Abstract
Introduction
Pyrazinamide (PZA) is essential in tuberculosis (TB) treatment. We describe the prevalence, trends and predictors of PZA resistance in Mycobacterium tuberculosis complex (MTBC) in the U.S.
Methods
We analyzed culture-positive MTBC cases with reported drug susceptibility tests (DST) for PZA in 38 jurisdictions routinely testing for PZA susceptibility from 1999-2009. National TB Genotyping Service data for 2004-2009 were used to distinguish Mycobacterium tuberculosis from Mycobacterium bovis and determine phylogenetic lineage.
Results
Overall 2.7% (2,167/79,321) of MTBC cases had PZA resistance, increasing annually from 2.0% to 3.3% during 1999-2009 (P<0.001), largely due to an increase in PZA monoresistance. PZA-monoresistant MTBC (versus drug-susceptible) was associated with age 0-24 years (adjusted prevalence ratio [aPR]=1.50, 95% CI 1.31-1.71), Hispanic ethnicity (aPR=3.52, 2.96-4.18), HIV infection (aPR=1.43, 1.15-1.77), extrapulmonary disease (aPR=3.02, 2.60-3.52), and normal chest radiograph (aPR=1.88, 1.63-2.16), and inversely associated with Asian (aPR=0.59, 0.47-0.73) and Black (aPR=0.37, 0.29-0.49) race. Among multidrug-resistant (MDR) cases 38.0% were PZA-resistant; PZA resistance in MDR MTBC was associated with female sex (aPR=1.25, 1.08-1.46) and previous TB diagnosis (aPR=1.37, 1.16-1.62). Of 28,080 cases with genotyping data, 925 (3.3%) had PZA resistance; 465/925 (50.3%) were M. bovis. In non-MDR M. tuberculosis cases, PZA resistance was higher in the Indo-Oceanic than the East Asian lineage (2.2% versus 0.9%; respectively; aPR=2.26, 1.53-3.36), but in MDR cases it was lower in the Indo-Oceanic lineage (22.0% versus 43.4%, respectively; aPR=0.54, 0.32-0.90).
Conclusions
Specific human and mycobacterial characteristics were associated with pyrazinamide-resistant MTBC, reflecting both specific subgroups of the population and phylogenetic lineages of the mycobacteria.
Keywords: tuberculosis, drug resistance, epidemiology, pyrazinamide
Introduction
Pyrazinamide (PZA) is an important component of first-line and second-line regimens for treatment of drug-susceptible and multidrug-resistant (MDR) tuberculosis (TB) [1-3]. PZA has remarkable sterilizing effects, playing a unique role in killing semi-dormant TB bacilli not easily killed by other antibiotics[4, 5]. In drug-susceptible TB, adding PZA to rifampin and isoniazid allowed shortening the duration of treatment from 9 to 6 months in most patients[6, 7]. Recent murine and human early bactericidal activity studies of novel drug regimens, demonstrated PZA was essential to well-performing regimens[8-10].
Growth-based testing for PZA resistance is difficult because the drug is active only in an acidic microenvironment (pH 5.5), but such low pH itself inhibits the growth of Mycobacterium tuberculosis complex (MTBC). Further, even modest variations in inoculum size can alter the pH and lead to differing results[11, 12]. For these technical reasons, most countries and some mycobacteriology laboratories in the U.S. do not test for PZA susceptibility, and the global extent of PZA resistance is largely unknown. The Clinical and Laboratory Standards Institute recommended the BACTEC 460TB (BD, Sparks, MD, USA) as a reference method for PZA susceptibility testing[13]. However, in 2011 BD stopped producing reagents for the BACTEC 460TB system, so BACTEC Mycobacteria Growth Indicator Tube® (MGIT) 960 system (BD, Sparks, MD, USA) and VersaTREK® (TREK Diagnostic Systems, Inc., Cleveland, OH, USA) are currently the only Food and Drug Administration (FDA) cleared systems for PZA drug susceptibility testing (DST)[14]. These systems, however, may have a higher potential for false-resistant test results for PZA resistance than the BACTEC 460TB[12, 15].
Mycobacterium bovis, a member of MTBC, is intrinsically resistant to PZA, and PZA-monoresistance is characteristic of M. bovis[16, 17]. In the U.S., M. bovis is transmitted to humans primarily by ingestion of unpasteurized dairy products and more often involves extrapulmonary sites of disease[18, 19].
Because PZA will likely remain a central component in the treatment of tuberculosis for the foreseeable future, it is critically important to understand the epidemiology of PZA resistance. We describe the prevalence, trends, and risk factors for initial resistance to PZA among MTBC cases in the U.S.
Methods
Case reporting
The U.S. National TB Surveillance System (NTSS) at the U.S. Centers for Disease Control and Prevention (CDC) has collected nationwide TB incidence data since 1953[20]. We analyzed data on all verified, culture-positive tuberculosis cases reported by the 50 states and the District of Columbia through the Report of Verified Case of Tuberculosis (RVCT) form between 1 January 1999 and 31 December 2009. The RVCT form includes socio-demographic and clinical information as well as DST results based on the initial positive culture of sputum or other specimen[21]. We examined overall and annual proportions of culture-positive MTBC cases with reported initial DST results for PZA. For this analysis, we excluded TB cases without reported DST results for isoniazid (INH), rifampin (RMP), and ethambutol (EMB). We also excluded states where the overall proportion of culture-positive TB cases with reported initial DST results to PZA was <85% during the study period.
Since data about sub-species are not available to most clinicians who treat TB (as the majority of the US public health labs use Accuprobe™ tests for culture identification reported as MTBC, and the genotype data which identifies sub-species are not immediately available)[22], we first characterize the epidemiology and factors associated with PZA-resistance among cases of MTBC as a whole. Because M. bovis has intrinsic resistance to PZA, we distinguished MTB and M. bovis for a sub-set of MTBC cases by linking NTSS data to data in the National TB Genotyping Service (NTGS) database[23] from 2004, when the NTGS was launched, to 2009. Spacer oligonucleotide typing (spoligotyping) and mycobacterial interspersed repetitive unit variable number tandem repeats (MIRU-VNTR) techniques distinguished M. tuberculosis (Mtb) and M. bovis and identified phylogenetic lineages for Mtb as previously described[18, 24, 25].
Definitions
”Drug-susceptible” was defined as susceptibility to INH, RMP, EMB, and PZA. MDR was defined as resistance to at least INH and RMP. “PZA monoresistance” was defined as resistance to PZA, and susceptibility to INH, RMP and EMB. “PZA polyresistance” was defined as resistance to PZA with additional resistance to INH, RMP, or EMB, but not both INH and RMP.
“Acquired” resistance to an anti-TB drug was used to describe a case in which the DST of the initial isolate was recorded as “susceptible,” and that of the final isolate was recorded as “resistant” to the same drug.
Statistical analyses
Statistical analyses were performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC). A P value of ≤0.05 was considered statistically significant. Risk factors associated with PZA resistance were determined among cases with MTBC, and among a subset of cases with infection with Mtb for 2004-2009 after matching with genotyping data. Prevalence ratios (PR) with 95% confidence intervals (CI) were calculated. Factors significant at a value of P≤0.05 and plausible epidemiological or biological associations with PZA resistance were included in a multivariable log-binomial regression model. We used backward selection starting with all candidate variables selected based on statistical criteria and plausible epidemiological or biological associations with PZA resistance and tested if the deletion of variables improved precision around point estimates in the model, repeating this process until no further improvement was possible.
For testing the significance of trends, we assessed the slope of the regression line using Poisson regression for event count data. Annual Percent Change (APC) in rates with confidence limits (CL) was calculated using Joinpoint Regression Program, Version 3.5.2. October 2011 (Statistical Research and Applications Branch, National Cancer Institute)[26].
Results
PZA resistance proportions, trends, and predictors in MTBC cases
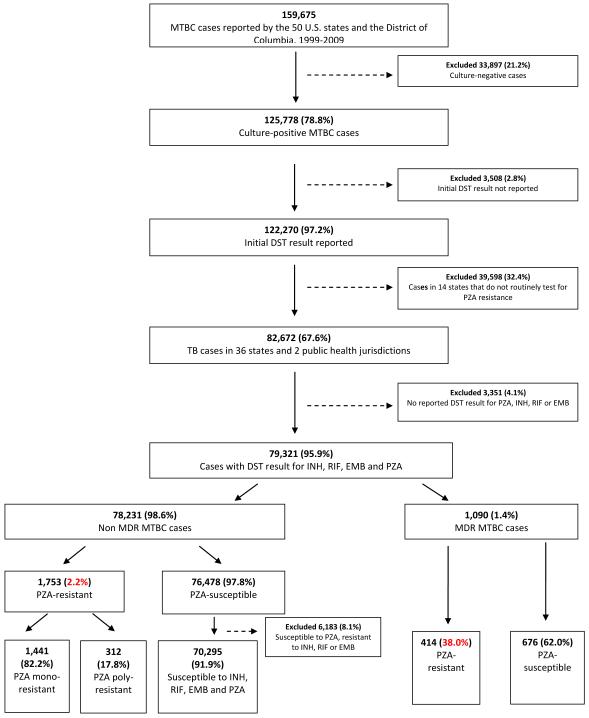
During the 11-year study period, 125,778 culture positive MTBC cases were reported by the 50 U.S. states and the District of Columbia (Figure 1). In 36 states and 2 other public health jurisdictions, the proportion of culture-positive MTBC cases that had DST results reported to the NTSS for PZA was ≥85% (Figure 2A), amounting to 82,672 TB cases. Of these, 79,321 (95.9%) had DST result for all 4 first-line drugs and were included in the analysis (Figure 1). A total of 2,167 (2.7%) cases had initial PZA resistance: 1,441 (66.5%) were PZA-monoresistant, 312 (14.4%) were PZA-polyresistant and 414 (19.1%) were PZA-resistant MDR cases. Resistance to PZA was reported in 2.2% of non-MDR cases, and 38.0% of MDR cases. In the 14 states excluded from analysis, <50% of cases per year had a DST result for PZA during the study period, with the exception of 2009 (Figure 2B).
Figure 1.
Selection of MTBC cases with resistance to pyrazinamide (PZA) reported in NTSS, 1999-2009
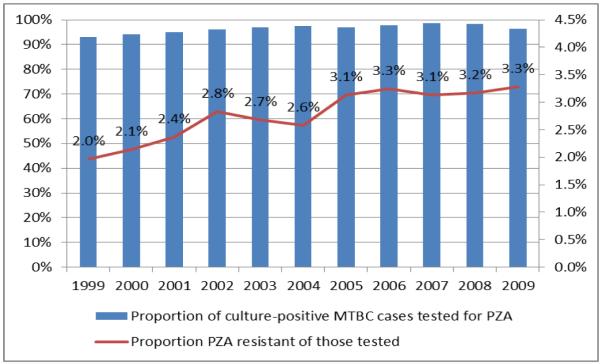
Figure 2.
Proportion of culture-positive MTBC cases with a reported PZA drug susceptibility test result, and proportion reported as resistant to PZA among those tested, 1999-2009 (N=122,270)
A. Data for 36 states and 2 public health jurisdictions with ≥85% TB cases with reported DST result for PZA (N=82,672)
Note. Overall 2.7% of cases had resistance to PZA. Proportions of resistance to PZA significantly increased (APC=5.0, CL: 3.2, 6.7; P<0.001).
B. Data for 14 states with <85% TB cases with reported DST result for PZA (N=39,598)
Note. Overall 2.9% of cases had resistance to PZA. Proportions of resistance to PZA significantly increased (APC=5.4, CL: 1.2, 9.6; P=0.02).
The proportion of any resistance to PZA increased significantly from 2.0% in 1999 to 3.3% in 2009 (P<0.001) (Figure 2A). Overall reported PZA resistance increased mainly because the proportion of PZA-monoresistance increased from 1.2% in 1999 to 2.5% in 2009 with the inflection point (“joinpoint”) for APC in 2002 (APC 1999-2002: 12.9, CL: 5.0, 19.7, P=0.005; APC 2002-2009: 6.0, CL: 4.3, 7.6; P<0.001), while the proportions were stable for PZA-polyresistance (P=0.40) and PZA resistance among MDR cases (P=0.98) (Figure 3A).
Figure 3.



Trends in proportions of PZA resistance in MTBC and M. tuberculosis.
Percent reflects proportion of cases with specific resistance pattern among all cases tested for PZA drug susceptibility in each sub-set.
A. All MTBC cases, with or without available genotype, 1999-2009 (N=79,321)
Increase in proportions of PZA-monoresistance was significant (APC during 1999-2002: 12.9, CL: 5.0, 19.7, P=0.005; APC during 2002-2009: 6.0, CL: 4.3, 7.6; P<0.001), while no significant change in proportions of PZA-polyresistance (APC=2.4, CL: −3.6, 8.7; P=0.40) and PZA resistance in MDR (APC=−0.04, CL: −4.2, 4.3; P=0.98) observed.
B. MTBC cases with available genotype M. bovis included, 2004-2009 (N=28,080)
For monoresistance APC=9.0, CL: 2.5, 15.9; P=0.02; for polyresistance APC=0.3, CL: −37.7, 61.5; P=0.99; for PZA resistance in MDR TB cases APC=−4.4, CL: −19.7, 13.8; P=0.51; for any PZA resistance APC=5.0, CL: −4.5, 15.3; P=0.23
C. M. tuberculosis cases only, 2004-2009 (N=27,428)
For monoresistance APC=11.0, CL: 2.6, 20.1; P=0.02; for polyresistance APC=−7.2, CL: −31.4, 25.6; P=0.53; for PZA resistance in MDR TB cases APC=−8.5, CL: −23.2, 8.8; P=0.23; for any PZA resistance APC=−0.1, CL: −8.4, 9.0; P=0.97.
A total of 4,961 cases were alive at diagnosis, initially treated with PZA and had both initial and final DST result to PZA reported to the NTSS. Of 4,769 (96.1% of 4961) cases with an initial isolate reported as PZA-susceptible, 36 (0.8%) cases had a final DST reported as resistant (i.e., “acquired” resistance to PZA).
Results of descriptive analysis of predictors of monoresistance, polyresistance, and PZA resistance with MDR in MTBC cases are shown in Table 1. The results of multivariable analysis are presented in Table 2. PZA-monoresistance (versus drug-susceptible) was associated with age 0-24 years (adjusted prevalence ratio [aPR]=1.50, 95% CI 1.31-1.71), Hispanic ethnicity (aPR=3.52, 2.96-4.18), HIV infection (aPR=1.43, 1.15-1.77), extrapulmonary disease (aPR=3.02, 2.60-3.52), normal chest radiograph (aPR=1.88, 1.63-2.16), and inversely associated with Asian (aPR=0.59, 0.47-0.73) and Black (aPR=0.37, 0.29-0.49) race, substance use (aPR=0.80, 0.67-0.97), homelessness (aPR=0.43, 0.28-0.65), and residence in correctional facility (aPR=0.41, 0.23-0.73). PZA-polyresistance (versus drug-susceptible) was associated with Hispanic ethnicity (aPR=2.49, 1.67-3.71), Asian (aPR=2.68, 1.82-3.96) race, previous TB diagnosis (aPR=1.78, 1.15-2.75), and normal chest x-ray (aPR=1.56, 1.17-2.08), and inversely associated with age ≥45 years (aPR=0.72, 0.56-0.92). PZA resistance in MDR cases (versus PZA-susceptible MDR) was associated with female sex (aPR=1.25, 1.08-1.46) and previous TB diagnosis (aPR=1.37, 1.16-1.62).
Table 1.
Socio-demographic and clinical characteristics associated with initial resistance to PZA in MTBC cases (N=73,138)
| Characteristics | No. (%) | Prevalence Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| PZA- mono-R (n=1,441) |
PZA- poly-R (n=312) |
PZA-R/ MDR (n=414) |
PZA-mono-R vs. Drug susceptible |
PZA-poly-R vs. Drug susceptible |
PZA-R in MDR vs. PZA-S in MDR |
||
| Sex | Female | 612 (2.2) | 122 (0.5) | 207 (42.7) | 1.20 (1.08-1.33) | 1.04 (0.83-1.31) | 1.25 (1.08-1.46) |
| Male | 826 (1.9) | 190 (0.4) | 206 (34.1) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Age categories, years | 0-4 | 77 (10.1) | 5 (0.7) | 7 (70.0) | 5.07 (4.04-6.38) | 1.31 (0.54-3.18) | 1.84 (1.21-2.81) |
| 5-14 | 85 (13.6) | 6 (1.1) | 9 (60.0) | 6.83 (5.50-8.47) | 1.98 (0.88-4.47) | 1.58 (1.03-2.42) | |
| 15-24 | 204 (2.6) | 30 (0.4) | 71 (40.8) | 1.29 (1.10-1.52) | 0.70 (0.47-1.03) | 1.07 (0.87-1.33) | |
| 25-44 | 503 (2.0) | 138 (0.6) | 196 (38.0) | 1.00 | 1.00 | 1.00 | |
| 45-64 | 309 (1.5) | 73 (0.4) | 98 (34.9) | 0.74 (0.65-0.86) | 0.64 (0.48-0.85) | 0.92 (0.76-1.11) | |
| ≥65 | 263 (1.6) | 60 (0.4) | 33 (35.1) | 0.81 (0.70-0.94) | 0.67 (0.50-0.91) | 0.92 (0.69-1.24) | |
|
| |||||||
| Race/ethnicity | Hispanic | 982 (5.3) | 107 (0.6) | 111 (38.5) | 4.31 (3.64-5.09) | 2.62 (1.74-3.95) | 0.97 (0.73-1.28) |
| Asian | 197 (1.0) | 128 (0.7) | 178 (38.2) | 0.83 (0.67-1.02) | 2.87 (1.92-4.29) | 0.96 (0.74-1.25) | |
| Non-Hispanic Black | 95 (0.5) | 45 (0.2) | 79 (35.4) | 0.40 (0.31-0.52) | 1.02 (0.64-1.63) | 0.89 (0.66-1.20) | |
| American Indian | 8 (0.7) | 2 (0.2) | 0 (0) | 0.55 (0.27-1.11) | 0.73 (0.17-3.06) | Undefined | |
| Non-Hispanic multiple | 1 (0.8) | 1 (0.8) | 3 (100) | 0.69 (0.10-4.87) | 3.66 (0.50-26.66) | 2.51 (1.98-3.19) | |
| Unknown | 2 (0.8) | 0 (0) | 2 (50.0) | 0.63 (0.16-2.52) | undefined | 1.26 (0.46-3.44) | |
| Non-Hispanic White | 156 (1.2) | 29 (0.2) | 41 (39.8) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Country of birth | Foreign-born nationals | 1017 (2.4) | 237 (0.6) | 316 (37.2) | 1.62 (1.45-1.82) | 2.15 (1.66-2.79) | 0.92 (0.77-1.10) |
| U.S.-born | 419 (1.5) | 74 (0.3) | 96 (40.5) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
|
Occupation during 2 years prior
to diagnosis |
Unemployed | 779 (2.1) | 155 (0.4) | 229 (40.4) | 1.10 (0.98-1.22) | 0.93 (0.74-1.17) | 1.14 (0.97-1.34) |
| Employed/not seeking empl. | 553 (1.9) | 130 (0.5) | 148 (35.4) | 1.00 | 1.00 | 1.00 | |
| Health care worker | 26 (1.2) | 7 (0.3) | 19 (37.3) | 0.65 (0.44-0.96) | 0.74 (0.34-1.57) | 1.05 (0.72-1.54) | |
| Other employment | 553 (1.9) | 130 (0.5) | 148 (35.4) | 1.00 | 1.00 | 1.00 | |
| Correctional facility resident | Yes | 15 (0.8) | 4 (0.2) | 6 (33.3) | 0.39 (0.23-0.65) | 0.47 (0.18-1.27) | 0.87 (0.45-1.69) |
| No | 1425 (2.0) | 308 (0.4) | 408 (38.2) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Homeless in the year prior to diagnosis | Yes | 28 (0.6) | 9 (0.2) | 15 (34.9) | 0.28 (0.19-0.41) | 0.41 (0.21-0.80) | 0.91 (0.60-1.38) |
| No | 1393 (2.1) | 300 (0.5) | 392 (38.2) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Substance use (alcohol, IDU, non-IDU) | Yes | 139 (1.1) | 33 (0.3) | 50 (38.2) | 0.50 (0.42-0.59) | 0.54 (0.38-0.78) | 1.01 (0.80-1.27) |
| No | 1299 (2.2) | 279 (0.5) | 363 (37.9) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Prior TB diagnosis | Yes | 35 (1.1) | 22 (0.7) | 96 (48.5) | 0.54 (0.39-0.76) | 1.63 (1.06-2.52) | 1.36 (1.15-1.61) |
| No | 1397 (2.1) | 289 (0.4) | 313 (35.6) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Location of TB disease | Extrapulmonary (EP) alone | 630 (4.7) | 70 (0.5) | 46 (39.0) | 4.34 (3.88-4.86) | 1.36 (1.04-1.78) | 1.02 (0.81-1.30) |
| Pulmonary & EP | 256 (3.6) | 39 (0.6) | 32 (34.8) | 3.29 (2.84-3.80) | 1.39 (0.99-1.96) | 0.91 (0.68-1.22) | |
| Pulmonary alone | 552 (1.1) | 203 (0.4) | 334 (38.0) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Sputum microscopy result for AFB | Positive | 426 (1.3) | 137 (0.4) | 237 (38.8) | 0.65 (0.58-0.74) | 1.01 (0.79-1.30) | 1.09 (0.92-1.29) |
| Negative | 544 (2.0) | 112 (0.4) | 140 (35.5) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Sputum culture result for MTBC | Positive | 673 (1.3) | 215 (0.4) | 341 (37.7) | 0.39 (0.34-0.45) | 1.13 (0.77-1.68) | 0.94 (0.72-1.21) |
| Negative | 260 (3.4) | 28 (0.4) | 39 (40.2) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| HIV test result | Positive | 113 (1.9) | 23 (0.4) | 50 (37.3) | 1.45 (1.18-1.79) | 0.92 (0.59-1.43) | 1.00 (0.78-1.28) |
| Unknown | 929 (2.7) | 160 (0.5) | 188 (38.7) | 2.08 (1.85-2.34) | 1.12 (0.89-1.42) | 1.03 (0.88-1.21) | |
| Negative | 399 (1.3) | 129 (0.4) | 176 (37.5) | 1.00 | 1.00 | 1.00 | |
|
| |||||||
| Vital Status at diagnosis | Alive | 1412 (2.0) | 300 (0.4) | 408 (38.0) | 1.25 (0.86-1.81) | 0.62 (0.35-1.11) | 1.14 (0.55-2.34) |
| Dead | 28 (1.6) | 12 (0.7) | 5 (33.3) | 1.00 | 1.00 | 1.00 | |
Note. MTBC=Mycobacterium tuberculosis complex. R=resistant, S=susceptible. IDU=injecting drug use. AFB=acid-fast bacillus.
Table 2.
Independent predictors of PZA resistance in MTBC cases in multivariable regression analysis (N=73,138)
| Adjusted prevalence ratio (95% CI) |
||||
|---|---|---|---|---|
| Characteristic | PZA-mono-R vs. Drug susceptible |
PZA-poly-R vs. Drug susceptible |
PZA-R/MDR vs. PZA-S/MDR |
|
| Sex | Female vs. male | - | - | 1.25 (1.08-1.46) |
|
| ||||
| Age group, years | 0-24 | 1.50 (1.31-1.71) | 0.81 (0.57-1.15) | - |
| 45+ | 0.85 (0.74-0.97) | 0.72 (0.56-0.92) | - | |
| 25-44 | 1.00 | 1.00 | - | |
|
| ||||
| Race/ethnicity | Hispanic | 3.52 (2.96-4.18) | 2.49 (1.67-3.71) | - |
| Asian | 0.59 (0.47-0.73) | 2.68 (1.82-3.96) | - | |
| Black | 0.37 (0.29-0.49) | 0.96 (0.61-1.52) | - | |
| White/Other | 1.00 | 1.00 | ||
|
| ||||
| Unemployed | Yes vs. No/Unknown | 1.27 (1.14-1.42) | - | - |
|
| ||||
| Substance use | Yes vs. No/Unknown | 0.80 (0.67-0.97) | - | - |
|
| ||||
| Homeless | Yes vs. No/Unknown | 0.43 (0.28-0.65) | - | - |
|
| ||||
|
Resident of
correctional facility |
Yes vs. No/Unknown | 0.41 (0.23-0.73) | - | - |
|
| ||||
| HIV status | Positive | 1.43 (1.15-1.77) | - | - |
| Unknown | 1.82 (1.61-2.06) | - | - | |
| Negative | 1.00 | - | - | |
|
| ||||
| Previous TB diagnosis | Yes | - | 1.78 (1.15-2.75) | 1.37 (1.16-1.62) |
| Unknown | - | 0.33 (0.05-2.38) | 1.12 (0.56-2.22) | |
|
| ||||
| No | - | 1.00 | 1.00 | |
| Site of TB disease | EPTB | 3.02 (2.60-3.52) | - | - |
| EPTB&PTB | 2.91 (2.50-3.39) | - | - | |
| PTB | 1.00 | - | - | |
|
| ||||
| Initial chest radiograph | Normal | 1.88 (1.63-2.16) | 1.56 (1.17-2.08) | - |
| Unknown | 1.48 (1.04-2.10) | 1.20 (0.54-2.70) | - | |
| Abnormal | 1.00 | 1.00 | - | |
Note. R=resistant, S=susceptible.
PZA resistance proportions and trends in MTBC cases with available genotyping
The NTGS began in 2004 and by the end of 2009, genotyping results were available for 28,080 (69.9%) of 40,151 TB cases during that period that had DST to 4 first-line drugs. Among the genotyped isolates, 27,428 (97.7%) were Mtb, 500 (1.8%) were M. bovis, and 152 (0.5%) were M. africanum. Of 28,080 MTBC isolates, 925 (3.3%) had any PZA resistance, including 465 (50.3%) that were M. bovis. Among 27,428 Mtb isolates, 458 (1.7%) had any resistance to PZA: 196 (42.8%) were PZA-monoresistant, 94 (20.5%) were PZA-polyresistant, and 168 (36.7%) were PZA-resistant MDR.
M. bovis accounted for 427 (68.3%) of 625 MTBC isolates with PZA-monoresistance, 37 (28.2%) of 131 with reported PZA-polyresistance, and 1 (0.6%) of 169 with PZA-resistant MDR cases. Of 500 M. bovis isolates, 35 (7.0%) were reported as PZA-susceptible.
During 2004-2009, the annual proportion of M. bovis among MTBC isolates ranged between 1.6-2.0% and did not significantly change over time (APC=2.1, CL: −4.6, 9.2; P=0.45) (Appendix. Figure 1A). Among 28,080 MTBC isolates, the proportions of PZA-monoresistance increased significantly (P=0.004) (Figure 3B). This trend remained significant when M. bovis was excluded (P=0.02), although proportions of monoresistance to PZA were approximately three times lower in the sub-set of Mtb cases compared to MTBC (Figure 3C).
Predictors of PZA resistance in M. tuberculosis cases
Further analyses were limited to the subset of cases with Mtb only, excluding M. bovis and M. africanum, in order to understand factors associated with PZA resistance in Mtb. The prevalence of any PZA resistance differed by Mtb lineage: 2.9% of East Asian (110/3,857), 2.4% of Indo-Oceanic (121/5,037), 1.8% of East African Indian (25/1,367), and 1.2% of Euro-American (206/17,314). The proportion of Mtb cases with Indo-Oceanic lineage significantly increased from 2004-2009 (APC=4.7, CL: 1.6, 7.9; P=0.01) (Appendix. Figure 1A). Similarly, the proportion of PZA-monoresistance significantly increased among Mtb cases infected with Indo-Oceanic (APC=14.2, CL: 7.0-21.8; P=0.005) and East African Indian (APC=38.6, CL: 1.0-90.3; P=0.05) isolates, but not among other lineages.
The results of multivariable analysis examining the associations between clinical and demographic characteristics and PZA resistance in Mtb cases are presented in Table 3. The patient characteristics associated with PZA resistance in the subset of cases with Mtb differed from those for all MTBC cases. Among cases with Mtb, PZA mono-resistance was associated with Asian race and extrapulmonary TB. When Mtb lineage was included in the multivariable model, PZA monoresistance was associated with Indo-Oceanic lineage and was no longer associated with race or site of disease (Table 4). MDR status significantly modified the association between PZA resistance and Mtb lineage (P<0.001, Breslow-Day test). Among non-MDR cases, PZA resistance was significantly higher in the Indo-Oceanic (2.2%) versus East Asian (0.9%) lineage (aPR=2.26, 95% CI 1.53-3.36), while in MDR cases, PZA resistance was significantly lower in the Indo-Oceanic (22.0%) versus East Asian (43.4%) lineage (aPR=0.54, 0.32-0.90), controlling for age, race, foreign birth, HIV status, previous TB diagnosis and site of TB disease (Table 4).
Table 3.
Independent predictors of PZA resistance in M. tuberculosis cases in multivariable regression analysis (N=27,428)
| Adjusted prevalence ratio (95% CI) |
||||
|---|---|---|---|---|
| Characteristic | PZA-mono-R vs. Drug susceptible |
PZA-poly-R vs. Drug susceptible |
PZA-R/MDR vs. PZA-S/MDR |
|
| Age group | 0-24 | - | 0.53 (0.26-1.09) | - |
| 25-44 | - | 1.00 | - | |
| 45+ | - | 0.55 (0.35-0.84) | - | |
|
| ||||
| Race/ethnicity | Hispanic | 0.95 (0.59-1.53) | 1.12 (0.41-3.09) | - |
| Asian | 1.79 (1.18-2.70) | 3.70 (1.49-9.22) | - | |
| Black | 0.91 (0.56-1.48) | 2.02 (0.75-5.47) | - | |
| White/Other | 1.00 | 1.00 | - | |
|
| ||||
| Foreign-born | Yes | - | 2.37 (1.24-4.70) | 0.72 (0.55-0.93) |
| No | - | 1.00 | 1.00 | |
|
| ||||
| Previous TB diagnosis | Yes | - | 2.42 (1.24-4.70) | - |
| No | - | 1.00 | - | |
|
| ||||
| Site of TB disease | EPTB | 1.42 (1.02-1.99) | - | 0.97 (0.66-1.43) |
| EPTB&PTB | 1.24 (0.80-1.92) | - | 0.60 (0.34-1.05) | |
| PTB | 1.00 | - | 1.00 | |
Note. R=resistant. S=susceptible
Table 4.
Association of PZA resistance with M. tuberculosis lineage in multivariable regression analysis (N=27,428)
| Characteristic | Adjusted prevalence ratio (95% CI) |
||
|---|---|---|---|
| Non MDR TB cases N=26,982 |
MDR TB cases N=446 |
||
| Phylogenetic lineage | EuroAmerican | 1.24 (0.80-1.92) | 0.83 (0.61-1.13) |
| IndoOceanic | 2.26 (1.53-3.36) | 0.54 (0.32-0.90) | |
| East African Indian | 1.17 (0.63-2.19) | 0.82 (0.49-1.40) | |
| East Asian | 1.00 | 1.00 | |
|
| |||
| Age group | 0-24 | 0.90 (0.63-1.30) | 1.12 (0.83-1.52) |
| 25-44 | 0.72 (0.57-0.93) | 0.85 (0.64-1.13) | |
| 45+ | 1.00 | 1.00 | |
|
| |||
| Race/ethnicity | Hispanic | 0.85 (0.52-1.38) | 1.14 (0.75-1.72) |
| Asian | 1.48 (0.89-2.46) | 0.93 (0.63-1.39) | |
| Black | 1.03 (0.67-1.59) | 0.87 (0.55-1.40) | |
| White/Other | 1.00 | 1.00 | |
|
| |||
| Foreign-born | Yes | 1.20 (0.84-1.71) | 0.71 (0.53-0.97) |
| No | 1.00 | 1.00 | |
|
| |||
| Previous TB diagnosis | Yes | 1.40 (0.87-2.26) | 1.14 (0.86-1.52) |
| No | 1.00 | 1.00 | |
|
| |||
| Site of TB disease | EPTB | 1.09 (0.82-1.46) | 1.06 (0.71-1.58) |
| EPTB&PTB | 1.05 (0.72-1.54) | 0.60 (0.34-1.06) | |
| PTB | 1.00 | ||
|
| |||
| HIV infection | Yes | 0.80 (0.43-1.46) | 1.00 (0.65-1.52) |
| No/unknown | 1.00 | 1.00 | |
Discussion
This large study of PZA resistance among MTBC cases in the U.S. demonstrates PZA resistance in 2.7% of tested MTBC cases in 38 public health jurisdictions routinely testing for PZA susceptibility: 2.2% of non-MDR and 38.0% of MDR cases. For comparison, Australia surveillance data for 2008-2009 reported PZA resistance in 1.0%-1.2% of all TB cases[27]. Four rounds of drug resistance surveys in South Korea conducted from 1999-2004 showed a significant increase of PZA resistance in new cases from 0.8% to 2.1% [28]. In re-treatment cases, PZA resistance varied from 3.5% to 15.9% with no clear trend[28]. A study from Thailand indicated PZA resistance in 6% of non-MDR and 49% MDR TB isolates[29]. The proportion of MDR TB cases with PZA resistance ranged from 36% to 85% in other reports[30-34].
Patient characteristics associated with PZA monoresistance among all cases with MTBC included Hispanic race, young age, and extrapulmonary disease. PZA monoresistance is typical of [0]M. bovis, and the patient characteristics we identified in this analysis are similar characteristics associated with TB due to M. bovis[18]. Indeed, two thirds of all PZA-monoresistant cases were among cases with M. bovis. In contrast, among MDR TB cases, PZA resistance was higher in females, in cases with previous TB, and almost exclusively in MTB species. Interestingly, the adjusted prevalence of PZA polyresistance, compared to drug-susceptible TB, included a mix of risk factors found for PZA monoresistance and PZA resistance in MDR, likely reflecting the broader mix of M. bovis and MTB species. Clinicians should be aware that patients with certain characteristics are more likely to have PZA-resistant TB. However, our findings confirm previous reports that PZA monoresistance is not a reliable marker of M. bovis[18, 35, 36]. Although not typically available for initial case management, species identification is important to better understand these findings.
Factors associated with PZA resistance among cases of Mtb are less well understood. In the analysis of only cases with Mtb we found that PZA monoresistance was associated with Asian race rather than Hispanic ethnicity, and with exclusively extrapulmonary disease. Because site of disease has been shown to be associated with Mtb lineage[24, 37], and these lineages are differentially associated with human populations globally[38], we sought to determine whether the patient characteristics associated with Mtb PZA monoresistance reflected differences in Mtb lineage. In multivariable analysis including Mtb lineage, only Indo-Oceanic lineage remained significantly associated with PZA monoresistance, suggesting that bacterial lineage, rather than host characteristic, was the primary association. Globally, Indo-Oceanic lineage is primarily localized to South and Southeast Asia[38]. Our findings suggest two possible hypotheses for the association between PZA monoresistance and Indo-Oceanic lineage. First, this may reflect a biological difference by lineage in the propensity towards development of resistance to this drug. Second, these results would also be consistent with international differences in rates of PZA resistance (e.g. due to regional or national programmatic differences in TB treatment). Over half of all TB cases in the US are foreign-born[20].
In contrast to other first-line drugs (including any resistance and monoresistance to INH or rifampin as well as MDR)[20], the proportion of cases with reported resistance to PZA increased in the U.S. from 2.0% to 3.3%, 65% relative increase, largely due to an increase in PZA-monoresistance. Since PZA-monoresistance is characteristic of M. bovis, we assessed proportions of M. bovis in a sub-set of MTBC cases with genotyping results available for 2004-2009. M. bovis did not significantly increase, and the proportion of PZA-monoresistance increased irrespective of M. bovis. The prevalence of PZA resistance differed among different lineages of Mtb and the relative prevalence of these lineages among TB isolates was changing over time in our study population. Among MDR TB cases, PZA resistance was significantly higher in the East Asian lineage, while in non-MDR cases PZA resistance was significantly higher in the Indo-Oceanic lineage. Even though the proportion of PZA resistance among isolates from MDR TB cases is much higher than those among non-MDR cases, there were only 1,090 MDR TB cases in this analysis compared with 78,231 non-MDR TB cases. Both the proportion of TB cases with Indo-Oceanic lineage and the proportion of Indo-Oceanic cases with PZA resistance increased over time. Therefore, given that increasing PZA monoresistance in the U.S. does not appear to be related to M. bovis, it may be related to increases in the proportion of Indo-Oceanic strains. An increase in the proportion of Indo-Oceanic strains could be related to an increase in the proportion of cases in the U.S. among foreign-born persons from regions in which the Indo-Oceanic lineage is common.
On the other hand, because MGIT 960 has been shown to have higher rates of false-resistant results compared to the BACTEC 460 system [15, 39], we considered that increasing proportions of PZA monoresistance might reflect the progressive change from BACTEC 460TB to MGIT 960 after FDA clearance in 2002[40]. However, PZA resistance began increasing 3 years earlier, in 1999, and did not accelerate after 2002, suggesting the change in technology may not be the main reason behind the trend in PZA DST results.
Our analysis was subject to several important limitations. The main limitation is restriction of the analysis to 38 public health jurisdictions that routinely test for susceptibility to PZA. For the other 14 states the prevalence and trends may have been due to selection bias. Thus, the study findings may not generalize to the whole country. However, the overall proportion of cases with reported PZA resistance during 1999-2009 was similar in the jurisdictions included in analysis (2.7%) and the 14 states excluded (2.9%); in both subsets, the proportions of resistance to PZA were significantly increasing. Second, the genotyping data was limited to 2004-2009. Genotyping in the U.S. is voluntary and coverage increased substantially from 51.2% to 88.2% during 2004-2010 [25]. Therefore selection bias cannot be excluded. Third, surveillance data have intrinsic limitations, and we could not exclude PZA susceptibility testing or reporting errors. For example, 7% of M. bovis cases were reported as PZA-susceptible, likely representing intrinsic variability of the PZA test itself and reporting errors[18]. PncA gene sequencing for PZA resistant strains is not routinely performed and surveillance data does not include this information, therefore we were not able to determine the correlation between phenotypic drug resistance result and PncA genotype.
PZA resistance in terms of absolute numbers represents a small fraction of U.S. TB cases, but the increasing trend in PZA resistance makes it an important public health problem because PZA is an essential part of treatment [1-3, 8-10]. Thus, DST for PZA is critical. The trend in PZA resistance calls attention to the limitations of growth-based DST for PZA, although meticulous attention to the size of the inoculum seems to improve its reproducibility[12]. Nonetheless, the development of faster, more reliable laboratory methods to detect PZA resistance is a priority[41, 42]. The phylogenetic diversity of Mtb may have important clinical consequences and implications for development of molecular assays for PZA resistance.
Key points.
In U.S. jurisdictions routinely testing Mycobacterium tuberculosis complex for pyrazinamide susceptibility, pyrazinamide resistance increased from 2.0% to 3.3% per year during 1999-2009. Changing human and mycobacterial characteristics were associated with this increase in resistance, including phylogenetic lineage of M. tuberculosis.
ACKNOWLEDGEMENTS
The authors thank Robert Pratt and Michael Chen from U.S. Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA for SAS programming expertise and statistical advice. We also would like to thank Michael F. Iademarco, Angela M. Starks and James E. Posey from the CDC for critical revision of the manuscript.
Financial support. None.
Appendix
Figure 1A. Time trends in proportion of MTBC species and M. tuberculosis lineages (N=28,080)

Note. Percents are shown using total number of MTBC as a denominator.
Among 27,428 M. tuberculosis cases the phylogenetic lineages were assigned as following: Euro-American 17,208 (62.7%), Indo-Oceanic - 5,019 (18.3%), East Asian - 3,839 (14.0%), East African Indian - 1,362 (5.0%). From 2004 to 2009, no significant changes in proportions of M. bovis (APC=2.1, CL: −4.6, 9.2; P=.45). The proportion of Indo-Oceanic lineage significantly increased (APC=4.7, CL: 1.6, 7.9; P=.01), and the proportion of Euro-American lineage significantly decreased (APC=−2.2, CL: −3.1, −1.3; P=.003). Proportions of East Asian (APC=2.1, CL: −0.6, 4.8; P=.10) and East African Indian (APC=5.6, CL: −0.6, 12.2; P=.07) lineages did not change significantly. No significant changes in M. africanum proportions (APC=−5.7, CL: −17.0, 7.1; P=.27).
Footnotes
Conflict of Interest. All authors declare no conflict of interest.
Institutional Review Board (IRB) approval. Data for the U.S. National TB Surveillance System (NTSS) and National TB Genotyping Service (NTGS) are collected as part of routine public health practice, and therefore this project was determined not to be human subjects research requiring institutional review board approval.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent official views of the U.S. Centers for Disease Control and Prevention.
Author Contributions:
Ekaterina V. Kurbatova had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ekaterina V. Kurbatova contributed to conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and administrative, technical, or material support, and supervision.
Joseph S. Cavanaugh contributed to analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support, and supervision.
Tracy Dalton contributed to conception and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support, and supervision.
Eleanor Click contributed to analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
J. Peter Cegielski contributed to conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, administrative, technical, or material support, and supervision.
References
- 1.Francis J. Curry National Tuberculosis Center and California Department of Public Health. Drug-Resistant Tuberculosis: A Survival Guide for Clinicians. (2nd) 2008 [Google Scholar]
- 2.World Health Organization . Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: 2011. 2011 update. [DOI] [PubMed] [Google Scholar]
- 3.The U.S. Centers for Disease Control and Prevention Treatment of tuberculosis. Morbidity and mortality weekly report Recommendations and reports. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 4.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. The international journal of tuberculosis and lung disease. 2003;7(1):6–21. [PubMed] [Google Scholar]
- 5.Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. The international journal of tuberculosis and lung disease. 2000;4(9):796–806. [PubMed] [Google Scholar]
- 6.British Thoracic Association A controlled trial of six months chemotherapy in pulmonary tuberculosis. Second report: results during the 24 months after the end of chemotherapy. The American review of respiratory disease. 1982;126(3):460–2. doi: 10.1164/arrd.1982.126.3.460. [DOI] [PubMed] [Google Scholar]
- 7.Singapore Tuberculosis Service/British Medical Research Council Clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis. The results up to 30 months. Tubercle. 1981;62:95–102. doi: 10.1016/0041-3879(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 8.Tasneen R, Li S-Y, Peloquin C, et al. Sterilizing Activity of Novel TMC207- and PA-824-Containing Regimens in a Murine Model of Tuberculosis. Antimicrobial agents and chemotherapy. 2011;55(12):5485–92. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams K, Minkowski A, Amoabeng O, et al. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother. 2012;56(6):3114–20. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diacon AH, Dawson R, von Groote-Bidlingmaier F, et al. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet. 2012 doi: 10.1016/S0140-6736(12)61080-0. [DOI] [PubMed] [Google Scholar]
- 11.McDermott W, Tompsett R. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. The American review of tuberculosis. 1954;70(4):748–54. doi: 10.1164/art.1954.70.4.748. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Permar S, Sun Z. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. Journal of medical microbiology. 2002;51(1):42–9. doi: 10.1099/0022-1317-51-1-42. [DOI] [PubMed] [Google Scholar]
- 13.NCCLS Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic Actimomycets; Approved standard. 2003 NCCLS document M24-A [ISBN 1-56238-500-3]. NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA. [Google Scholar]
- 14.NCCLS Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic Actimomycets; Approved standard. 2011 NCCLS document M24-A2 [ISBN 1-56238-746-4]. NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA. [PubMed] [Google Scholar]
- 15.Chedore P, Bertucci L, Wolfe J, Sharma M, Jamieson F. Potential for erroneous results indicating resistance when using the Bactec MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. Journal of clinical microbiology. 2010;48(1):300–1. doi: 10.1128/JCM.01775-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konno K, Feldmann FM, McDermott W. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis. 1967;95(3):461–9. doi: 10.1164/arrd.1967.95.3.461. [DOI] [PubMed] [Google Scholar]
- 17.Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nature medicine. 1996;2(6):662–7. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 18.Hlavsa MC, Moonan PK, Cowan LS, et al. Human tuberculosis due to Mycobacterium bovis in the United States, 1995-2005. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47(2):168–75. doi: 10.1086/589240. [DOI] [PubMed] [Google Scholar]
- 19.LoBue PA, Enarson DA, Thoen CO. Tuberculosis in humans and animals: an overview. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14(9):1075–8. [PubMed] [Google Scholar]
- 20.CDC . Reported Tuberculosis in the United States, 2010. U.S. Department of Health and Human Services; Atlanta, GA: Oct, 2011. CDC. [Google Scholar]
- 21.The U.S. Centers for Disease Control and Prevention Report of Verified Case of Tuberculosis (RVCT) instruction manual. Available at: Available at http://ftp.cdc.gov/pub/software/tims/2009%20rvct%20documentation/rvct%20training%20materials/rvct%20instruction%20manual.pdf. Accessed July 13.
- 22.The U.S. Centers for Disease Control and Prevention Tuberculosis Laboratory Aggregate Report. Available at: http://www.cdc.gov/tb/publications/reportsarticles/2009aggregatereport.pdf.
- 23.National TB Controllers Association / CDC Advisory Group on Tuberculosis Genotyping . Guide to the Application of Genotyping to Tuberculosis Prevention and Control. US Department of Health and Human Services; Atlanta, GA: 2004. CDC. [Google Scholar]
- 24.Click ES, Moonan PK, Winston CA, Cowan LS, Oeltmann JE. Relationship between Mycobacterium tuberculosis phylogenetic lineage and clinical site of tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(2):211–9. doi: 10.1093/cid/cir788. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease C, Prevention Tuberculosis genotyping - United States, 2004-2010. MMWR Morbidity and mortality weekly report. 2012;61:723–5. [PubMed] [Google Scholar]
- 26.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine. 2000;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Lumb R, Bastion I, Carter R, Jelfs P, Keehner T, Sievers A. Tuberculosis in Australia: bacteriologically confirmed cases and drug resistance, 2008 and 2009. A report of the Australian Mycobacterium Reference Laboratory Network. Communicable diseases intelligence. 2011;35(2):154–61. doi: 10.33321/cdi.2011.35.11. [DOI] [PubMed] [Google Scholar]
- 28.Bai GH, Park YK, Choi YW, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2007;11(5):571–6. [PubMed] [Google Scholar]
- 29.Jonmalung J, Prammananan T, Leechawengwongs M, Chaiprasert A. Surveillance of pyrazinamide susceptibility among multidrug-resistant Mycobacterium tuberculosis isolates from Siriraj Hospital, Thailand. BMC microbiology. 2010;10:223. doi: 10.1186/1471-2180-10-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierre-Audigier C, Surcouf C, Cadet-Daniel V, et al. Fluoroquinolone and pyrazinamide resistance in multidrug-resistant tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16(2):221–3. doi: 10.5588/ijtld.11.0266. i-ii. [DOI] [PubMed] [Google Scholar]
- 31.Mphahlele M, Syre H, Valvatne H, et al. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. Journal of clinical microbiology. 2008;46(10):3459–64. doi: 10.1128/JCM.00973-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HJ, Kwak HK, Lee J, et al. Patterns of pncA mutations in drug-resistant Mycobacterium tuberculosis isolated from patients in South Korea. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16(1):98–103. doi: 10.5588/ijtld.10.0739. [DOI] [PubMed] [Google Scholar]
- 33.Chiu YC, Huang SF, Yu KW, Lee YC, Feng JY, Su WJ. Characteristics of pncA mutations in multidrug-resistant tuberculosis in Taiwan. BMC infectious diseases. 2011;11:240. doi: 10.1186/1471-2334-11-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan RC, Hui M, Chan EW, et al. Genetic and phenotypic characterization of drug-resistant Mycobacterium tuberculosis isolates in Hong Kong. The Journal of antimicrobial chemotherapy. 2007;59(5):866–73. doi: 10.1093/jac/dkm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jong BC, Onipede A, Pym AS, et al. Does resistance to pyrazinamide accurately indicate the presence of Mycobacterium bovis? Journal of clinical microbiology. 2005;43(7):3530–2. doi: 10.1128/JCM.43.7.3530-3532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannan MM, Desmond EP, Morlock GP, Mazurek GH, Crawford JT. Pyrazinamide-monoresistant Mycobacterium tuberculosis in the United States. Journal of clinical microbiology. 2001;39(2):647–50. doi: 10.1128/JCM.39.2.647-650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pareek M, Evans J, Innes J, et al. Ethnicity and mycobacterial lineage as determinants of tuberculosis disease phenotype. Thorax. 2012 doi: 10.1136/thoraxjnl-2012-201824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagneux S, Small PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. The Lancet infectious diseases. 2007;7(5):328–37. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- 39.Simons SO, van Ingen J, van der Laan T, et al. Validation of pncA gene sequencing in combination with the mycobacterial growth indicator tube method to test susceptibility of Mycobacterium tuberculosis to pyrazinamide. Journal of clinical microbiology. 2012;50(2):428–34. doi: 10.1128/JCM.05435-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angra PK, Taylor TH, Iademarco MF, Metchock B, Astles JR, Ridderhof JC. Performance of tuberculosis drug susceptibility testing in U.S. laboratories from 1994 to 2008. Journal of clinical microbiology. 2012;50(4):1233–9. doi: 10.1128/JCM.06479-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell PJ, Morlock GP, Sikes RD, et al. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2011;55(5):2032–41. doi: 10.1128/AAC.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin A, Takiff H, Vandamme P, Swings J, Palomino JC, Portaels F. A new rapid and simple colorimetric method to detect pyrazinamide resistance in Mycobacterium tuberculosis using nicotinamide. The Journal of antimicrobial chemotherapy. 2006;58(2):327–31. doi: 10.1093/jac/dkl231. [DOI] [PubMed] [Google Scholar]