Circadian clocks provide the means to coordinate behavior and physiology into rhythms that integrate information from the external environment. The generation of circadian timing in mammals arises from a transcription/translation feedback loop driven by the basic helix-loop-helix PER-ARNT-SIM (bHLH-PAS) transcription factor, CLOCK:BMAL1. This heterodimeric complex binds to the promoters of dedicated clock genes Period (Per) and Cryptochrome (Cry) in the morning to induce their expression. PER:CRY proteins return to the nucleus at night to inhibit CLOCK:BMAL1 activity, closing the feedback loop to generate 24-hour rhythms in transcription [1].
From head to toe, an astonishing 40% of the mouse genome is controlled by this molecular clock, which gives rise to a single peak of mRNA expression once per day. The identity of clock-controlled genes varies widely by tissue, with transcriptional regulation of the core clock genes conserved across tissues [2]. While some mRNA cycling occurs due to circadian transcriptional regulation by CLOCK:BMAL1 or its downstream targets, there is growing appreciation for the role of post-transcriptional processes in sculpting circadian expression profiles of mRNA [3]. Remarkably, the molecular oscillator also regulates ribosome biogenesis through rhythmic transcription of translation initiation factors and ribosomal protein mRNA, demonstrating that the clock exploits many pathways to elicit temporal changes in mRNA and/or protein expression. [4,5]
At the cellular level, BMAL1 pairs up with CLOCK in the cytosol to translocate the active, heterodimeric transcription factor to the nucleus [6]. Perhaps due to the transcription-centric focus of the clock, no one has looked for activity within the cytosolic fraction of BMAL1. In a recent study, Sahin and colleagues now demonstrate that cytosolic BMAL1 acts as a translation factor to enhance protein synthesis during the metabolically active nighttime period in mice [7]. This unexpected finding demonstrates that core clock proteins can moonlight in other pathways to confer circadian regulation to processes outside of the core timekeeping mechanism.
BMAL1 is primarily found in large complexes of clock proteins within the nucleus [1], but little was known about its status in the cytosol. Using mass spectrometry of cytosolic BMAL1 complexes from mouse embryonic fibroblasts (MEFs), the authors found a surprising enrichment of translation factors, including eukaryotic translation initiation and elongation factors (eIF and eEFs) and polyadenylate binding protein 1 (PABP1), which are generally regarded as components of the cap-binding complex (CBC). Complexes of BMAL1 with these factors bound to immobilized 7-methyl guanosine (m7-GTP), which mimics the “cap” at the 5’ end of most mRNAs. Correspondingly, Bmal1−/− MEFs had a striking decrease in steady-state levels of de novo protein synthesis, and the addition of BMAL1 stimulated cap-dependent translation. Given that cap-binding complexes containing BMAL1 were detected in cytosolic fractions of mouse liver and brain, there is some evidence that translational regulation by BMAL1 may occur widely across tissues.
Clock-regulated processes, by definition, have peak activity at a specific time of day. Sahin and colleagues found that de novo protein synthesis was increased in the liver in a Bmal1-dependent manner at the end of the dark cycle (Zeitgeber time 0, ZT0) when cytosolic BMAL1 was also found to preferentially associate with cap-binding complexes. What factors determine the temporal basis of translation regulation by BMAL1? Translation regulation integrates responses from many signaling pathways, including the mechanistic Target of Rapamycin (mTOR) pathway. The authors identified a conserved phosphorylation motif within the BMAL1 N terminus that is targeted by the mTOR-dependent kinase, S6K1. Phosphorylation of Serine 42 (S42) was required for association with translation factors and stimulation of cap-dependent translation. Activation of the mTOR pathway oscillates on a circadian basis, as demonstrated by cycling levels of activated S6K1 that peak during the evening in mouse liver [8]. Here, Sahin and colleagues show that phosphorylation of BMAL1 S42 also occurs on a circadian basis in liver and MEFs, and can be upregulated by deletion of the mTOR suppressor Tsc1, thereby linking translation regulation by BMAL1 to mTOR.
Sahin and colleagues investigated the degree of overlap between the two activities of BMAL1, asking whether its nuclear and cytosolic roles were independent, and whether the two roles represented distinct pools of BMAL1 protein. They showed that the ability of BMAL1 to regulate translation is independent of its transcriptional role, as a transcriptionally dead mutant (lacking the bHLH DNA binding domain) could still enhance cap-dependent translation. Moreover, while phosphorylation at S42 is necessary to prime BMAL1 for association with the translational machinery, the S42G mutant does not affect the transcriptional activity of BMAL1 or timing of the circadian clock itself, suggesting that the transcriptional and translational roles of BMAL1 are completely separable.
Interesting links between the molecular clock and translation regulation remain unexplored. Bmal1-dependent stimulation of protein synthesis appears to be dependent on the rhythmic cycling of Bmal1 mRNA, because rhythms of translation regulation were blunted when BMAL1 was constitutively overexpressed, whereas the transcription-based clock functioned normally. Notably, some apparent rhythms in translation appeared to persist in Bmal1−/− MEFs, in which the molecular clock is completely disrupted [9]. This demonstrates that while rhythms of protein synthesis are clearly dependent on cycling BMAL1 levels, they also receive input from other stimuli that occur on a daily basis even in the absence of a molecular clock. The identification of these daily stimuli (e.g., feeding, sleep/wake cycle and/or other daily behaviors) and how they interface with translation regulation remain to be determined.
Bmal1−/− mice also exhibit severe co-morbid phenotypes such as premature aging with decreased activity and body weight [9]. Some of these phenotypes are rescued by tissue-specific restoration of Bmal1 expression, while others are not [10]. This new insight into the moonlighting role of BMAL1 in translational regulation could help to explain the particularly strong phenotypes seen with deletion of Bmal1, which may perturb cellular function beyond disruption of the transcription-based circadian clock. Future studies investigating the effects of circadian disruption or metabolic perturbation on the translational activity of BMAL1 may provide insight into how circadian translational interference promotes disease.
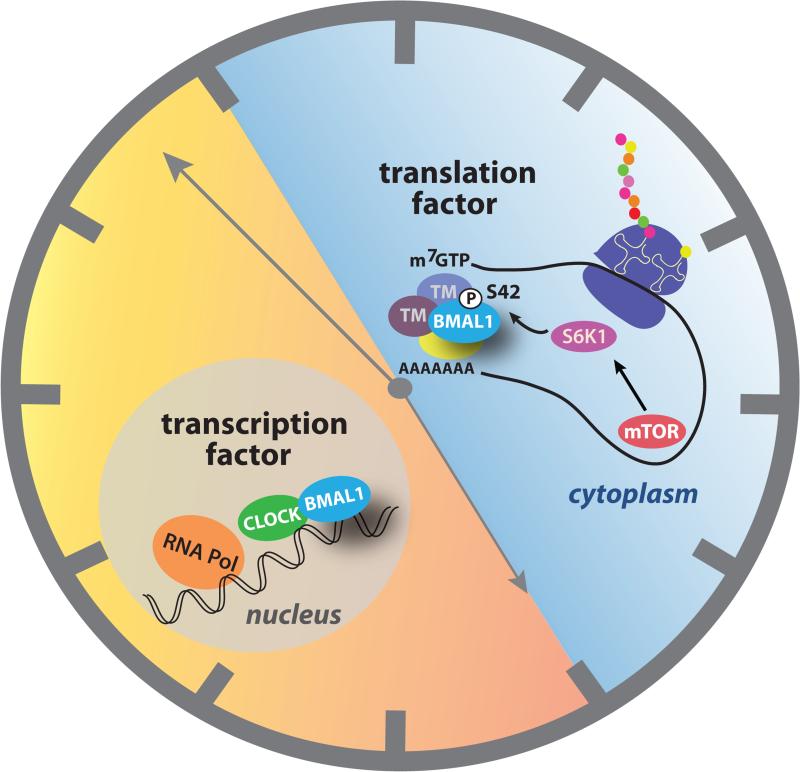
Figure 1. BMAL1 works around the clock to control transcription and translation.
During the day, the heterodimeric transcription factor CLOCK:BMAL1 is located in the nucleus, where it transactivates clock-controlled genes to control temporal regulation of physiology, including a daily peak in expression of mTOR [5]. During the night, cytosolic BMAL1 interacts with components of the translational machinery (TM) to stimulate cap-dependent protein synthesis [7]. Phosphorylation of BMAL1 Ser42 by the mTOR-dependent kinase, S6K1, promotes the interaction of BMAL1 with translational machinery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gustafson CL, Partch CL. Emerging models for the molecular basis of mammalian circadian timing. Biochemistry. 2014;54(2):134–149. doi: 10.1021/bi500731f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R, et al. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl.Acad. Sci. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menet JS, et al. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife. 2012;1:e00011. doi: 10.7554/eLife.00011. doi:10.7554/eLife.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jouffe C, et al. The Circadian Clock Coordinates Ribosome Biogenesis. PLoS Biol. 2013;11(1):e1001455. doi: 10.1371/journal.pbio.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao R, et al. Circadian regulation of mTOR signaling in the mouse SCN. Neuroscience. 2011;181:79–88. doi: 10.1016/j.neuroscience.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondratov RV, et al. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17(15):1921–1932. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipton JO, et al. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell. 2015;161(5):1138–1151. doi: 10.1016/j.cell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khapre RV, et al. Metabolic clock generates nutrient anticipation rhythms in mTOR signaling. Aging (Albany NY) 2014;6(8):675–689. doi: 10.18632/aging.100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunger MK, et al. Mop3 Is an Essential Component of the Master Circadian Pacemaker in Mammals. Cell. 2000;103(7):1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDearmon EL, et al. Dissecting the Functions of the Mammalian Clock Protein BMAL1 by Tissue-Specific Rescue in Mice. Science (New York, N.Y.) 2006;314(5803):1304–1308. doi: 10.1126/science.1132430. [DOI] [PMC free article] [PubMed] [Google Scholar]