-sensitive” genes are not known.
The long-term regulation of blood pressure rests on renal and non-renal mechanisms (19–21). The impaired renal sodium handling in essential hypertension and salt sensitivity are caused by aberrant counter-regulatory natriuretic and antinatriuretic pathways. The nervous system, including renal nerves (22–25) and the parasympathetic and sympathetic nervous systems (26), renin-angiotensin-aldosterone system (24, 25, 27–29), and endothelin via the ETA receptor (30) are examples of antinatriuretic pathways. An important counter-regulatory natriuretic pathway is afforded by the renal dopaminergic system. Aberrations of this system are involved in the pathogenesis of hypertension (26, 31–35), including that associated with obesity (36–38). However, the gastrointestinal tract has to be integrated in the overall regulation sodium balance and blood pressure because it the first organ exposed to ingested sodium (39, 40). Inhibition of gastrointestinal sodium transport is now being considered in the treatment of essential hypertension (41). Moreover, the gut microbiota can modify the expression of the hypertensive phenotype (42–44).
Gut microbiota and hypertension
The gut microbiota, dominated to a large extent by Firmicutes and Bacteroidetes and to a lesser extent by Actinobacteria and Proteobacteria (45), constantly adapt to lifestyle modifications, such as diet (46, 47) and even exercise (48). The gut microbiota can regulate about 10% of the host’s transcriptome, especially those genes related to immunity, cell proliferation, and metabolism (49, 50). The gut microbiota may play a role in the development of cardiovascular disease, including arteriosclerosis and hypertension. Female C57BL/6J Apoe−/− mice develop atherosclerosis related to increased trimethylamine N-oxide (TMAO) levels following fecal microbial transplantation from atherosclerosis-prone C57BL/6J mice fed choline diet (51). Toxic metabolites, such as p-cresol, indoxyl sulfate, and TMAO, are produced following fermentation of protein by gut microbiota (52–54). Chronic kidney disease patients have elevated plasma levels of TMAO that are derived from the metabolism of dietary choline, phosphatidylcholine (lecithin), and l-carnitine by microbiota (55). This elevation in plasma TMAO levels is probably mainly due to gut microbial action, because genes play a minor role in determining TMAO levels in humans (56).
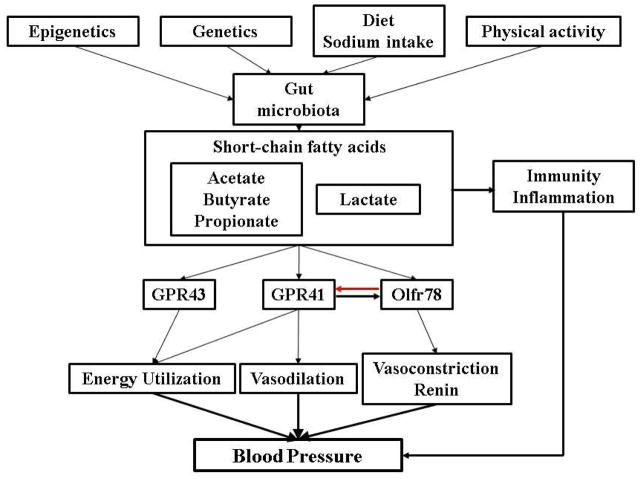
Short chain fatty acids (SCFA) produced by the gut microbiota (40) influence blood pressure that is related to renal sensory nerves (43, 57). These SCFAs activate two orphan G protein-coupled receptors, GPR41 (aka Free Fatty Acid Receptor 3), GPR43 (aka Free Fatty Acid Receptor 2), and olfactory receptor 78 (Olfr78). The increase in blood pressure caused by SCFA-induced renin release from the afferent arteriole is mediated by Olfr78. This, in turn, can be counteracted by the vasodilatory action of GPR43 (43, 57). SCFA, via GPR43, also suppresses insulin signaling in adipocytes, improving metabolism, in part, by inhibiting accumulation of fat in adipose tissue (58). By contrast, GPR41 increases energy expenditure by stimulating the sympathetic nervous system, but this could also lead to an increase blood pressure (59).
Chronic low-grade inflammation can be a cause or consequence of hypertension (60). Low-grade inflammation can be the result of a reduction in microbial gene richness (61). Preeclampsia is associated with hypertension and inflammation, the incidence of which is decreased by chronic intake of probiotics (62). Changes in the ratio of the microbes Firmicutes and Bacteroidetes have been used as a biomarker for pathological conditions. The Firmicutes and Bacteroidetes ratio was recently reported to be increased in spontaneously hypertensive rats, angiotensin II- induced hypertension in rats, and small group of humans with essential hypertension. The oral administration of minocycline normalized the Firmicutes and Bacteroidetes ratio and blood pressure of spontaneously hypertensive rats and rats with angiotensin II- induced hypertension (63). Angiotensin converting enzyme type 2 (ACE2)-mediated regulation of gut microbiota is important in epithelial immunity (64). Lactobacilli also produce biologically active peptides capable of inhibiting ACE1 (65); ACE2-mediated production of angiotensin 1–7 decreases while ACE1-mediated production of angiotensin II increases blood pressure (28).
Consumption of milk fermented with Lactobacilli lowered blood pressure in hypertensive humans (66). The antihypertensive effect of blueberries may also be due to Lactobacilli in the gut (67). Oral administration of sour milk to spontaneously hypertensive rats has been reported to lower systolic blood pressure. Phenylacetylglutamine is a gut microbial metabolite that is negatively associated with pulse wave velocity and systolic blood pressure (68). A meta-analysis of randomized, controlled trials in humans showed that probiotic consumption modestly decreased both systolic and diastolic blood pressures with a greater effect when at least 1011 colony-forming units are taken for at least 8 weeks and if multiple species of probiotics are consumed (69).
The role of a particular species of gut microbiota on blood pressure regulation needs to be sorted. For example, both the Dahl salt-sensitive and salt-resistant rats on a high salt diet have more Firmicutes than Bacteroidetes but the ratio may be the same in these two Dahl rat strains. This is in contrast to the aforementioned increased Firmicutes and Bacteroidetes ratio in spontaneously hypertensive rats, angiotensin II- induced hypertension in rats, and hypertensive humans (63). The amount of Bacteroidetes, especially the S24-7 family, and the family Veillonellaceae of the Firmicutes phylum was higher in Dahl salt-sensitive than Dahl salt-resistant rats. Dahl salt-sensitive rats given cecal content from Dahl salt-resistant rats had higher blood pressure, higher Veillonellaceae, higher plasma acetate and heptanoate, lower sodium excretion, and shorter life span that those that received cecal content from Dahl salt-sensitive rats (70). These effects were not found in Dahl salt-sensitive rats fed a low salt diet or antibiotics. By contrast, the blood pressures of Dahl salt-resistant rats on high salt diet were not affected by cecal content from Dahl salt-sensitive or salt-resistant rats. There are also no differences in Olfr78 and Gpr41 sequences between these two rat strains (108). However, antibiotic treatment resulting in a reduction in the biomass of the gut microbiota elevated the blood pressure in Olfr78 knockout but not wild-type mice (43). Thus, the influence of that gut microbiota on blood pressure is modulated by genetics.
Gut microbiota and gastrorenal axis
There are monoamine-containing enterochromaffin cells in the mucosa and submucosa of different portions of the stomach and small intestines (71). The gut microbiota can influence the ability of enterochromaffin cells to produce serotonin, dopamine, and norepinephrine that can influence the behavior of the host, termed brain gut microbiome axis (72, 73) and renal function, termed gastrorenal reflex (74, 75). The absence of gut microbiota has been reported to increase anxiety-like behavior and decreased dopamine turnover in the frontal cortex, hippocampus, and striatum in response to acute stress in rats (76). Norepinephrine, released in response to stress, can also increase the growth and production of virulence-associated factors of gram-negative bacteria. Gut-germ-free stress-sensitive F344 rats had abnormal behavior associated with increased glucocorticoid mRNA, but decreased dopamine turnover in the hippocampus (77). However, in BALB/c salt-resistant mice, the oral administration of antibiotics increased exploratory behavior that was not due to changes in gastrointestinal transmitters, such as serotonin, norepinephrine, and dopamine (78). By contrast, specific-pathogen free mice had increased production of norepinephrine and dopamine in the cecum and colon (79). Dopamine, via D1-like receptors, can inhibit Na+, K+ ATPase activity and electrolyte transport in the jejunum of young but not adult rats (80). In adult rats, D1-like receptors stimulate potassium secretion in the duodenum (81) and inhibit ileal ion transport (82).
Dietary factors may also influence intestinal L-3,4- dihydroxyphenylalanine (L-DOPA) concentrations, although the effect of gut microbiota in this process is unknown. A two-week intake of a low salt diet was associated with increased dopamine but decreased L-DOPA levels in the jejunal mucosa. By contrast, high salt intake markedly increased the tissue levels of both dopamine and L-DOPA without changes in dopamine/L-DOPA ratios (83). The major mechanism for the increase in renal dopamine production with salt loading has been suggested to be caused by neural L-DOPA spill-over into the circulation (84, 85). Dopamine, produced in the kidney, and not converted to norepinephrine, is responsible for at least 50% of sodium excretion during conditions of moderate sodium excess (32–34). However, gastrin secreted by G-cells in the stomach and duodenum and released into the circulation (39, 86) may aid in this process. Gastrin is taken up by renal cortical tubules to a greater extent than the other enterokines released after a meal (87). Gastrin then acts on its receptor, the cholecystokinin B receptor expressed in several nephron segments (88) to increase renal dopamine production by increasing the renal tubular uptake of L-DOPA (unpublished data). Gastrin synergistically interacts with renal D1 receptors to inhibit sodium transport, enabling the excretion of a sodium load (74, 88–90).
Gut microbiota, genetics, hypertension, and salt sensitivity
The gut microbiota is influenced not only by nutrition and environment but also by genetic factors (91, 92). The gut microbiota can modify the expression of the hypertensive phenotype in mice with germ-line deletion of Slc26a6, which encodes an anion exchanger, Olfr78, which encodes an olfactory receptor, or toll-like receptor 5 (Tlr5), a gene component of the innate immune system expressed in the gut mucosa (42–44). Dietary nutrients have also been reported to affect microRNA (miR) and DNA methylation and acetylation and affect blood pressure. The biological function of probiotics has been suggested to be a consequence of epigenetic modification (93).
As aforementioned, the increase in blood pressure with an increase in sodium intake occurs in normotensive as well as hypertensive humans and is predictive of increased cardiovascular events and mortality, irrespective of basal blood pressure levels (10, 11). The mechanisms underlying salt sensitivity are not well understood (94–98). However, genetics can determine the blood pressure response to salt intake (31, 99–105). We have recently reported that intronic variants (intron 22–23 [rs7571842] and intron 25–26 [rs1017783]) of SLC4A5 and GRK4 (GRK4 65R>L rs2960306]) are associated with salt sensitivity in two Euro-American populations (99). GRK4 is important in the regulation of the dopamine receptors and as aforementioned, dopamine receptors are important in the regulation of renal sodium transport and blood pressure (21, 31–38, 105, 106). Human GRK4 65 R>L and two other human GRK4 gene variants (GRK4 142 A>V rs1024323, GRK4 486 A>V rs1801058) constitutively impair dopamine receptor (types 1 and 3) function (105). GRK4 gene variants cause hypertension in transgenic mice (105, 106) and salt sensitivity (unpublished) and thus fulfill the essential test for the demonstration that these genetic variants are causal of a complex trait (107), e.g., hypertension and salt sensitivity.
Gut microbiota, epigenetics, hypertension, and salt sensitivity
Genome-wide association studies (GWAS), which have identified only 2% of the genetic factors believed to influence blood pressure variation (3, 5, 6, 108), did not report GRK4 or SLC4A5 to be associated with hypertension. However, the failure to identify GRK4 and SLC4A5 in GWAS does not, by itself, eliminate GRK4 and SLC4A5 gene variants or any particular gene as causative of hypertension or salt sensitivity (or any phenotype). The current presentation of GWAS data often fails to report all truly associating variants if they do not meet arbitrary P-value cutoffs (5, 109). Moreover, the chips may not contain the gene of interest. For example, SLC4A5 rs10177833 is not in any of the Affymetrics chips and rs7571842 is found in only 3 of the 6 Affymetrix chips. Illumina chips have both variants only in Human1M-Duo-v3 and each variant in only 1 of 7 chips. Affymetrix chips do not have GRK4142V and the only Affymetrix chip that has GRK4486V is Genomewide 6. The Illumina chips, except for Illumina Human 1M-Duo-v3, do not have GRK4486V; not all the chips have GRK465L. The failure of GWAS to identify the association of GRK4 or other genes with hypertension in some studies (109–112) may also be due to a failure to examine gene-gene and gene-environment (salt sensitivity) interaction.
The lack of powerful genetic association in essential hypertension, especially salt-sensitive hypertension, as with type 2 diabetes and metabolic syndrome, may indicate the importance of gene modifiers, such as epigenetics, especially resulting from environmental influence (113–115). Diet, including salt and gut microbiota can influence epigenetics (116–119); salt can increase oxidative stress (120, 121) and oxidative stress can influence epigenetics (e.g., histone deacetylase activity) (122). Lysine-specific demethylase 1 regulates histone methylation by demethylating histone H3 at lysine residues 4 and 9 and is involved in salt-sensitive hypertension (7, 8, 114, 118, 123). Certain miRs have been implicated in salt sensitivity and inverse salt sensitivity of blood pressure (114, 124–128). For example, miR-320 and miR-26b are increased in the aorta while miR-21 and miR-1331 are decreased in the aorta and myocardium, respectively, in Dahl salt-sensitive rats fed a high salt diet (125, 126). Several miRs in human renal proximal tubule cells were found to distinguish salt-resistant from salt-sensitive human subjects, including miR-3661, miR-3126, miR-3183, and miR-615-5p while miR-4516 was able to distinguish salt sensitivity from inverse salt sensitivity (127). Mir-124 expression is also increased in urinary exosomes of salt-sensitive subjects (127) and can regulate c-Myc (128, 129). C-Myc, being a proto-oncogene (130, 131), is of interest because there is a positive association of hypertension and cancer, at least in males (132) and increased dietary salt intake increases the risk of gastric cancer (133).
Conclusion
In summary, microbiota can be controlled by many factors including diet, physical activity, genetics, and epigenetics. The influence of gut microbiota on the host may be partially explained by the generation of SCFA, including the beneficial SCFAs (acetate, butyrate, and propionate) and the non-beneficial lactate. These SCFA acting on cell surface receptors, including GPR43, GPR41, and Olfr78 regulate blood pressure (Figure 1). Gut microbiota can also influence the state of immunity and inflammation, cell metabolism, and proliferation that may eventually affect blood pressure.
Figure 1.
Microbiota can be controlled by many factors including diet, physical activity, genetics, and epigenetics. The influence of gut microbiota on the host may be partially explained by the generation of short chain fatty acids, including the beneficial acetate, butyrate and propionate, and non-beneficial lactate. These short chain fatty acids acting on cell surface receptors, including GPR43, GPR41, and Olfr78 regulate blood pressure. GPR41 and Olfr78 counter-regulate each other.
Key Points.
Genome-wide association studies on blood pressure do not take into account the effect of life-style or intake of salt and antibiotics. Salt sensitivity of blood pressure should take into account not only the ability of a high sodium intake to increase blood pressure and a low sodium intake to decrease blood pressure, but also the ability of a marked decrease in sodium intake to increase blood pressure. The intake of antibiotics at the time blood pressure is measured should be taken into account because antibiotics, by altering the gut microbiota, can affect blood pressure.
The lack of powerful genetic association in essential hypertension, especially salt-sensitive hypertension, as with type 2 diabetes and metabolic syndrome, suggests the importance of gene modifiers, such as epigenetics, especially resulting from environmental influence. Nutrition, including salt, and gut microbiota can influence epigenetics.
Gut microbiota can influence the production of monoamines by enterochromaffin cells. The gut production of serotonin, dopamine, and norepinephrine can affect not only the behavior of the host (brain-gut axis) but also the ability of the kidney to excrete a sodium load (gastro-renal axis).
Gut microbiota can regulate genes related to immunity, inflammation, and metabolism. Toxic metabolites produced following fermentation of protein, such as trimethylamine N-oxide, can also lead to chronic renal disease; the latter may be independent of genetic make-up of the host.
Acknowledgments
Financial support
This work was supported, in part, by grants from the National Institutes of Health, R37 HL023081, R01DK039308, R01HL092196, P01HL068686, and P01 HL074940 (PAJ) and R01DK073665, U01DK099924, and 01DK099914 (DR).
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Pickering TG. Modern definitions and clinical expressions of hypertension. In: Laragh JH, Brenner BM, editors. Hypertension. Pathophysiology, Diagnosis, and Management. 2. Raven Press; NY: 1995. pp. 17–21. [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;21:129 e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrap SB. Where are all the blood pressure genes? Lancet. 2003;361:2149–2151. doi: 10.1016/S0140-6736(03)13694-X. [DOI] [PubMed] [Google Scholar]
- 4.Williams SM, Haines JL, Moore JH. The use of animal models in the study of complex disease: all else is never equal or why do so many human studies fail to replicate animal findings? Bioessays. 2004;26:170–179. doi: 10.1002/bies.10401. [DOI] [PubMed] [Google Scholar]
- 5.Williams SM, Canter JA, Crawford DC, Moore JH, Ritchie MD, Haines JL. Problems with genome-wide association studies. Science. 2007;316:1840–1842. [PubMed] [Google Scholar]
- 6▪.Padmanabhan S, Caulfield M, Dominiczak AF. Genetic and molecular aspects of hypertension. Circ Res. 2015;116:937–959. doi: 10.1161/CIRCRESAHA.116.303647. Up-to-date review on the genetics of hypertension and salt sensitivity. [DOI] [PubMed] [Google Scholar]
- 7.Cowley AW, Jr1, Nadeau JH, Baccarelli A, et al. Report of the National Heart, Lung, and Blood Institute Working Group on epigenetics and hypertension. Hypertension. 2012;59:899–905. doi: 10.1161/HYPERTENSIONAHA.111.190116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friso S, Carvajal CA, Fardella CE, Olivieri O. Epigenetics and arterial hypertension: the challenge of emerging evidence. Transl Res. 2015;165:154–165. doi: 10.1016/j.trsl.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension. 1991;17(1 suppl):I61–I68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 10.Weinberger MH, Fineberg NS, Fineberg SE, et al. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 11.Strazzullo P, D’Elia L, Kandala NB, et al. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.He FJ, MacGregor GA. Salt and sugar: their effects on blood pressure. Pflugers Arch. 2015;467:577–586. doi: 10.1007/s00424-014-1677-x. Review on the interaction of sugar and salt in the pathogenesis of hypertension. [DOI] [PubMed] [Google Scholar]
- 13.Felder RA, White MJ, Williams SM, et al. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens. 2013;22:65–76. doi: 10.1097/MNH.0b013e32835b3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suematsu N, Ojaimi C, Recchia FA, et al. Potential mechanisms of low-sodium diet-induced cardiac disease: superoxide-NO in the heart. Circ Res. 2010;106:593–600. doi: 10.1161/CIRCRESAHA.109.208397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montasser ME, Douglas JA, Roy-Gagnon MH, et al. Determinants of blood pressure response to low-salt intake in a healthy adult population. J Clin Hypertens (Greenwich) 2011;13:795–800. doi: 10.1111/j.1751-7176.2011.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harsha DW, Sacks FM, Obarzanek E, et al. Effect of dietary sodium intake on blood lipids: results from the DASH-sodium trial. Hypertension. 2004;43:393–398. doi: 10.1161/01.HYP.0000113046.83819.a2. [DOI] [PubMed] [Google Scholar]
- 17.Stolarz-Skrzypek K, Kuznetsova T, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 19.Hall JE, Granger JP, do Carmo JM, et al. Hypertension: physiology and pathophysiology. Compr Physiol. 2012;2:2393–2442. doi: 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- 20.Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asico L, Zhang X, Jiang J, Cabrera D, Escano CS, Sibley DR, Wang X, Yang Y, Mannon R, Jones JE, Armando I, Jose PA. Lack of renal dopamine D5 receptors promotes hypertension. J Am Soc Nephrol. 2011;22:82–89. doi: 10.1681/ASN.2010050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlaich MP, Esler MD, Fink GD, et al. Targeting the sympathetic nervous system: critical issues in patient selection, efficacy, and safety of renal denervation. Hypertension. 2014;63:426–432. doi: 10.1161/HYPERTENSIONAHA.113.02144. [DOI] [PubMed] [Google Scholar]
- 23.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coble JP, Grobe JL, Johnson AK, Sigmund CD. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: importance of the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 2015;308:R238–249. doi: 10.1152/ajpregu.00486.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimoto M, Fujita T. Renal mechanisms of salt-sensitive hypertension: contribution of two steroid receptor-associated pathways. Am J Physiol Renal Physiol. 2015;308:F377–387. doi: 10.1152/ajprenal.00477.2013. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Pires NM, Igreja B, Moura E, et al. Blood pressure decrease in spontaneously hypertensive rats folowing renal denervation or dopamine β-hydroxylase inhibition with etamicastat. Hypertens Res. 2015 Apr 9; doi: 10.1038/hr.2015.50. Epub ahead of print Dopamine β-hydroxylase inhibition by etamicastat that does not cross the blood brain barrier decreases blood pressure by preventing the conversion of dopamine to norepinephrine. This results in a decrease in sympathetic activity and increase in dopamine availability. [DOI] [PubMed] [Google Scholar]
- 27.Te Riet L, van Esch JH, Roks AJ, et al. Hypertension: Renin-Angiotensin-Aldosterone system alterations. Circ Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RJ, Lanaspa MA, Gabriela Sánchez-Lozada L, et al. The discovery of hypertension: evolving views on the role of the kidneys, and current hot topics. Am J Physiol Renal Physiol. 2015;308:F167–178. doi: 10.1152/ajprenal.00503.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prieto MC, Gonzalez AA, Navar LG. Evolving concepts on regulation and function of renin in distal nephron. Pflugers Arch. 2013;465:121–132. doi: 10.1007/s00424-012-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollock DM. 2013 Dahl Lecture: American Heart Association council for high blood pressure research clarifying the physiology of endothelin. Hypertension. 2014;63:e110–117. doi: 10.1161/HYPERTENSIONAHA.114.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayner B, Ramesar R. The importance of G protein-coupled receptor kinase 4 (GRK4) in pathogenesis of salt sensitivity, salt sensitive hypertension and response to antihypertensive treatment. Int J Mol Sci. 2015;16:5741–5749. doi: 10.3390/ijms16035741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Villar VA, Jones JE, et al. G protein-coupled receptor kinase 4: role in hypertension. Hypertension. 2015;65:1148–1155. doi: 10.1161/HYPERTENSIONAHA.115.05189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chugh G, Lokhandwala MF, Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension. 2012;59:1029–1036. doi: 10.1161/HYPERTENSIONAHA.112.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang MZ, Harris RC. Antihypertensive mechanisms of intra-renal dopamine. Curr Opin Nephrol Hypertens. 2015;24:117–122. doi: 10.1097/MNH.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu MC, Di Sole F, Zhang J, et al. Chronic regulation of the renal Na+/H+ exchanger NHE3 by dopamine: translational and posttranslational mechanisms. Am J Physiol Renal Physiol. 2013;304:F1169–1180. doi: 10.1152/ajprenal.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhammad AB, Lokhandwala MF, Banday AA. Exercise reduces oxidative stress but does not alleviate hyperinsulinemia or renal dopamine D1 receptor dysfunction in obese rats. Am J Physiol Renal Physiol. 2011;300:F98–104. doi: 10.1152/ajprenal.00386.2010. [DOI] [PubMed] [Google Scholar]
- 37.Fang YJ, Thomas GN, Xu ZL, Fang JQ, Critchley JA, Tomlinson B. An affected pedigree member analysis of linkage between the dopamine D2 receptor gene TaqI polymorphism and obesity and hypertension. Int J Cardiol. 2005;102:111–116. doi: 10.1016/j.ijcard.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Li F, Jose PA, Ecelbarger CM. Reduction of renal dopamine receptor expression in obese Zucker rats: role of sex and angiotensin II. Am J Physiol Renal Physiol. 2010;299:F1164–1170. doi: 10.1152/ajprenal.00604.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michell AR, Debnam ES, Unwin RJ. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu Rev Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330. [DOI] [PubMed] [Google Scholar]
- 40.Furness JB1, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729–740. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- 41.Spencer AG1, Labonte ED, Rosenbaum DP, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med. 2014;6:227ra36. doi: 10.1126/scitranslmed.3007790. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Perez I, Villaseñor A, Wijeyesekera A, et al. Urinary metabolic phenotyping the slc26a6 (chloride-oxalate exchanger) null mouse model. J Proteome Res. 2012;11:4425–4435. doi: 10.1021/pr2012544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;3(28):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin J, Li R, Raes J, Arumugam M, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪▪.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. Fecal deoxycholic acid, a byproduct of microbial metabolism that promotes liver, is increased in animal-based diet. Deoxycholic acid causes stress of endoplasmic reticulum and can cause hypertension (J Clin Invest. 2012; 122:3960–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Petriz BA, Castro AP, Almeida JA, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;21:15, 511. doi: 10.1186/1471-2164-15-511. Non-obese Wistar-Kyoto and spontaneously hypertensive rats have gut microbiota that are different from obese rats. Lactobacilli, greatly increased by exercise, produces lactate that is converted by gut bacteria into butyrate, a beneficial short chain fatty acid. The conversion of lactate into butyrate is also enhanced after exercise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sommer F, Nookaew I, Sommer N, et al. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol. 2015;16:62. doi: 10.1186/s13059-015-0614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Min YW, Rhee PL. The role of microbiota on the gut immunology. Clin Ther. 2015 doi: 10.1016/j.clinthera.2015.03.009. S0149–2918(15)00146-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–760. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuso P, Stoll SR, Li WW. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm J. 2015;19:62–67. doi: 10.7812/TPP/14-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartiala J, Bennett BJ, Tang WH, et al. Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler Thromb Vasc Biol. 2014;34:1307–1313. doi: 10.1161/ATVBAHA.114.303252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014;126:267–174. doi: 10.1042/CS20130407. [DOI] [PubMed] [Google Scholar]
- 61.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–858. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 62.Brantsaeter AL, Myhre R, Haugen M, et al. Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2011;174:807–815. doi: 10.1093/aje/kwr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura Y, Yamamoto N, Sakai K, et al. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J Dairy Sci. 1995;78:1253–1257. doi: 10.3168/jds.S0022-0302(95)76745-5. [DOI] [PubMed] [Google Scholar]
- 66.Seppo L1, Jauhiainen T, Poussa T, et al. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr. 2003;77:326–330. doi: 10.1093/ajcn/77.2.326. [DOI] [PubMed] [Google Scholar]
- 67.Ahrén IL, Xu J, Onning G, et al. Antihypertensive activity of blueberries fermented by Lactobacillus plantarum DSM 15313 and effects on the gut microbiota in healthy rats. Clin Nutr. 2014 doi: 10.1016/j.clnu.2014.08.009. pii: S0261–5614 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 68.Menni C1, Mangino M, Cecelja M, et al. Metabolomic study of carotid-femoral pulse-wave velocity in women. J Hypertens. 2015;33:791–796. doi: 10.1097/HJH.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69▪▪.Khalesi S, Sun J, Buys N, et al. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. A meta-analysis of nine trials showed that consuming probiotics decreased systolic blood pressure by 3.56 mm Hg and diastolic blood pressure by 2.38 mm Hg similar to that reported with an intake of less than 2 g of sodium/day. [DOI] [PubMed] [Google Scholar]
- 70.Mell B, Jala VR, Mathew AV, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat model. Physiol Genomics. 2015 Mar 31; doi: 10.1152/physiolgenomics.00136.2014. physiolgenomics. 00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ridaura V, Belkaid Y. Gut microbiota: the link to your second brain. Cell. 2015;161:193–194. doi: 10.1016/j.cell.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 73.O’Mahony SM, Clarke G, Borre YE. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Asico LD, Zheng S, et al. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension. 2013;62:927–933. doi: 10.1161/HYPERTENSIONAHA.113.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banday AA, Lokhandwala MF. Novel gastro-renal axis and sodium regulation during hypertension. Hypertension. 2013;62:834–835. doi: 10.1161/HYPERTENSIONAHA.113.01799. [DOI] [PubMed] [Google Scholar]
- 76.Crumeyrolle-Arias M, Jaglin M, Bruneau A. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 77.Lyte M, Bailey MT. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J Surg Res. 1997;70:195–201. doi: 10.1006/jsre.1997.5130. [DOI] [PubMed] [Google Scholar]
- 78.Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 79.Asano Y1, Hiramoto T, Nishino R, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 80.Vieira-Coelho MA1, Soares-da-Silva P. Ontogenic aspects of D1 receptor coupling to G proteins and regulation of rat jejunal Na+, K+ ATPase activity and electrolyte transport. Br J Pharmacol. 2000;129:573–581. doi: 10.1038/sj.bjp.0703065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng XY, Li Y, Li LS, et al. Dopamine D1 receptors mediate dopamine-induced duodenal epithelial ion transport in rats. Transl Res. 2013;161:486–494. doi: 10.1016/j.trsl.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 82.Fraga S, Luo Y, Jose P, Zandi-Nejad K, Mount DB, Soares-da-Silva P. Dopamine D1-like receptor-mediated inhibition of Cl/HCO3- exchanger activity in rat intestinal epithelial IEC-6 cells is regulated by G protein-coupled receptor kinase 6 (GRK 6) Cell Physiol Biochem. 2006;18:347–360. doi: 10.1159/000097612. [DOI] [PubMed] [Google Scholar]
- 83.Lucas-Teixeira V, Serrão MP, Soares-Da-Silva P. Effect of salt intake on jejunal dopamine, Na+,K+-ATPase activity and electrolyte transport. Acta Physiol Scand. 2000;168:225–231. doi: 10.1046/j.1365-201x.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 84.Pestana M, Jardim H, Correia F, et al. Renal dopaminergic mechanisms in renal parenchymal diseases and hypertension. Nephrol Dial Transplant. 2001;16 (Suppl 1):53–59. doi: 10.1093/ndt/16.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- 85.Grossman E1, Hoffman A, Tamrat M, et al. Endogenous dopa and dopamine responses to dietary salt loading in salt-sensitive rats. J Hypertens. 1991;9:259–263. doi: 10.1097/00004872-199103000-00010. [DOI] [PubMed] [Google Scholar]
- 86.Jiang X, Wang W, Ning B, et al. Basal and postprandial serum levels of gastrin in normotensive and hypertensive adults. Clin Exp Hypertens. 2013;35:74–78. doi: 10.3109/10641963.2012.690474. [DOI] [PubMed] [Google Scholar]
- 87.Melis M, Krenning EP, Bernard BF, et al. Renal uptake and retention of radiolabeled somatostatin, bombesin, neurotensin, minigastrin and CCK analogues: species and gender differences. Nucl Med Biol. 2007;34:633–641. doi: 10.1016/j.nucmedbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 88.von Schrenck T, Ahrens M, de Weerth A, Bobrowski C, Wolf G, Jonas L, Jocks T, Schulz M, Bläker M, Neumaier M, Stahl RA. CCKB/gastrin receptors mediate changes in sodium and potassium absorption in the isolated perfused rat kidney. Kidney Int. 2000;58:995–1003. doi: 10.1046/j.1523-1755.2000.00257.x. [DOI] [PubMed] [Google Scholar]
- 89.Liu T, Jose PA. Gastrin induces sodium-hydrogen exchanger 3 phosphorylation and mTOR activation via a phosphoinositide 3-kinase-/protein kinase C-dependent but AKT-independent pathway in renal proximal tubule cells derived from a normotensive male human. Endocrinology. 2013;154:865–875. doi: 10.1210/en.2012-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pisegna JR, Tarasova NI, Kopp JA, et al. Postprandial changes in renal function are mediated by elevated serum gastrin acting at cholecystokinin type B receptors (CCKBR) in the kidney (Abstract) Gastroenterology. 1996;110:1106A. [Google Scholar]
- 91.Hehemann JH, Correc G, Barbeyron T, et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 92.Slack E, Hapfelmeier S, Stecher BV, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Canani RB, Costanzo MD, Leone L, et al. Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev. 2011;24:198–205. doi: 10.1017/S0954422411000102. [DOI] [PubMed] [Google Scholar]
- 94.Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol. 2014;25:1148–1155. doi: 10.1681/ASN.2013121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yatabe MS, Yatabe J, Yoneda M, et al. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am J Clin Nutr. 2010;92:77–82. doi: 10.3945/ajcn.2009.29028. [DOI] [PubMed] [Google Scholar]
- 96.Unlap MT, Bates E, Williams C, et al. Na+/Ca2+ exchanger: target for oxidative stress in salt-sensitive hypertension. Hypertension. 2003;42:363–368. doi: 10.1161/01.HYP.0000084060.54314.F5. [DOI] [PubMed] [Google Scholar]
- 97.Kanbay M, Chen Y, Solak Y, Sanders PW. Mechanisms and consequences of salt sensitivity and dietary salt intake. Curr Opin Nephrol Hypertens. 2011;20:37–43. doi: 10.1097/MNH.0b013e32834122f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(3 Suppl):247S–255S. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 99.Carey RM, Schoeffel CD, Gildea JJ, et al. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. doi: 10.1161/HYPERTENSIONAHA.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Padmanabhan S, Graham L, Ferreri NR, et al. Uromodulin, an emerging novel pathway for blood pressure regulation and hypertension. Hypertension. 2014;64:918–923. doi: 10.1161/HYPERTENSIONAHA.114.03132. [DOI] [PubMed] [Google Scholar]
- 101.Garza AE, Rariy CM, Sun B, et al. Variants in striatin gene are associated with salt-sensitive blood pressure in mice and humans. Hypertension. 2015;65:211–217. doi: 10.1161/HYPERTENSIONAHA.114.04233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Svetkey LP, McKeown SP, Wilson AF. Heritability of salt sensitivity in black Americans. Hypertension. 1996;28:854–858. doi: 10.1161/01.hyp.28.5.854. [DOI] [PubMed] [Google Scholar]
- 103.Beeks E, Kessels AG, Kroon AA, van der Klauw MM, de Leeuw PW. Genetic predisposition to salt-sensitivity: a systematic review. J Hypertens. 2004;22:1243–1249. doi: 10.1097/01.hjh.0000125443.28861.0d. [DOI] [PubMed] [Google Scholar]
- 104.Kelly TN, He J. Genomic epidemiology of blood pressure salt sensitivity. J Hypertens. 2012;30:861–873. doi: 10.1097/HJH.0b013e3283524949. [DOI] [PubMed] [Google Scholar]
- 105.Felder RA, Sanada H, Xu J, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Z, Armando I, Asico LD, et al. The elevated blood pressure of human GRK4gamma A142V transgenic mice is not associated with increased ROS production. Am J Physiol Heart Circ Physiol. 2007;292:H2083–2092. doi: 10.1152/ajpheart.00944.2006. [DOI] [PubMed] [Google Scholar]
- 107.Glazier AM, Nadeau JH, Aitman TJ. Finding genes that underlie complex traits. Science. 2002;298:2345–2349. doi: 10.1126/science.1076641. [DOI] [PubMed] [Google Scholar]
- 108.Ehret GB, Caulfield MJ. Genes for blood pressure: an opportunity to understand hypertension. Eur Heart J. 2013;34:951–961. doi: 10.1093/eurheartj/ehs455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams SM, Haines JL. Correcting away the hidden heritability. Ann Hum Genet. 2011;75:348–50. doi: 10.1111/j.1469-1809.2011.00640.x. [DOI] [PubMed] [Google Scholar]
- 110.Hiura Y, Tabara Y, Kokubo Y, et al. A genome-wide association study of hypertension-related phenotypes in a Japanese population. Circ J. 2010;74:2353–2359. doi: 10.1253/circj.cj-10-0353. [DOI] [PubMed] [Google Scholar]
- 111.Rana BK, Insel PA, Payne SH, et al. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 112.Staessen JA, Kuznetsova T, Zhang, et al. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008;51:1643–1650. doi: 10.1161/HYPERTENSIONAHA.107.109611. [DOI] [PubMed] [Google Scholar]
- 113.Udali S, Guarini P, Moruzzi S, et al. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med. 2013;34:883–901. doi: 10.1016/j.mam.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 114.Friso S, Carvajal CA, Fardella CE, et al. Epigenetics and arterial hypertension: the challenge of emerging evidence. Transl Res. 2015;165:154–165. doi: 10.1016/j.trsl.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 115.Chen LJ, Wei SY, Chiu JJ. Mechanical regulation of epigenetics in vascular biology and pathobiology. J Cell Mol Med. 2013;17:437–448. doi: 10.1111/jcmm.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schwenk RW, Vogel H, Schürmann A. Genetic and epigenetic control of metabolic health. Mol Metab. 2013;2:337–347. doi: 10.1016/j.molmet.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tammen SA, Friso S, Choi SW. Epigenetics: the link between nature and nurture. Mol Aspects Med. 2013;34:753–764. doi: 10.1016/j.mam.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y, Liu P, Yang C, et al. Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in Dahl S rats: effect of salt and genomic sequence. Hypertension. 2014;63:827–838. doi: 10.1161/HYPERTENSIONAHA.113.02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119▪▪.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. This article reviews the importance of gut microbiota in mediating gene-environment interactions. [DOI] [PubMed] [Google Scholar]
- 120.Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension. 2008;51:367–375. doi: 10.1161/HYPERTENSIONAHA.107.102111. [DOI] [PubMed] [Google Scholar]
- 121▪.Ando K1, Matsui H, Fujita M, et al. Protective effect of dietary potassium against cardiovascular damage in salt-sensitive hypertension: possible role of its antioxidant action. Curr Vasc Pharmacol. 2010;8:59–63. doi: 10.2174/157016110790226561. Dietary potassium may protect against salt-induced hypertension by the reduction of reactive oxygen species production. [DOI] [PubMed] [Google Scholar]
- 122.Doyle K, Fitzpatrick FA. Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function. J Biol Chem. 2010;285:17417–17424. doi: 10.1074/jbc.M109.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Williams JS1, Chamarthi B, Goodarzi MO, et al. Lysine-specific demethylase 1: an epigenetic regulator of salt-sensitive hypertension. Am J Hypertens. 2012;25:812–817. doi: 10.1038/ajh.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rezaei M, Andrieu T, Neuenschwander S, et al. Regulation of 11β-hydroxysteroid dehydrogenase type 2 by microRNA. Hypertension. 2014;64:860–866. doi: 10.1161/HYPERTENSIONAHA.114.00002. [DOI] [PubMed] [Google Scholar]
- 125.Ling S, Nanhwan M, Qian J, et al. Modulation of microRNAs in hypertension-induced arterial remodeling through the beta1 and beta 3-adrenoreceptor pathways. J Mol Cell Cardiol. 2013;65:127–136. doi: 10.1016/j.yjmcc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Guo TS, Zhang J, Mu JJ, et al. High-salt intake suppressed microRNA-133a expression in Dahl SS rat myocardium. Int J Mol Sci. 2014;15:10794–10805. doi: 10.3390/ijms150610794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA. Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem. 2013;46:1131–1134. doi: 10.1016/j.clinbiochem.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mu P, Han YC, Betel D, et al. Genetic dissection of the miR-17~92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 2009;23:2806–2811. doi: 10.1101/gad.1872909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Z, Van Calcar S, Qu C, et al. A global transcriptional regulatory role for c-Myc in Burkitt’s lymphoma cells. Proc Natl Acad Sci USA. 2003;100:8164–8169. doi: 10.1073/pnas.1332764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Z, Van Calcar S, Qu C, et al. A global transcriptional regulatory role for c-Myc in Burkitt’s lymphoma cells. Proc Natl Acad Sci USA. 2003;100:8164–8169. doi: 10.1073/pnas.1332764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heideman MR, Wilting RH, Yanover E, et al. Dosage-dependent tumor suppression by histone deacetylases 1 and 2 through regulation of c-Myc collaborating genes and p53 function. Blood. 2013;121:2038–2050. doi: 10.1182/blood-2012-08-450916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stocks T, Van Hemelrijck M, Manjer J, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension. 2012;59:802–810. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 133.D’Elia L, Galletti F, Strazzullo P. Dietary salt intake and risk of gastric cancer. Cancer Treat Res. 2014;159:83–95. doi: 10.1007/978-3-642-38007-5_6. [DOI] [PubMed] [Google Scholar]