Abstract
Background
Our aim was to study the effect of caffeic acid phenethyl ester (CAPE) on iNOS and cystathionine gamma-lyase (CSE) of hepatic fibrosis rat, and discuss the anti-hepatic fibrosis mechanism of caffeic acid phenethyl ester.
Material/Methods
We observed changes of NO and H2S in serum of hepatic fibrosis rats. Enzyme-linked immunosorbent assay was used to test OD value of iNOS and CSE in serum of each. The expressions of iNOS and CSE protein in the liver were also detected by immunohistochemistry.
Results
Compared with the model group, the expression of NO and iNOS was decreased obviously and the level of H2S and CSE was increased in the CAPE group.
Conclusions
CAPE has the effect of anti-hepatic fibrosis, which can be realized through adjusting the expression level of iNOS and CSE.
MeSH Keywords: Caffeic Acids, Liver Cirrhosis, Nitric Oxide Synthase
Background
Liver fibrosis and its end-stage manifestation of cirrhosis represent clinical challenges worldwide. Hepatic stellate cell (HSC) activation is the cardinal feature that results in hepatic fibrosis [1]. When stimulated by reactive oxygen species or cytokines in response to various hepatic insults, quiescent HSCs are transformed to myofibroblasts (MF-HSCs) that proliferate and secrete collagen [2–4]. Hepatic fibrosis (HF) is a compensatory response of the liver to repair injuries caused by a variety of factors. These injury-induced factors may cause the proliferation and activation of hepatic stellate cells (HSC), resulting in the excessive deposition of the extracellular matrix (ECM) in the liver, which is the main cause of liver fibrosis [5]. Recent studies have proved that hepatic fibrosis is a reversible disease; therefore, studying the stages and mechanism of its reversion is of great significance in treating liver fibrosis. Caffeic acid phenethyl ester (CAPE) is a kind of flavonoid extracted from propolis [6]. It has been reported that CAPE can inhibit the hepatotoxicity caused by carbon tetrachloride (CC14) [7]. However, there is limited research on its exact molecular target. NO (nitric oxide) and hydrogen sulfide (H2S) are 2 gaseous signaling molecules that play an important role in the formation liver fibrosis [8]. Induced nitric oxide synthase (iNOS) and cystathionine gamma-lyase (CSE) are synthetic enzymes of NO and H2S [9]. The present study may further clarify the possible anti-fibrosis mechanisms of CAPE by elucidating its effects on the iNOS and CSE expression of rats with liver fibrosis.
Material and Methods
Reagents
The reagents used were: Colchicine (Xishuangbanna Pharmaceutical Co., Ltd.); CC14 (Tianjin Bodie Chemical Co.); SP immunohistochemical kit (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.); ELISA kit (Santa Cruz); NO kit (Nanjing Jiancheng Bioengineering Institute); and iNOS, CSE polyclonal antibody (Wuhan Boster Biological Engineering Co., Ltd.).
Establishment of animal model and grouping
We randomly assigned 60 male SD rats of clean grade (provided by the Green Leaf Pharmaceutical Co., Ltd. in Yantai, Shandong), weighing (180±30 g) into 4 groups: the normal control group, the liver fibrosis model group, the CAPE group, and the colchicine group. Each group included 15 rats. Rats in the normal control group had free access to food and water and were given 3 ml/kg of soybean oil by gavage once every 2 days. CC14 compound modeling method was used on the other 3 groups to create rat models with hepatic fibrosis [10]; and 3 ml/kg of 30% oil solution made by mixing CC14 with soybean oil in a proportion of 3:7 were administered by gavage once every other 2 days. From the second week of modeling, water with 10% ethanol was given to the rats as the only source of drinking water, and lard was added to the food in the proportion of 2:8. From the third week, the 3 model groups were given 1 ml/kg of saline, C10 mg/kg of APE, and 0. 1 mg/kg of colchicine sequentially; intraperitoneal injections were given 5 times a week for 8 consecutive weeks.
Sampling methods
In the 10th week, all rats were anesthetized by 45 mg/kg of 2% pentobarbital sodium through intraperitoneal injection. Serum samples were centrifuged after 30 minutes and were preserved at 20°C until use. The right lobe tissues of the rats were taken after being fixed with 40% paraformaldehyde perfusion. The tissue samples were fixed in 4% paraformaldehyde and then were preserved after being routinely embedded in paraffin and sliced into sections5-μm thick.
Determination of contents of NO and H2S in serum
According to instructions of the NO kit, nitrate reductase method was used to measure the contents of nitrate (N02−) and nitrite (N03−) in serum. Sensitive sulfur electrode assay was used to measure the content of sulfide in serum and to calculate the concentration of H2S.
Enzyme-linked immunosorbent assay (ELISA)
The double-antibody sandwich assay was performed as follows. (1) Coating: the antibodies were diluted with 0.05 mol/L of PH 9. 6 carbonate coating buffer solution to decrease its protein content to 1~10 μg/ml and 0.1 ml of the solution was added into each polystyrene plate, staying overnight at 4°C. The solution was discarded the next day. The reaction hole was washed by buffer solution 3 times for 3 min each time. (2) Sample adding: 0. 1 ml of serum was added into the coated reaction holes. The reaction holes were incubated for 1 h in 37°C and then washed. (3) Adding HRP: 0.1 ml of freshly diluted HRP was added into each reaction hole. The solution was incubated in 37°C for 0.5~1 h and then washed. (4) Adding a substrate VIS color: TMB substrate is was added to each well with extemporaneous 0.1 ml, 37°C 10~30 min (5). Terminating the reaction: 0.05 ml of 2 mol/L sulfuric acid was added to each reaction hole. (6) Results: the OD value was measured by the ELISA detector at 450 nm after the blank control hole was set to zero. It was positive if the OD value was 2.1 times the negative value.
Immunohistochemistry SP method and image analysis
The immunohistochemical SP kit was used. After paraffin sectioning and dewaxing, the samples were processed by the following steps. (1) Incubated for 30 min in 3% hydrogen peroxide and methanol at room temperature and repaired by microwave into citrate buffer solution for 10 min. (2) Incubated at 37°C with 10% goat serum blocking solution for 30 min. (3) Adding rabbit anti-iNOS, CSE polyclonal antibody, and kept overnight at 4°C. (4) Adding the secondary antibody dropwise and incubating for 30 min in 37°C. (5) Adding DAB color liquid dropwise and developing for 10 min at room temperature (after observing the coloration under a microscopic). (6) Dehydrating by graded ethanol, making transparent by xylene, mounting with the neutral gum, and examining under a microscopic. PBS replaced the primary antibody in the negative control test. Other tests followed similar steps as those described above.
Protein expression in each group was observed under a microscope and the IMAGE-PROPLUS image analysis system was used to analyze the test results.
Statistical analysis
The Statistical Package for Social Sciences software (SPSS, Inc., Chicago, IL, USA), version 16.0 for Windows was used for statistical analysis. The clinical data are presented as mean ±SD and compared between groups by the Student’s t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Morphological observation
Livers of rats in the normal group were reddish brown and soft with smooth surface and were normal size. Livers of rats in the model groups were significantly shrunken and hardened; they were dark red with greasy and grainy surface and slightly blunt edges. Liver surfaces of rats in the CAPE group were dark red, and were less rough, grainy, and greasy than that of the model group.
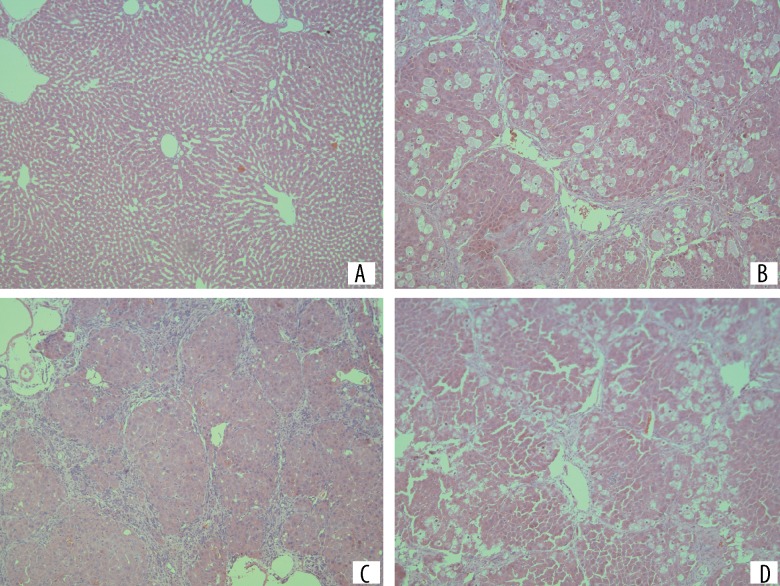
Lobular structure of rats in the normal group could be observed clearly under a light microscope. The liver cells were morphologically intact, arranged radially along the central vein, and interwoven like a net; liver cells of the model group were edematous with significant fatty degeneration, and most of the normal lobular architecture was destroyed or had disappeared. Liver lobules was segmented and surrounded by the collagen fibers produced by the liver tissue hyperplasia, forming the pseudolobules; the liver injuries in the CAPE group were significantly improved. The fibrosis hyperplasia, liver cell edema, and fatty degeneration were significantly alleviated. Pseudolobules could be clearly seen (Figure 1).
Figure 1.
Liver tissue stained by hematoxylin and eosin in each group, ×100. (A) Control group; (B–D) Colchicine.
Determination of serum
The NO content in serum of the liver fibrosis group was higher than that of the normal group, and its H2S level was lower than that of the normal group (P<0.05). NO contents in serum of CAPE group and the colchicine group were lower than that of the model group, and its H2S level was higher than that of the model group (P<0.05) (Table 1).
Table 1.
Concentration of NO and H2S in serum of rats in each group (n=15, mean ±SD, μmol/L).
| Group | NO | H2S |
|---|---|---|
| Control | 21.83±8.75 | 314.39±23.22 |
| Model | 76.69±12.46* | 101.64±13.36* |
| CAPE | 42.75±7.59*,** | 245.57±18.95*,** |
| Colchicine | 39.73±2.54*,** | 269.49±15.23*,** |
P<0.05 vs. control;
P<0.05 vs. model.
ELISA determination
The OD values of CSE iNOS in serum of rats in each group are shown in Figure 2. The iNOS level of the liver fibrosis model group was higher than that of the normal group, and CSE was lower than that of the normal group (P<0.05). The iNOS levels of the CAPE group and the colchicine group were lower than that of the model group, and their CSE levels were higher than that of the model group (P<0.05).
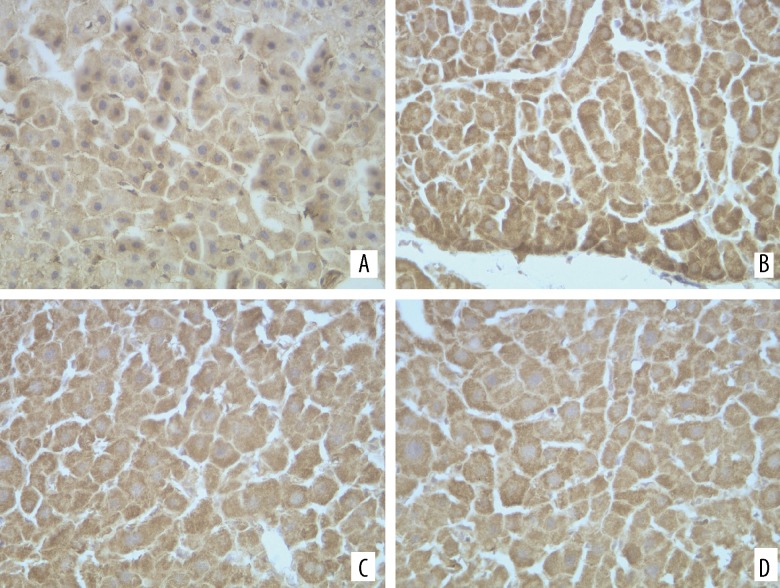
Figure 2.
Expression of iNOS in liver of each group, ×400. (A) Control group; (B) Model group; (C) CAPE; (D) Colchicine
Immunohistochemical tests
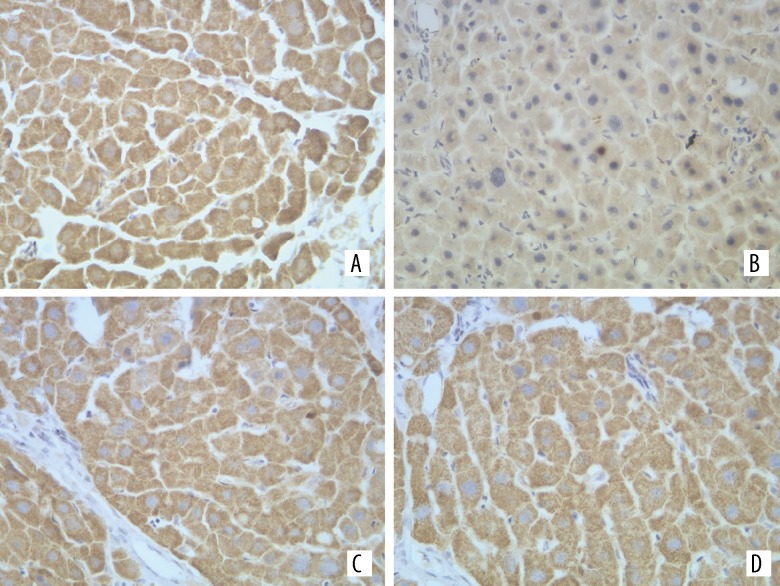
The iNOS and CSE expression could be mainly seen in the liver cells (Figures 2, 3). The iNOS protein expression of the model group increased more than that of the normal group, while its CSE protein expression became less than that of the normal group. The differences between the 2 groups were statistically significant (P<0.05). Compared with the model group, the iNOS protein expression of liver tissues in the CAPE group became less, and its CSE protein expression increased (P<0.05) (Table 2).
Figure 3.
Expression of CSE in liver of each group, ×400. (A) Control group; (B) Model group; (C) CAPE; (D) Colchicine.
Table 2.
OD in the serum of rats and expression of iNOS and CSE in the liver of each group (n=15, mean ±SD).
| Group | Serum (OD) | Liver (A value) | ||
|---|---|---|---|---|
| iNOS | CSE | iNOS | CSE | |
| Control | 29.57±5.39 | 53.64±3.74 | 41.00±1.65 | 68.17±1.86 |
| Model | 38.16±2.24* | 32.76±4.15* | 57.04±3.65* | 44.2±2.80* |
| CAPE | 32.53±2.25** | 42.15±3.53** | 49.68±2.14** | 56.02±3.41** |
| Colchicine | 30.42±3.68** | 46.46±4.42** | 45.63±1.48** | 58.23±2.34** |
P<0.05 vs. control;
P<0.05 vs. model.
Discussion
In Western medicine, liver fibrosis is commonly treated by colchicine and interferon. However, due to the serious adverse effects, they are strictly prescribed, which make traditional Chinese medicine the focus of research. CAPE is a major active component of propolis. It is a popular topic of research outside China on the active components of propolis in recent years. It can combat oxidation, inflammations, and cancers, and can regulate the immune system [11,12]. For instance, it can inhibit the expression of matrix metalloproteinases (MMP-2), playing a key role in preventing the metastasis of liver cancer cells [13]. It can also reduce the consumption of superoxide dismutase enzyme to resist oxidation by scavenging reactive oxidative substances, inhibiting the activity of xanthine oxidase and NOS [14]. It is a classic method to build the liver fibrosis models with CC14, which has been widely used in studying the screening and mechanisms of the anti-hepatic fibrosis drugs. Our study suggests that after getting liver fibrosis induced by CC14 and being given the CAPE treatment, the liver structures and liver injuries of rats in the liver fibrosis group improved more significantly than in the model group, indicating that CAPE has some therapeutic effects on liver fibrosis.
NO is a substance with a variety of bioactivities. Endogenous NO is generated by L-arginine (L-Arg) decomposition substrated by nitric oxide synthase (NOS). NOS includes neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). Under normal circumstances, eNOS is the main type of liver tissue NOS. It is secreted by the liver sinusoidal endothelial cells and acts on HSC through the paracrine to maintain its diastolic state, inhibit proliferation and migration, and to promote apoptosis of HSC [15,16]. iNOS is induced by liver toxins and some cytokines. The cytotoxic effects caused by the over expression of NO can stimulate the inflammatory process and may be involved in the pathological processes of liver fibrosis and other liver diseases [17,18]. According to our study, increases in NO and iNOS levels can be seen in rats with liver fibrosis, which is consistent with the study results of Liuch and other researchers [5,19], indicating that the liver fibrosis is promoted by the inflammation of and damage to liver cells caused by NO. The immunohistochemical tests show that iNOS levels increase degree of damage degree, indicating that the high expression of iNOS in liver cells is associated with the occurrence of liver fibrosis. iNOS is involved in the chronic liver fibrosis damage of rats induced by CC14. After adding CAPE, the expression of iNOS decreases significantly and the liver cell damages are also greatly improved, indicating that CAPE may reduce NO synthesis by inhibiting the expression of iNOS in damaged liver cells to exert an anti-fibrosis function.
Endogenous H2S is the third gaseotransmitter that can cause vasodilation, following NO and CO. It is generated mainly through cystathionine p-synthase and CSE catalytic L-cysteine in the body [20,21]. HSC is a major source of cells generating ECM during liver injuries, playing an important role in mechanisms of occurrence and development of liver fibrosis. Studies have shown that H2S could inhibit the proliferation of HSC, reduce the oxidative damages of oxygen under stress, and can protect liver fibers. H2S can inhibit the synthesis of the type I and III procollagen [22], by which it can suppress the formation of liver fibrosis. This study shows that H2S content of the liver fibrosis group was reduced, which is consistent with the study results mentioned above. After being given CAPE, the production of H2S increased, thereby inhibiting the synthesis of collagen and alleviating the liver fibrosis. The increased expression of CSE could be observed in the liver tissues, suggesting that the CAPE managed to reduce the liver tissue damages caused by CC14 by promoting the synthesis of H2S. However, more attention should be paid to its mechanism, as well as safety and effectiveness in clinical use.
Conclusions
Our study results indicate that the CAPE can alleviate the liver damages by affecting the generation of H2S and NO through interfering with the expressions of iNOS and CSE. CAPE can inhibit the formation of liver fibrosis in rats, which provides new ideas for the treatment of liver fibrosis.
Footnotes
Source of support: The Science and Technology Project for the Universities of Shandong Province (J10LF57) Natural fund of Shandong Province (ZR2011HL063)
References
- 1.Li M, Wang XF, Shi JJ, et al. Caffeic acid phenethyl ester inhibits liver fibrosis in rats. World J Gastroenterol. 2015;21:3893–903. doi: 10.3748/wjg.v21.i13.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torok NJ. Recent advances in the pathogenesis and diagnosis of liver fibrosis. J Gastroenterol. 2008;43:315–21. doi: 10.1007/s00535-008-2181-x. [DOI] [PubMed] [Google Scholar]
- 5.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao WX, Wang L, Yang JL, et al. Caffeic acid phenethyl ester attenuates pro-inflammatory and fibrogenic phenotypes of LPS-stimulated hepatic stellate cells through the inhibition of NF-kappaB signaling. Int J Mol Med. 2014;33:687–94. doi: 10.3892/ijmm.2013.1613. [DOI] [PubMed] [Google Scholar]
- 7.Lee KJ, Choi JH, Hwang YP, et al. Protective effect of caffeic acid phenethyl ester on tert-butyl hydroperoxide-induced oxidative hepatotoxicity and DNA damage. Food Chem Toxicol. 2008;46:2445–50. doi: 10.1016/j.fct.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Hassan MI, Kassim SK, Ali HS, et al. Evaluation of nitric oxide (NO) levels in hepatitis C virus (HCV) infection: relationship to schistosomiasis and liver cirrhosis among Egyptian patients. Dis Markers. 2002;18:137–42. doi: 10.1155/2002/647961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomur A, Kanter M, Gurel A, Erboga M. The efficiency of CAPE on retardation of hepatic fibrosis in biliary obstructed rats. J Mol Histol. 2011;42:451–58. doi: 10.1007/s10735-011-9350-6. [DOI] [PubMed] [Google Scholar]
- 10.Jang JH, Kang KJ, Kim YH, et al. Reevaluation of experimental model of hepatic fibrosis induced by hepatotoxic drugs: an easy, applicable, and reproducible model. Transplant Proc. 2008;40:2700–703. doi: 10.1016/j.transproceed.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 11.Tekin A, Turkyilmaz S, Kucukkartallar T, et al. Effects of caffeic acid phenethyl ester (CAPE) on hepatopulmonary syndrome. Inflammation. 2011;34:614–19. doi: 10.1007/s10753-010-9270-8. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Cha DG, Liu YL, Zhou SF. Differential effects of selective and non-selective nitric oxide synthase inhibitors on the blood perfusion of ischemia-reperfused myocardium in dogs. Med Sci Monit Basic Res. 2013;19:181–86. doi: 10.12659/MSMBR.883964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KW, Kang NJ, Kim JH, et al. Caffeic acid phenethyl ester inhibits invasion and expression of matrix metalloproteinase in SK-Hep1 human hepatocellular carcinoma cells by targeting nuclear factor kappa B. Genes Nutr. 2008;2:319–22. doi: 10.1007/s12263-007-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armutcu F, Akyol S, Ustunsoy S, Turan FF. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review) Exp Ther Med. 2015;9:1582–88. doi: 10.3892/etm.2015.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langer DA, Das A, Semela D, et al. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology. 2008;47:1983–93. doi: 10.1002/hep.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho YJ, Lee AS, Chen WP, et al. Caffeic acid phenethyl amide ameliorates ischemia/reperfusion injury and cardiac dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2014;13:98. doi: 10.1186/1475-2840-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh BJ, Tan BT, Hon WM, et al. Nitric oxide synthase and heme oxygenase expressions in human liver cirrhosis. World J Gastroenterol. 2006;12:588–94. doi: 10.3748/wjg.v12.i4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: Part I. General considerations and redox biology in hepatitis. J Surg Res. 2010;162:95–109. doi: 10.1016/j.jss.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lluch P, Torondel B, Medina P, et al. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J Hepatol. 2004;41:55–59. doi: 10.1016/j.jhep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Geng B, Yang J, Qi Y, et al. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004;313:362–68. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 21.Ince H, Kandemir E, Bagci C, et al. The effect of caffeic acid phenethyl ester on short-term acute myocardial ischemia. Med Sci Monit. 2006;12(5):BR187–93. [PubMed] [Google Scholar]
- 22.Hongfang J, Cong B, Zhao B, et al. Effects of hydrogen sulfide on hypoxic pulmonary vascular structural remodeling. Life Sci. 2006;78:1299–309. doi: 10.1016/j.lfs.2005.07.009. [DOI] [PubMed] [Google Scholar]