Abstract
In response to DNA damage, the tumor suppressor p53 elicits a complex cellular response. In this issue of Cell, Wu et al. (2005) show that the transcription factor SLUG is induced by p53 and protects hematopoietic progenitor cells from apoptosis triggered by DNA damage. SLUG exerts this protective role by repressing Puma, a proapoptotic target of p53. PUMA is also a key coordinator of apoptosis mediated by both nuclear and cytoplasmic functions of p53 (Chipuk et al., 2005).
The tumor suppressor p53 is frequently mutated in cancer cells, and its roles and regulation have been the topic of much research and debate. p53 is a transcription factor that is activated by cellular stress, leading to increases in p53 mRNA translation (Takagi et al., 2005), protein stability, and sequence-specific DNA binding (reviewed in Yee and Vousden, 2005). Once activated, p53 induces or represses various target genes, leading to a myriad of cellular outcomes, including apoptosis, growth arrest, cellular senescence, and DNA repair. Thus, p53 integrates cellular stress responses, and loss of p53 function leads to the aberrant proliferation of damaged cells. p53 can be remarkably effective at eliminating incipient cancer cells; however, inappropriate or sustained p53 activation can damage normal tissues and, in fact, may contribute to the aging of tissues and organisms (Febeyre and Lowe, 2002). The p53 network has therefore evolved a complex control system that keeps p53 expressed and available when needed but builds in safeguards that prevent “surges” in its network from wreaking havoc on normal cells.
One important p53 effector is PUMA (p53-upregulated modulator of apoptosis) (Yee and Vousden, 2005). PUMA is a BH3-only member of the Bcl-2 family that is a potent inducer of apoptosis mediated by p53. It initiates the cell-death cascade by modulating Bax activity to facilitate cytochrome c release from the mitochondria. Its significance is underscored by the observation that thymocytes from PUMA-deficient and p53-deficient mice show comparable defects in apoptosis induced by radiation. Additionally, suppression of PUMA, like loss of p53, cooperates with Myc to promote the formation of lymphomas. Two new reports highlight how PUMA acts to fine tune and coordinate the p53 apoptotic network. In this issue of Cell, Wu, Look, and coworkers (Wu et al., 2005) describe a new p53 target gene, Slug, which acts as a transcriptional repressor of Puma expression to antagonize apoptosis mediated by p53. In a recent issue of Science, Chipuk et al. (2005) identify a critical role for PUMA in mediating apoptosis through both nuclear (transcription-dependent) and cytoplasmic (transcription-independent) functions of p53.
Look and colleagues (Inoue et al., 2002) originally identified SLUG, a zinc-finger transcription factor, as a downstream target of the oncogenic transcription factor E2A-HLF in human pro-B acute cell leukemia. They showed that SLUG-deficient mice are radiosensitive because their hematopoietic compartment fails to regenerate following sublethal doses of radiation. Wu et al. (2005) extend these findings by showing that SLUG acts by repressing the transcriptional activation of Puma by p53 and that loss of either p53 or PUMA rescues the radiosensitivity phenotype of SLUG-deficient mice. Thus, the key factor that SLUG targets to protect hematopoietic progenitors from DNA-damage-induced programmed cell death is PUMA.
Dissection of apoptotic pathways that are conserved in evolution provided important clues to elucidating the action of SLUG. The Caenorhabditis elegans protein CES-1 (SLUG homolog) acts in specific cell types to inhibit EGL-1, a BH3-only proapoptotic protein (PUMA homolog). ces-1 is transcriptionally repressed by CES-2, a member of the bZIP family of transcription factors, ultimately leading to cell death. E2A-HLF may promote cancer by preventing the action of the putative ces-2 homolog, leading to more SLUG and aberrant pro-B cell survival. There is likely to be a CES-2-like protein, possibly a tumor suppressor, that inhibits Slug in hematopoietic progenitor cells to allow for apoptosis of defective pro-B cells. The definitive mammalian ces-2 homolog remains uncertain, although TEF and/or E4BP4 are candidates (Ikushima et al., 1997; Inukai et al., 2005).
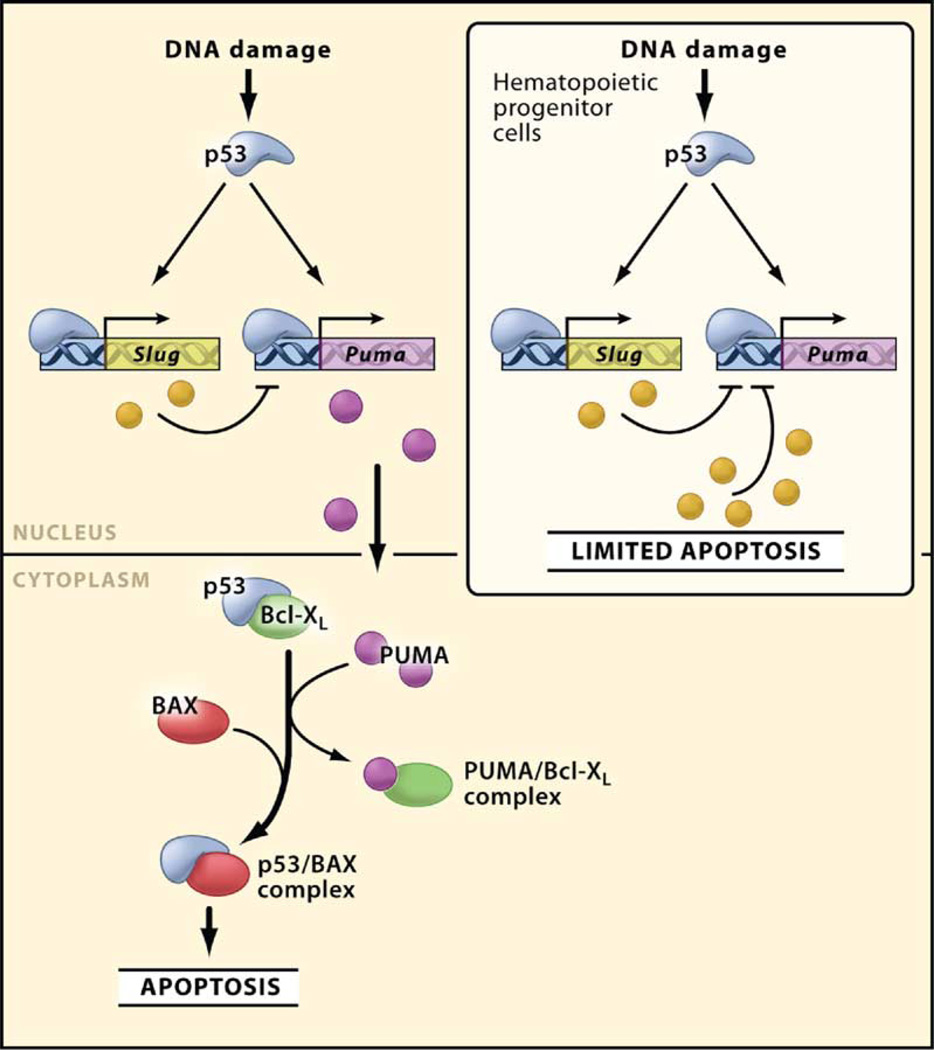
Surprisingly, Wu et al. (2005) also show that Slug is a p53 target gene. Thus, following DNA damage of hematopoietic progenitor cells, p53 not only upregulates Puma to mediate apoptosis but simultaneously upregulates Slug. SLUG represses Puma, thereby preventing apoptosis (Figure 1). At first glance, these data are paradoxical: why would p53, a protein known for its potent apoptotic activity, also promote cell survival? The authors propose that hematopoietic progenitor cells, owing to their regenerative value to the organism, must be relatively protected from culling through p53-mediated apoptosis. The decision to arrest and repair or undergo apoptosis is controlled by SLUG levels, and, consistent with this view, SLUG is expressed at lower levels in mature hematopoietic cells that undergo apoptosis following DNA damage. In addition to high endogenous amounts of the SLUG protein in hematopoietic progenitor cells, transactivation of Slug by p53 may also contribute to the suppression of Puma transcription (Figure 1).
Figure 1.
Role of PUMA and SLUG in Modulating Apoptosis Mediated by p53 Once activated by DNA damage, p53 induces various target genes, including Puma (which encodes a proapoptotic BH3-only protein) and Slug (which encodes a transcription factor that represses Puma transcription). In most cells, the amount of SLUG may not be sufficient to repress Puma and prevent apoptosis. Importantly, in hematopoietic progenitor cells, the endogenous amount of SLUG protein is sufficient to repress Puma and limit apoptosis induced by DNA damage. Also shown is the role of PUMA and p53 in coordinating cell death in the cytoplasm. Here, PUMA binds Bcl-xL and displaces p53, thereby allowing p53 to directly activate Bax and induce permeabilization of mitochondria and cell death.
The duality of p53 pro- and antiapoptotic signaling on the SLUG-PUMA connection may influence cell fate in the hematopoietic system. However, p53 can simultaneously promote cell death and survival through other mechanisms as well. In fact, the best characterized example of p53 “survival” signaling involves the ability of p53 to induce Mdm2, a p53 ubiquitin ligase that acts in a negative-feedback loop to terminate a p53 response (Yee and Vousden, 2005). Moreover, p53 activates “general” survival pathways, perhaps to alleviate the adverse effects of DNA damage (Fang et al., 2001). Thus, by integrating cell-type- or context-dependent layers of regulation into the canonical death pathway, the system takes into account different cellular needs and coordinates damage responses in the organism as a whole. Such dual signaling appears to be a common theme in cell-death regulation. For example, the apoptotic activity of tumor necrosis factor (TNF) is balanced by the ability of TNF receptor signaling to simultaneously activate NF-κB, which activates targets important for cell survival (Aggarwal, 2003).
Whereas Wu et al. (2005) emphasize the intricacy with which p53 regulates cell-survival and cell-death decisions through regulating Puma expression, Chipuk et al. (2005) highlight how PUMA coordinates the death process once it is induced. Specifically, the authors report that PUMA provides a critical link that couples p53 transcription-dependent and transcription-independent functions (Chipuk et al., 2005). In transcription-independent apoptosis, p53 in the cytoplasm directly binds to proapoptotic Bcl-2-family proteins, leading to permeabilization of mitochondria and apoptosis (Moll et al., 2005). Chipuk et al. show that, after genotoxic stress, the antiapoptotic protein Bcl-xL sequesters cytoplasmic p53, whereas nuclear p53 induces transcription of Puma. PUMA then binds to Bcl-xL and displaces p53, thereby allowing p53 to directly activate Bax and induce mitochondrial permeabilization and cell death. Importantly, the authors showed that a mutant Bcl-xL impaired at binding PUMA but capable of binding p53 rendered cells resistant to apoptosis mediated by p53.
These findings go a long way toward resolving a controversy in the p53 field. The transcription-dependent apoptotic functions of p53 are well established in part because p53 directly regulates so many genes linked to the apoptotic process. Thus, p53 transcriptionally induces proapoptotic members of the Bcl-2 family, components of death receptor signaling pathways, cell-death effectors, and negative regulators of cell-survival pathways (Yee and Vousden, 2005). Indeed, genetic studies demonstrating the importance of PUMA for p53-mediated cell death reinforce this view (Yee and Vousden, 2005). However, although evidence for transcription- independent functions of p53 goes back over a decade, it is only recently that a plausible mechanism (direct targeting of mitochondria) has emerged (Moll et al., 2005). However, genetic evidence was lacking. By linking PUMA to the transcription-independent functions of p53, Chipuk et al. (2005) associate the phenomenon with established genetics and reveal how the transcription-dependent and -independent functions of p53 act in concert to control apoptosis (Figure 1). Thus, the role of Bcl-xL in preventing initiation of apoptosis at the mitochondria is analogous to SLUG—each controls PUMA to modulate the p53 response. It will be interesting to determine whether the transcription-independent function of p53 operates in hematopoietic progenitor cells.
Despite the clear importance of PUMA in the p53-mediated apoptotic program, p53 also targets other apoptotic regulators, including NOXA and BAX (Yee and Vousden, 2005). Although none have been as strongly linked to apoptosis as PUMA, it seems unlikely that the system would have evolved to target so many irrelevant genes. Perhaps p53 targets multiple steps in the apoptotic cascade to ensure that cell death proceeds efficiently. And given that each of these effectors is also embedded in other signaling networks, the decisive mediators of p53 action may be influenced by other cell type and microenvironment factors that impact these components. For example, factors influencing the levels of SLUG, Bcl-xL, or PUMA may alter the importance of other p53 effectors in a given cell-death program or may dictate the balance between apoptosis mediated by p53 and its other effector functions.
Finally, these results suggest ways to modulate the p53 pathway for therapeutic purposes. For example, based on insights from Chipuk et al. (2005) and others, one would predict that, in tumors with wild-type p53, the overexpression of a peptide consisting of the domain in PUMA that mediates interaction with Bcl-xL, the BH3 domain, would sequester Bcl-xL, thereby releasing p53 to induce cell death. The data of Wu et al. (2005) provide another example of a potential therapeutic application. Their data suggest that increasing the amount of SLUG to inhibit apoptosis may be a good strategy to circumvent the death of hematopoietic stem cells and progenitors induced by chemotherapy. Of note, the induction of apoptosis by p53 in normal tissues is responsible for some side effects of DNA-damaging chemotherapeutic agents, and p53 inhibitors have been developed to thwart these effects (Komarov et al., 1999). Although such drugs should have no effect on tumors bearing a mutant form of p53 and should reduce the dose-limiting toxicities of chemotherapy, there are concerns that these such therapies would be mutagenic because they would also disable p53 checkpoint functions. Alternatively, activation of the SLUG pathway would prevent apoptosis without compromising repair and, in principle, provide a less mutagenic strategy to achieve similar ends. A limitation to such therapy is that the SLUG pathway may also be activated in tumor cells and consequently may confer resistance to chemotherapeutic agents. Further insights into the complexities of p53 regulation will eventually provide a foothold toward exploiting this potent tumor-suppressor network for treating cancer.
Selected Reading
- Aggarwal BB. Nat. Rev. Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- Fang L, Li G, Liu G, Lee SW, Aaronson SA. EMBO J. 2001;20:1931–1939. doi: 10.1093/emboj/20.8.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febeyre G, Lowe SW. Nature. 2002;415:26–27. doi: 10.1038/415026a. [DOI] [PubMed] [Google Scholar]
- Ikushima S, Inukai T, Inaba T, Nimer SD, Cleveland JL, Look AT. Proc. Natl. Acad. Sci. USA. 1997;94:2609–2614. doi: 10.1073/pnas.94.6.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Seidel MG, Wu W, Kamizono S, Ferrando AA, Bronson RT, Iwasaki H, Akashi K, Morimoto A, Hitzler JK, et al. Cancer Cell. 2002;2:279–288. doi: 10.1016/s1535-6108(02)00155-1. [DOI] [PubMed] [Google Scholar]
- Inukai T, Inaba T, Dang J, Kuribara R, Ozawa K, Miyajima A, Wu W, Look AT, Arinobu Y, Iwasaki H, et al. Blood. 2005;105:4437–4444. doi: 10.1182/blood-2004-08-2976. [DOI] [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. Science. 1999;285:1651–1653. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Moll UM, Wolff S, Speidel D, Deppert W. Curr. Opin. Cell Biol. 2005;17:1–6. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Takagi M, Absalon MJ, McLure KG, Kastan MB. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Yee KS, Vousden KH. Carcinogenesis. 2005;26:1317–1322. doi: 10.1093/carcin/bgi122. [DOI] [PubMed] [Google Scholar]
- Wu W-S, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. this issue. [DOI] [PubMed] [Google Scholar]