Abstract
Background & Aims
Emerging data suggest that changes in intestinal permeability and increased gut microbial translocation contribute to the inflammatory pathway involved in nonalcoholic steatohepatitis (NASH) development. Numerous studies have investigated the association between increased intestinal permeability and NASH. Our meta-analysis of this association investigates the underlying mechanism.
Methods
A meta-analysis was performed to compare the rates of increased intestinal permeability in patients with NASH and healthy controls. To further address the underlying mechanism of action, we studied changes in intestinal permeability in a diet-induced (methionine-and-choline-deficient; MCD) murine model of NASH. In vitro studies were also performed to investigate the effect of MCD culture medium at the cellular level on hepatocytes, Kupffer cells, and intestinal epithelial cells.
Results
Nonalcoholic fatty liver disease (NAFLD) patients, and in particular those with NASH, are more likely to have increased intestinal permeability compared with healthy controls. We correlate this clinical observation with in vivo data showing mice fed an MCD diet develop intestinal permeability changes after an initial phase of liver injury and tumor necrosis factor-α (TNFα) induction. In vitro studies reveal that MCD medium induces hepatic injury and TNFα production yet has no direct effect on intestinal epithelial cells. Although these data suggest a role for hepatic TNFα in altering intestinal permeability, we found that mice genetically resistant to TNFα-myosin light chain kinase (MLCK)–induced intestinal permeability changes fed an MCD diet still develop increased permeability and liver injury.
Conclusions
Our clinical and experimental results strengthen the association between intestinal permeability increases and NASH and also suggest that an early phase of hepatic injury and inflammation contributes to altered intestinal permeability in a fashion independent of TNFα and MLCK.
Keywords: Meta-Analysis, Myosin Light Chain Kinase, Steatosis, Tight Junctions
Abbreviations used in this paper: ALT, alanine aminotransferase; CI, confidence interval; FITC, fluorescein isothiocyanate; IL, interleukin; MCD, methionine and choline deficient; MLCK, myosin light chain kinase; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NPC, nonparenchymal cells; OR, odds ratio; PBS, phosphate-buffered saline; qRT-PCR, quantitative real-time polymerase chain reaction; TEER, transepithelial electrical resistance; TNFα, tumor necrosis factor-α; ZO-1, zona occludens-1
Summary.
This study comprehensively defines the clinical association between intestinal permeability increases and nonalcoholic steatohepatitis. The results suggest that early-phase hepatic injury and inflammation contribute to altered intestinal permeability in a fashion independent of tumor necrosis factor-α and myosin light chain kinase.
Nonalcoholic fatty liver disease (NAFLD) has become an increasingly common clinical condition, with an estimated prevalence of 30% in the U.S. population.1 Despite this high prevalence, only a minority of NAFLD patients develop nonalcoholic steatohepatitis (NASH) and fibrosis, which account for significant morbidity and mortality.2 The clinical challenge remains identifying the patients who are more likely to develop NASH, as these patients are at greater risk for liver-related adverse events. Unfortunately, to date there is no reliable predictor of progression to NASH, nor are therapies approved by the U.S. Food and Drug Administration for this condition. Accordingly, a more fundamental understanding of the pathophysiology of NASH is critical to help identify high-risk NAFLD patients and therapeutic targets.
There are accumulating data that suggest a role for alterations in intestinal permeability in the pathogenesis of NASH. Specifically, it is hypothesized that an increase in intestinal epithelial cell permeability allows for translocation of microbial products into the portal vein, which propagate inflammation in a susceptible liver primed for injury.3, 4 These data suggest that communication between the intestine and liver, the so-called gut-liver axis, plays a role in NASH development. Accordingly, there is building momentum to study the contribution of changes in intestinal homeostasis to liver injury and inflammation.
Although our understanding of the gut-liver axis is rapidly evolving, there remain many unanswered questions. Multiple studies have examined have examined the incidence of intestinal permeability changes in NASH patients; however, a comprehensive and systemic assessment of this relationship has yet to be performed. Furthermore, the inciting event responsible for intestinal permeability changes in patients with primary liver disease has not been identified. Obesity is common in patients with NASH and has been associated with intestinal inflammation and up-regulation of tumor necrosis factor-α (TNFα), both of which may contribute to intestinal leakage of microbial products.5 Additionally, differences in the gut microbiome in patients with NASH may alter intestinal permeability through inflammation-based and bacterial metabolite-driven pathways.4, 6 The potential contribution of liver pathology to intestinal permeability in NASH, however, has yet to be investigated.
In an effort to comprehensively assess the rates of increased intestinal permeability in patients with and without NASH, we performed a meta-analysis of literature examining this question. We then sought to mechanistically explain the association between intestinal permeability and NASH using an animal model for NASH as a means to eliminate possible confounders of the clinical data, such as antibiotic exposure and medical comorbidities. We focused our attention on the potential contribution of liver injury to intestinal permeability, as we hypothesized that an initial liver injury may lead to systemic disturbances, including an increase in intestinal permeability, which further propagates liver inflammation.
Materials and Methods
Meta-analysis
We performed a meta-analysis to compare the rates of increased intestinal permeability in patients with and without NAFLD. For inclusion in the meta-analysis, a study had to meet the following criteria: 1) measurement in vivo of intestinal permeability with a validated test substance (monosaccharide, nonhydrolyzed or hydrolyzed disaccharide, 51Cr-EDTA, or 99mTc-DTPA); 2) documentation of NAFLD with imaging or histology; 3) documentation of minimal to no alcohol use in both groups; and 4) lack of coexisting liver disease in both groups.
To find relevant articles, a systematic review of English and non-English articles was performed using PubMed (1946 to July 2014) and EMBASE (1988 to 2014 week 15). The PubMed search was performed by the authors (J.L., M.D., and S.J.P.), and an information library specialist at the Mayo clinic library performed the EMBASE search. To reduce reporting bias and error in data collection, two independent reviewers (J.L. and S.J.P.) extracted data from selected studies using standardized data extraction forms. We contacted the primary investigators of articles with questions that arose during data extraction. We identified additional studies by searching bibliographies of all the reviewed articles and abstracts presented at the Digestive Disease Week and the Liver Meeting from 2007 to 2014. We used crude odds ratio (OR) for increased intestinal permeability, comparing NAFLD patients to controls as our parameter of interest. We pooled estimates by random-effects meta-analysis according to the method of DerSimonian and Laird7 and fixed-effect meta-analysis by calculating the weighted average of study estimates with the inverse of estimates variance used as the study weight. Q statistic and I2 values were estimated to evaluate the heterogeneity among the studies.8
We used Comprehensive Meta-Analysis software for these analyses. The methodological quality of the studies was assessed by two investigators (J.L. and S.J.P.) independently using the Newcastle-Ottawa scale.9 For meta-analyses of a small number of studies (generally less than 10), the power to detect publication bias is poor and is not recommended.10
In Vivo Induction of Nonalcoholic Steatohepatitis
Male C57/BL6 mice aged 8 to 10 weeks were obtained from Jackson Laboratory (Bar Harbor, ME). To study the role of TNF-α in NASH pathogenesis, male C57/BL6 mice genetically deficient in the long isoform of myosin light chain kinase (MLCK) were used. All animal experiments were reviewed and approved by the Massachusetts General Hospital and the University of Chicago subcommittees on research animal care. To induce NASH, mice were fed a methionine-and-choline-deficient (MCD) diet (Research Diets, New Brunswick, NJ). Mice were euthanized at multiple time points up to 21 days.
Biochemical Analysis of Liver Injury
For the animal experiments, immediately after euthanasia, we collected systemic blood from the inferior vena cava. Serum was obtained by centrifugation of whole blood at 10,000 rpm for 10 minutes. For the in vitro experiments, cell-free culture supernatant was concentrated to 500 μL using Microcon centrifugal filter devices (Millipore, Billerica, MA). To determine the extent of hepatocyte injury, quantification of alanine aminotransferase (ALT) was performed from serum and concentrated culture supernatant using the Infinity ALT Liquid Stable Reagent (Thermo Scientific, Middletown, VA).
Histologic Analysis of Liver Injury
The intact liver was excised immediately after mouse sacrifice, fixed in formalin for 24 hours, and then embedded in paraffin. Histologic analysis was performed on liver sections stained with H&E.
The severity of liver injury was determined using the NAFLD activity score as previously described elsewhere with slight modifications.11 Briefly, using the most affected area of tissue, a steatosis score was determined by the extent of steatosis: 0 (<5%), 1 (5%–33%), 2 (34%–66%), and 3 (>66%). An inflammation score was determined based upon the number of inflammatory foci in a 200× field: 0 (0 foci), 1 (<2 foci), 2 (2–4 foci), and 3 (>4 foci). The degree of hepatocyte ballooning was not scored as we have not found this histologic feature to be a characteristic finding of the MCD model. The modified NAFLD activity score was calculated by summation of the steatosis and inflammation scores. The examinations were performed in a blinded fashion by two independent investigators (J.L. and V.D.).
In Vivo Intestinal Permeability Assay
To measure intestinal paracellular permeability, the serum level of fluorescein isothiocyanate-4 kD dextran was measured as previously described elsewhere.12 Briefly, mice were denied access to food and water for 8 hours before sacrifice. At 4 hours before sacrifice, fluorescein isothiocyanate-4 kD dextran (Sigma-Aldrich, St. Louis, MO) at a dose of 60 mg/100 g body weight was orally gavaged. Immediately after the mice were sacrificed, we collected serum and measured the fluorescence intensity in serum (excitation, 490 nm; emission, 525 nm) using the Synergy 2 plate reader (BioTek, Winooski, VT).
Immunofluorescence Microscopy
The small intestine was excised and immediately placed in optimal cutting temperature compound (Tissue Tek; Sakura, Torrance, CA) and frozen in liquid nitrogen. We collected 5-μm frozen sections on coated slides and fixed them with 4% paraformaldehyde. Immunostaining was performed as previously described elsewhere.13 Briefly, the fixed sections were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 20 minutes and washed five times with 1% bovine serum albumin in PBS. Zona occludens-1 (ZO-1) was localized by incubating with rabbit anti-ZO-1 rabbit polyclonal antisera (Upstate Biotechnology, Waltham, MA) at a 1:200 dilution for 1 hour at room temperature. Subsequently, tissue sections were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG antibody (Sigma-Aldrich) at a 1:200 dilution for 1 hour. Standard epifluorescence microscopy was performed using a fluorescence microscope (EVOS FL; Life Technologies, Grand Island, NY).
Quantitative Real-Time Polymerase Chain Reaction
Mouse liver tissues were mechanically homogenized using the PowerGen 125 Homogenizer (Fisher Scientific, Fair Lawn, NJ). Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). Total RNA (500 ng) was converted into cDNA using the RT2 First Strand Kit (SA Biosciences, Valencia, California). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the RT2 Master Mix Kit (SA Biosciences, Valencia, CA) and the iQ 5 system (Bio-Rad Laboratories, Hercules, CA). Quantitative RT-PCR was performed for mRNA expression of β-actin and TNF-α, interleukin-1β (IL1β), and IL6 using primers designed by SA Biosciences (Qiagen). Expression of β-actin was used to standardize the samples, and the results are expressed as a ratio relative to control.
Detection of Cytokines in the Systemic Circulation
The levels of multiple cytokines (TNF-α, IL1β, and IL6) was assessed from serum using magnetic bead-based multiplex assays (Millipore) coupled with the Luminex-200 System Analyzer (Luminex, Austin, TX), as recommended by the manufacturer’s overnight protocol. The mean fluorescence intensity was expressed as the ratio relative to the average mean fluorescence intensity value obtained at day 0.
Cell Lines and Primary Cell Isolation
We maintained the H35 hepatocyte-derived cells as previously described elsewhere.14 The Caco2 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin G and 100 U/mL streptomycin) (Life Technologies, Grand Island, NY).
Rat Kupffer cells were separated from the nonparenchymal cell (NPC) fraction using centrifugal elutriation, as previously described elsewhere.15 Briefly, the NPC fraction was centrifuged at 300g for 15 minutes. The supernatant was discarded, and the pellet was resuspended in ice-cold PBS and passed through a cell strainer (40-μm pore) to remove any large debris. The elutriator was sterilized by circulating water (45 mL/min, 0g, 5 minutes), 6% H2O2 (10 mL/min, 50g, 5 minutes), 15 mg/mL catalase solution (10 mL/min, 0g, 5 minutes), sterile water (10 mL/min, 40g, 5 minutes), and Hank’s balanced salt solution (10 mL/min, 40g, 5 minutes). The elutriator was then ramped to 600g, and a flow of 10 mL/min was maintained. We introduced 10 mL of the NPC fraction into the elutriator and washed for 10 minutes to remove any cell debris while we maintained the rotor at 600g. Kupffer cells were eluted at 45 mL/min, 600g, and 100 mL of the cell suspension was collected. The cells were pelleted at 500g for 7 minutes (no brake) and used as an enriched fraction in experiments.
In Vitro Induction of Steatohepatitis
Media identical to standard Dulbecco’s modified Eagle’s medium culture yet deficient in l-methionine and choline chloride (Life Technologies) (MCD medium) was used to induce steatohepatitis in vitro as previously described elsewhere.14 Briefly, H35 cells were cocultured with rat primary Kupffer cells at a 2:1 ratio in 12-well plates in standard medium for 24 hours, after which the standard medium was replaced by MCD medium for 24 hours. Cell-free culture supernatant was harvested for the ALT measurement. Additionally, the protein level of TNF-α in the culture supernatant was measured using a TNF-α Platinum ELISA kit (eBioscience, San Diego, CA).
In Vitro Intestinal Permeability Assay
To assess the effect of MCD medium on intestinal permeability in vitro, Caco2 cells were added to the apical side of collagen-coated Transwells (Corning, Lowell, MA). Caco2 cells were grown for 21 days to allow for monolayer and tight junction formation. We then added the MCD medium to the culture system. After 24 hours, the intestinal permeability was assessed by measuring the transepithelial electrical resistance (TEER) using the Millicell-ERS electrical resistance system (Millipore). The resistance obtained from each experimental well was subtracted from a blank value obtained by inserting the electrodes in a transwell harboring a cell-fee medium. This value was multiplied by the area of the membrane to obtain TEER (Ω × cm2).
Statistical Analyses for In Vivo and In Vitro Experiments
Data are expressed as mean ± standard error and analyzed by unpaired Student t tests. Two-tailed P values were calculated, and P < .05 was considered statistically significant.
Results
Nonalcoholic Steatohepatitis Patients Are More Likely to Have Increased Intestinal Permeability Compared With Healthy Controls
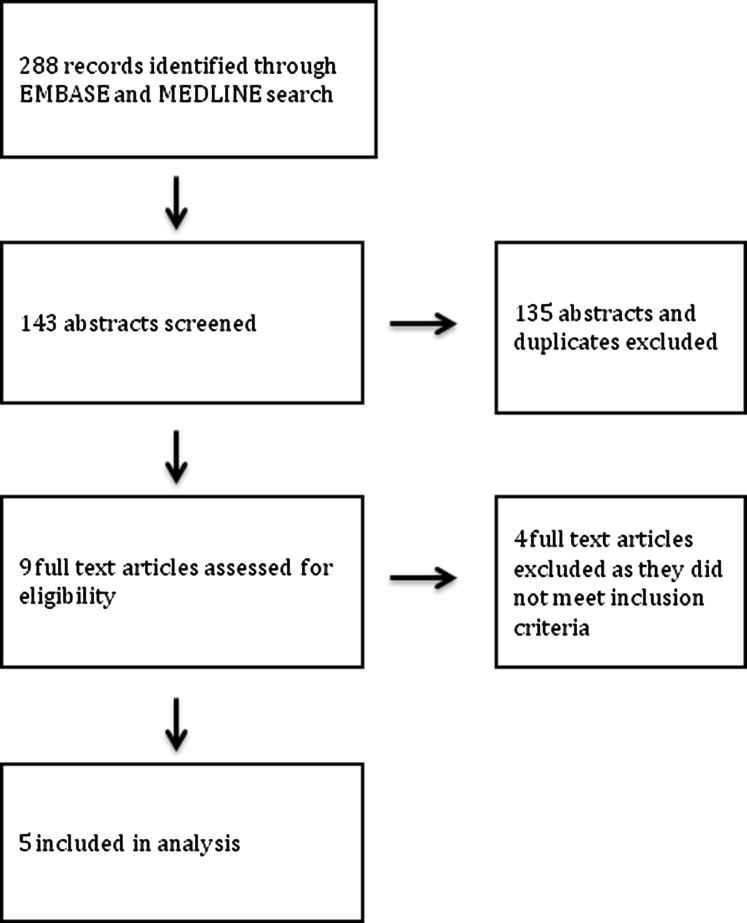
The initial search strategy for the meta-analysis yielded 288 potential articles for inclusion (Supplementary Table 1). Although there were multiple reasons for exclusion, the most common included studies involved animals and studies that did not directly measure intestinal permeability but rather used a surrogate measure such as serum lipopolysaccharide. After analysis of the selected articles, nine were reviewed in further detail. Subsequently, five studies (N = 128 patients) met our inclusion criteria (Supplementary Figure 1).16, 17, 18, 19, 20, 21 The characteristics of the included studies are summarized in Table 1. Normal values for tests measuring intestinal permeability were determined by the included study or from previously defined values.22
Table 1.
Characteristics of Included Nonalcoholic Fatty Liver Disease (NAFLD) Studies
| Author | Year | Location | NAFLD Diagnosis | Intestinal Permeability Assay | Single versus Multicenter | NAFLD (n) | Control (n) |
|---|---|---|---|---|---|---|---|
| Wigg et al17 | 2001 | Australia | Histology | Urinary lactulose/rhamnose at 5 hours | Single | 18 | 16 |
| Farhadi et al18 | 2008 | United States | Histology | Urinary lactulose/mannitol at 5 hours | Single | 16 | 12 |
| Miele et al19 | 2009 | Italy | Histology | Urinary chromium-51 ethylene diamine excretion over 24 hours | Single | 35 | 24 |
| Volynets et al20 | 2012 | Germany | Ultrasound and serology | Urinary lactulose/mannitol at 6 hours | Single | 20 | 10 |
| Giorgio et al21 | 2014 | Italy | Histology | Urinary lactulose/mannitol at 6 hours | Single | 39 | 21 |
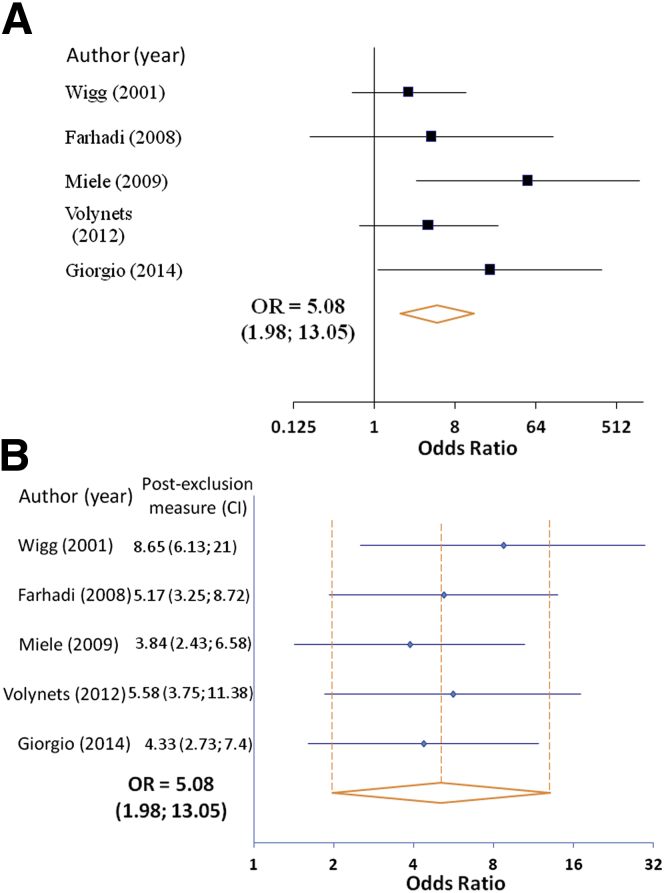
We found that 39.1% of NAFLD patients in our analysis had evidence for increased intestinal permeability, compared to 6.8% of healthy controls. The OR of NAFLD patients having increased intestinal permeability compared with controls was 5.08 (95% confidence interval [CI], 1.98–13.05) (Figure 1A). There was minimal heterogeneity in the included studies (I2 = 9.8%, Q statistic 4.43, P = .35), which was further strengthened by an exclusion sensitivity analysis (Figure 1B). The assessment of study quality is outlined in Table 2.
Figure 1.
Meta-analysis of increased intestinal permeability rates in nonalcoholic fatty liver disease (NAFLD) patients versus healthy controls. (A) Forest plot of increased intestinal permeability in patients with NAFLD as compared to healthy controls using a fixed-effects model. (B) Exclusion sensitivity plot of increased intestinal permeability in NAFLD patients versus healthy controls. CI, confidence interval; OR, odds ratio.
Table 2.
Newcastle-Ottawa Scale for Assessment of Quality of Included Studies
| Quality Assessment Criteria | Wigg et al17 | Farhadi et al18 | Miele et al19 | Volynets et al20 | Giorgio et al21 |
|---|---|---|---|---|---|
| Is the case definition adequate? (Yes, with independent validation) | + | + | + | + | + |
| Representatives of cases? (Consecutive or obviously representative series of cases) | + | − | + | − | + |
| Selection of controls? (Community controls) | + | + | + | − | + |
| Definition of controls? (No history of studied end point) | + | + | + | + | + |
| Study controls for age/sex? (Matching or multivariable analysis) | + | − | + | − | + |
| Study controls for at least three additional factors? | − | − | − | − | − |
| Ascertainment of exposure? (Secure record, structured interview by health-care practitioner, blind to case/control status) | + | + | + | + | + |
| Same method of ascertainment of cases/controls? (Yes) | − | + | + | + | + |
| Nonresponse rate? (Same for both groups) | − | + | + | + | + |
| Total overall score (Maximum = 9) | 6 | 6 | 8 | 5 | 8 |
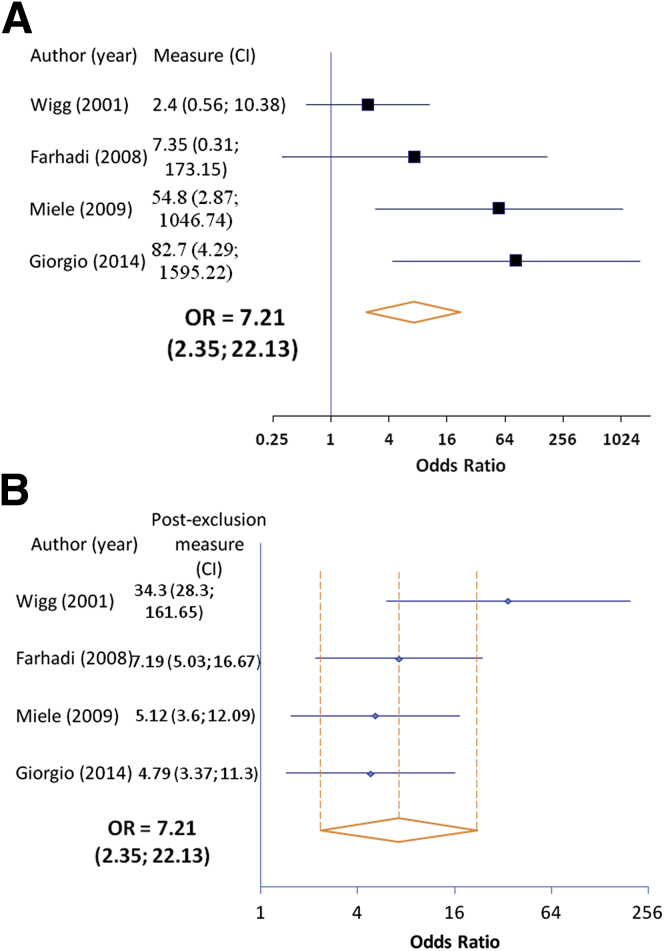
We next performed a subgroup analysis comparing the frequency of increased intestinal permeability in patients with NASH with that of the healthy controls. Four of the five studies (n = 83 patients) allowed for this analysis. We found that patients with NASH are also more likely to have increased intestinal permeability compared to healthy controls (OR 7.21; 95% CI, 2.35–22.13). The incidence of permeability changes in this subgroup (49.2%) was higher as compared to NAFLD patients as a whole, which includes patients with bland steatosis (Figure 2A). Although no statistically significant heterogeneity was detected (P = .09), there was moderate heterogeneity (I2 = 54.5%) across the studies. Interestingly, exclusion sensitivity analysis revealed the study by Wigg et al17 markedly reduced the observed OR (OR when excluded: 34.3; 95% CI, 28.3–161.65) (Figure 2B). Overall, these data show that NAFLD patients, and in particular those with NASH, are more likely to exhibit increased intestinal permeability compared with healthy controls.
Figure 2.
Meta-analysis of increased intestinal permeability rates in nonalcoholic steatohepatitis (NASH) patients versus healthy controls. (A) Forest plot of increased intestinal permeability in patients with NASH as compared with healthy controls using a fixed-effects model. (B) Exclusion sensitivity plot of increased intestinal permeability in NASH patients versus healthy controls. CI, confidence interval; OR, odds ratio.
A Methionine-Choline-Deficient Diet Induces Intestinal Permeability Changes After Liver Injury
To further investigate the clinical association between intestinal permeability and NASH, we examined this relationship in a dietary animal model (the MCD model) for NASH. The MCD model was chosen for its well-established ability to induce steatosis and inflammation and do so reproducibly in a relatively short period of time. However, it must be noted that the MCD model does not induce the weight gain, lipid abnormalities, or insulin resistance that are common in NASH patients.
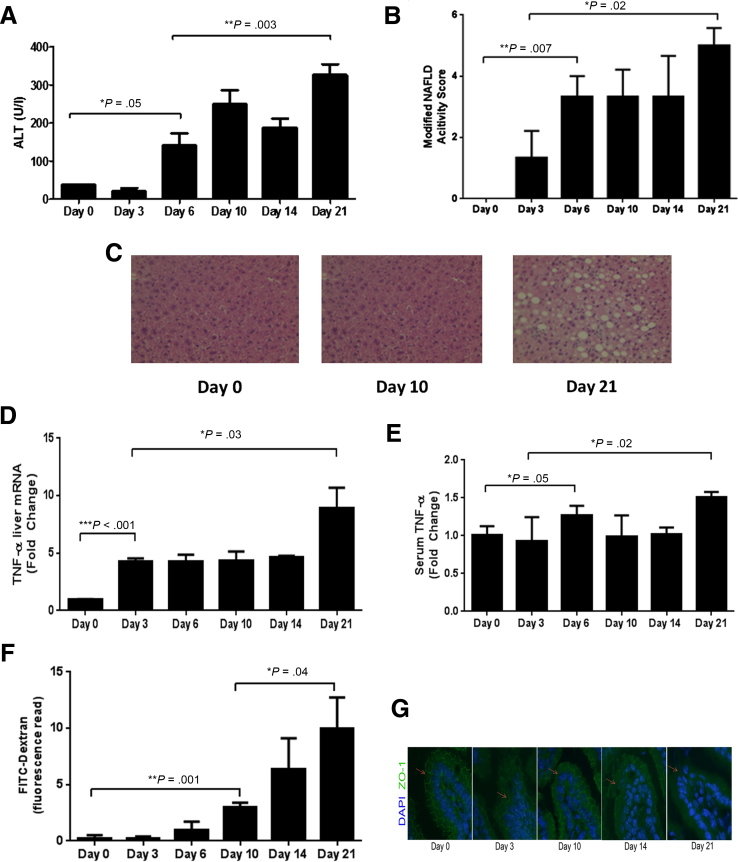
We found that mice fed an MCD diet developed liver injury rapidly, as early as 6 days into the diet, at which time serum levels of ALT were elevated and histologic evidence for hepatic steatosis and inflammation was also observed (Figure 3A–C). This early phase of liver injury and inflammation was followed by a secondary phase of injury, with peak ALT and histologically based modified NAFLD activity scores occurring at day 21. In parallel, we found that both hepatic and systemic levels of TNFα rose at an early time point with a subsequent secondary rise at day 21 (Figure 3D and E). Interestingly, we were unable to detect a difference in hepatic or systemic levels of IL6 or IL1β over the 21-day course, suggesting that these cytokines, which have been implicated in NASH pathogenesis, may contribute to a later phase of injury and inflammation.23, 24
Figure 3.
Temporal characterization of liver injury and intestinal permeability changes in a murine dietary nonalcoholic steatohepatitis (NASH) model. C57BL/6 mice (N = 5 mice/group) were fed a diet deficient in methionine and choline (MCD) and were sacrificed at multiple time points up to 21 days. We found evidence for significant MCD-induced liver injury as early as day 6 based on both (A) serum alanine aminotransferase (ALT) and (B, C) H&E liver histology (original magnification: 20×) that progressed to a peak value at day 21. Specifically, liver histologic examination revealed a progressively increasing number of inflammatory foci and steatosis throughout the experiment. In parallel, hepatic mRNA expression and systemic levels of tumor necrosis factor-α (TNF-α) were elevated at an early phase of the diet (D, E).Temporal evaluation of intestinal permeability changes, based on (F) fluorescein isothiocyanate (FITC) dextran serum measurements, and tight junction architecture based on (G) immunofluorescence staining for zona-occludens-1 (ZO-1), revealed evidence for a significant increase in intestinal permeability and disruption of normal tight junction architecture (loss of chicken-wire appearance of ZO-1: arrows) at day 10. DAPI, 4′,6-diamidino-2-phenylindole.
We next examined the temporal pattern of intestinal permeability changes during the course of the MCD diet. Although permeability steadily increased as the experiment progressed, a significant increase in intestinal permeability, as measured by both the serum signal intensity of FITC-dextran, was not observed until day 10 (Figure 3F), well after the initial phase of liver injury, inflammation, and TNFα production. Furthermore, we first noted evidence for disruption of ZO-1 localization in the small intestine at day 10, suggesting injury to the epithelial tight junction complex. These findings are consistent with the clinical association between increased intestinal permeability and NASH, but suggest that increased intestinal permeability is an effect rather than cause of NASH. One potential explanation could be that an initial phase of hepatic injury and inflammation releases intermediates, such as cytokines, that increase intestinal permeability, and that the latter further contribute to the progression of liver injury.
Methionine and Choline Deficient Culture Medium Directly Induces Hepatocyte Injury and Inflammation but Does Not Affect Intestinal Epithelial Paracellular Permeability
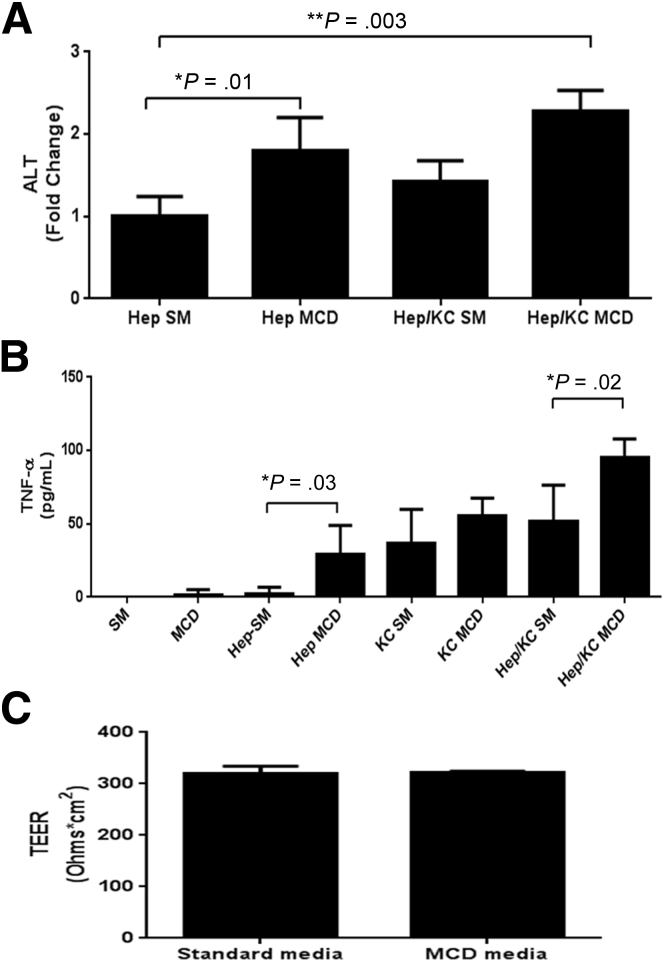
To address our in vivo observation of early liver injury and TNFα production subsequently influencing intestinal permeability, we studied the effect of MCD medium on hepatocytes, Kupffer cells, and intestinal epithelial cells in vitro. We cocultured rat hepatocytes (H35 cells) and primary rat Kupffer cells together in an attempt to more appropriately model the physiologic inflammatory dynamic within the liver. We found that exposing hepatocytes and Kupffer cells to MCD medium induced significant hepatocyte injury and TNFα production as compared with cells grown in standard medium (Figure 4A and B). These data reinforced our in vivo observation that MCD directly induces liver injury and inflammation.
Figure 4.
In vitro assessment of MCD-induced changes to the liver and intestine. Rat hepatocytes (H35) were grown in coculture with primary rat Kupffer cells (KC) at a ratio of 2:1. Cells were exposed to standard medium (SM) or methionine-and-choline-deficient (MCD) medium for 24 hours, after which the supernatant was harvested for further analysis. (A) Coculturing of hepatocytes and KCs in the presence of MCD caused the most significant elevation in hepatocyte injury, based on supernatant levels of alanine aminotransferase (ALT). (B) Further, MCD-exposed hepatocytes and KCs produced significantly more tumor necrosis factor-α (TNF-α) compared to cells grown in standard medium. (C) Intestinal epithelial cells (Caco2 cells) were grown to confluence and allowed to form strong tight junctions, after which they were exposed to either MCD medium or SM. We found no difference in tight junction function between cells grown in MCD medium versus SM, suggesting MCD medium is not directly toxic to these cells. TEER, transepithelial electrical resistance.
In contrast, we did not observe any deleterious effect of exposing intestinal epithelial cells to MCD medium. Specifically, intestinal epithelial cells exposed to the MCD medium showed no reduction in TEER, suggesting that the MCD medium does not directly disrupt intestinal tight junction function. Taken together, these in vitro data show that MCD medium induces hepatocyte injury and TNFα production yet does not independently disrupt intestinal epithelial tight junction function, suggesting that our in vivo observation of increased intestinal permeability in the MCD model may rely on MCD-induced hepatic injury and TNFα production.
Mice Resistant to TNFα-Induced Intestinal Permeability Changes Develop Significant Intestinal Permeability and Liver Injury When Fed a NASH-Inducing Diet
Our data thus far suggest that MCD-induced hepatic TNFα production may play a role in altering intestinal permeability and contribute to ongoing liver injury and inflammation. To test this hypothesis, we exposed mice deficient in the long isoform of myosin light chain kinase (MLCK) to the MCD diet. These mice are resistant to acute-TNFα-induced intestinal permeability increases13, 25 and also are protected from barrier loss in experimental inflammatory bowel disease.26 We hypothesized that long MLCK-deficient mice would exhibit early hepatic injury induced by the MCD diet but be protected against intestinal permeability changes and ongoing hepatic injury and inflammation.
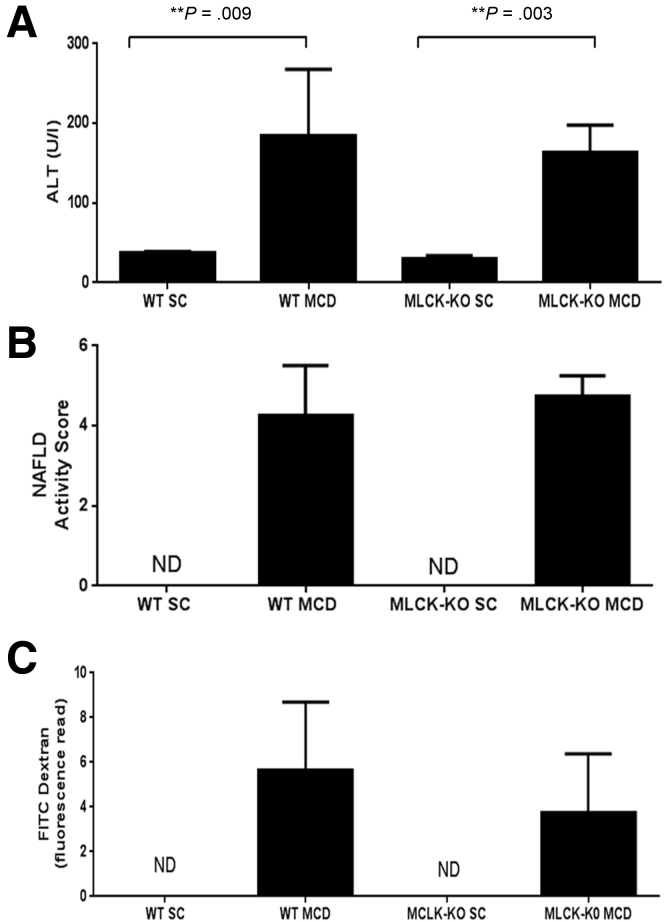
However, we found that MCD-fed MLCK-deficient mice developed equally significant liver injury compared with wild-type mice fed an MCD diet for 21 days (Figure 5A and B). Furthermore, long MLCK-deficient mice exhibited increased intestinal permeability compared with the MLCK-deficient mice fed a standard chow (Figure 5C). Taken together, these data strongly suggest that TNFα is not predominantly responsible for the observed changes in intestinal permeability seen during the MCD diet.
Figure 5.
In vivo assessment of tumor necrosis factor-α (TNF-α) on intestinal permeability in nonalcoholic steatohepatitis (NASH) pathogenesis. TNF-α-induced increases in intestinal permeability are mediated through myosin light chain kinase (MLCK); therefore, genetic deletion of MLCK renders mice impervious to intestinal permeability changes caused by TNF-α.22 We tested whether mice deficient in the long isoform of MLCK would be protected against MCD-induced liver injury and intestinal permeability changes. MLCK-knockout (KO) and wild-type (WT) mice were fed the MCD diet for 24 days and then euthanized (N = 5 mice per group). There were no differences in (A) serum levels of ALT or (B) in H&E liver histology (original magnification: 20×) between MLCK-KO and WT mice. (C) Furthermore, we were unable to detect a difference in intestinal permeability changes between the MCD-fed MLCK-KO and WT mice.
Discussion
In this study we demonstrate that NAFLD patients, and in particular patients with NASH, are more likely to exhibit increased intestinal permeability compared with healthy controls. We correlate this clinical observation with in vivo data showing that MCD-fed mice develop intestinal permeability changes after an initial phase of liver injury and TNFα induction.
In vitro studies reveal that MCD medium induces hepatic injury and TNFα production yet has no direct effect on intestinal epithelial cells. Although these data suggest a role for hepatic TNFα in altering intestinal permeability, we found that MCD-fed mice genetically resistant to TNFα-induced intestinal permeability changes were not protected against liver injury. Taken together, our clinical and experimental results strengthen the association between intestinal permeability changes and NASH as well as suggest that an early phase of hepatic injury and inflammation contributes to altered intestinal permeability in a TNFα- and MLCK-independent fashion.
Although the pathogenesis of NASH is likely multifactorial, there are emerging data that suggest alterations in intestinal permeability can contribute to liver injury. The emergence of the “gut-liver axis” implicates changes in intestinal physiology in the pathogenesis of liver injury. For example, Gabele et al3 demonstrated that induction of colitis in mice fed a high-fat diet caused more severe steatohepatitis as compared with mice fed a high-fat diet without colitis. They further showed that increased intestinal translocation of microbial products into the portal vein of colitis-affected mice occurred, which suggests that disruption of the intestinal barrier allowed proinflammatory substrates access to the liver. Similarly, Henao-Mejia et al4 associated changes in gut physiology and microbiota with elevated levels of Toll-like receptor agonists in the portal vein and more severe steatohepatitis in an MCD model.
In parallel with these animal data, clinical experience also supports the notion of a gut-liver axis. Pathological intestinal conditions with documented changes in intestinal permeability have been associated with NASH, including celiac disease and inflammatory bowel disease.27, 28, 29 Furthermore, probiotic treatment has been shown to improve liver aminotransferases in patients with NAFLD, suggesting a role for intestinal microbes in NASH pathogenesis.30 On the other hand, there is less evidence suggesting that end-stage liver disease or cirrhosis can affect intestinal physiology, and the mechanism for this is unclear.31, 32
Our results further strengthen the potential role of intestinal permeability in the pathogenesis of NASH, but uniquely suggest that early changes in liver physiology may also affect intestinal homeostasis. We show that liver injury is induced early in the course of the MCD diet, before any change in intestinal permeability, which suggests that the initial liver injury phase may be contributing to the observed intestinal permeability changes in a TNFα-independent manner.
The mechanism by which hepatic injury may affect intestinal permeability, however, remains elusive. We focused our study on TNFα as higher levels of this cytokine have been associated with NASH in clinical studies.33, 34, 35 Additionally, its role in modulation of intestinal permeability has been well-established through its activation of long MLCK and the subsequent effect on tight junction permeability.24, 25, 36, 37 However, despite the initially encouraging data implicating hepatic TNFα, ultimately it does not appear to be responsible for altering intestinal permeability through an MLCK-dependent mechanism.
Alternatively, another liver-produced mediator may be responsible for the observed intestinal changes. Besides TNFα, several other cytokines have been implicated in altering intestinal permeability. We did not find any differences in hepatic and systemic levels of IL1β, which can alter intestinal permeability through an MLCK-dependent mechanism.38 Furthermore, there were no differences in IL6, which has been associated with liver-related mortality.39 and can alter intestinal permeability through claudin-2 up-regulation, although there is no expected increase in FITC-dextran permeability.40 Our findings should not underscore the potential importance of these cytokines to NASH pathogenesis, as elevated serum and hepatic levels of IL1β have been reported at different time points in the MCD model.41 Several other mediators, such as transforming growth factor β and plasminogen activator inhibitor-1, deserve study. Further investigation into identifying a novel hepatic-produced modulator of intestinal permeability is desirable.
Although the contribution of TNFα to NASH pathogenesis is debated,42 there are data highlighting its key role. Tomita et al43 showed mice deficient in both TNF receptors 1 and 2 developed attenuated liver steatosis and fibrosis when fed the MCD diet, implicating the TNFα/TNF-receptor-mediated pathway in NASH pathogenesis. Koca et al44 demonstrated that administration of an antibody to TNFα lessened the severity of steatohepatitis induced by the MCD diet. Thalidomide, an immunosuppressant with anti-TNFα properties, has also been shown to reduce the inflammatory profile induced in a murine NAFLD model.45 Furthermore, NAFLD patients receiving etanercept, an antibody to TNF, have significant reductions in the AST/ALT ratio and serum C-reactive protein levels.46
Our data demonstrate that hepatic TNFα is not responsible for altering intestinal permeability through an MLCK-dependent mechanism. It is possible, however, that TNFα modulates intestinal permeability independent of MLCK and tight junctions by accelerating intestinal epithelial cell turnover.26 Additionally, it is also possible that TNFα alters permeability through activation of TNF-receptor 1, which has been recently been shown as important to the pathogenesis of alcoholic liver disease.47 Therefore, the potential role for TNFα in NASH pathogenesis and intestinal barrier loss should not be fully discounted.
Our study does have limitations. First, defining normal values for intestinal permeability is difficult and was not uniform throughout the clinical studies included in our meta-analysis. For example, the normal value chosen for 51Cr-EDTA testing in the study by Miele et al19 of 4.88%, which was based on the median value obtained from the NAFLD group in the study, is higher than normal values previously reported.48 As another example, the normal value for lactulose-rhamnose intestinal permeability testing of 0.18 used to analyze data from the study by Wigg et al17 has been shown to have sensitivity and specificity values of 78%.22 Along these lines, intestinal permeability is difficult to assess in vivo. Although multiple methodologies have been used to assess permeability, measurement of serum FITC dextran is commonly employed. These analyses, however, may not sensitively detect small changes in intestinal permeability to large probes, such as FITC-dextran, and are unable to measure paracellular permeability increases limited to small solutes such as ions. Last, although the MCD diet as a model for NASH is effective in recapitulating the histologic changes seen in NASH, it does not induce weight gain, lipid abnormalities, or insulin resistance, which are common in NASH patients. Therefore, it is difficult to comment on the potential impact of these clinical variables on our results.
In summary, our clinical and experimental results strengthen the association between intestinal permeability increases and NASH and also suggest that an early phase of hepatic injury and inflammation contributes to altered intestinal permeability in a TNFα- and MLCK-independent fashion. As evidence continues to mount supporting the notion of gut-liver cross-talk, further research is needed to decipher the mechanism by which this cross-talk occurs. It is likely that the findings of such research will have significant clinical implications for NASH patients.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded by a Sheila Sherlock Clinical and Translation Research Award (to J.L.); and the National Institutes of Health NIH R01DK61931 and R01DK68271 (to J.R.T.) and DK078772 (to R.T.C.).
Supplementary Material
Supplementary Figure 1.
Flow diagram for meta-analysis.
Supplementary Table 1.
Search Strategy and Results
| No. | Search Strategy | Results |
|---|---|---|
| 1 | Fatty Liver/ | 35,304 |
| 2 | exp nonalcoholic fatty liver/ | 13,602 |
| 3 | (“fatty liver” or steatohepatitis or ((NAFLD or NASH) and (liver∗ or hepat∗)) or steatohepatitides or (liver adj2 steatos∗) or “visceral steatos∗”).mp. [mp=ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, ui] | 57,349 |
| 4 | 1 or 2 or 3 | 57,349 |
| 5 | exp Intestines/ | 51,8207 |
| 6 | exp Intestinal Absorption/ | 56,039 |
| 7 | exp Permeability/ | 247,453 |
| 8 | (5 or 6) and 7 | 10,664 |
| 9 | ((intestin∗ or bowel∗ or gut or gastrointestin∗) and (permeab∗ or leakiness)).mp. | 25,612 |
| 10 | 8 or 9 | 27,656 |
| 11 | 4 and 10 | 288 |
| 12 | 11 not (exp animals/ not exp humans/) | 193 |
| 13 | limit 12 to (book or book series or editorial or erratum or letter or note or addresses or autobiography or bibliography or biography or comment or dictionary or directory or interactive tutorial or interview or lectures or legal cases or legislation or news or newspaper article or overall or patient education handout or periodical index or portraits or published erratum or video-audio media or webcasts) [Limit not valid in Embase, Ovid MEDLINE(R),Ovid MEDLINE(R) In-Process; records were retained] | 10 |
| 14 | 12 not 13 | 183 |
| 15 | remove duplicates from 14 | 143 |
Note: Database(s): EMBASE 1988 to 2014 week 15; Ovid MEDLINE In-Process and Other Non-Indexed Citations; and Ovid MEDLINE 1946 to Present.
References
- 1.Szczepaniak L.S., Nurenberg P., Leonard D. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 2.Dixon J.B., Bhathal P.S., O’Brien P.E. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 3.Gabele E., Dostert K., Hofmann C. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol. 2011;55:1391–1399. doi: 10.1016/j.jhep.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Henao-Mejia J., Elinav E., Jin C. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pendyala S., Neff L.M., Suarez-Farinas M., Holt P.R. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93:234–242. doi: 10.3945/ajcn.110.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L., Baker S.S., Gill C. Characterization of gut microbiome in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 7.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Health Research Institute, nd. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 10.Sterne J.A., Gavaghan D., Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner D.E., Brunt E.M., Van Natta M. Design and validation of a histological scoring system for nonalocholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 12.Napolitano L.M., Koruda M.J., Meyer A.A., Baker C.C. The impact of femur fracture with associated soft tissue injury on immune function and intestinal permeability. Shock. 1996;5:202–507. doi: 10.1097/00024382-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Clayburgh D.R., Barrett T.A., Tang Y. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell-activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King K.R., Wang S., Irimia D. A high-throughput microfluidic real-time gene expression living cell array. Lab Chip. 2007;7:77–85. doi: 10.1039/b612516f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMullen M., Pritchard M., Nagy L. Isolation of Kupffer cells from rates fed chronic ethanol. In: Nagy L.E., editor. Alcohol: methods and protocols. Humana Press; Totowa, NJ: 2008. pp. 199–212. [DOI] [PubMed] [Google Scholar]
- 16.Sahai A., Pan X., Paul R. Roles of phosphatidylinositol 3-kinase and osteopontin in steatosis and aminotransferase release by hepatocytes treated with methionine-choline-deficient medium. Am J Physiol Gastrointest Liver Physiol. 2006;291:G55–G62. doi: 10.1152/ajpgi.00360.2005. [DOI] [PubMed] [Google Scholar]
- 17.Wigg A.J., Roberts-Thomson I.C., Dymock R.B. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor α in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhadi A., Gundlapalli S., Shaikh M. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miele L., Valenza V., La Torre G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 20.Volynets V., Kuper M.A., Strahl S. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:1932–1941. doi: 10.1007/s10620-012-2112-9. [DOI] [PubMed] [Google Scholar]
- 21.Giorgio V., Miele L., Principessa L. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46:556–560. doi: 10.1016/j.dld.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 22.van Wijck K., Bessems B.A., van Eijk H.M. Polyethylene glycol versus dual sugar assay for gastrointestinal permeability analysis: is it time to choose? Clin Exp Gastroenterol. 2012;5:139–150. doi: 10.2147/CEG.S31799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura K., Kodama Y., Inokucki S. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology. 2010;139:323–334. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mas E., Danjoux M., Garcia V. IL-6 deficiency attenuates murine diet-induced non-alcoholic steatohepatitis. PLoS ONE. 2009;4:e7929. doi: 10.1371/journal.pone.0007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchiando A.M., Shen L., Graham W.V. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su L., Nalle S.C., Shen L. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardella M.T., Valenti L., Pagliari C. Searching for coeliac disease in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2004;36:333–336. doi: 10.1016/j.dld.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 28.McGowan C.E., Jones P., Long M.D., Barritt A.S., 4th Changing shape of disease: nonalcoholic fatty liver disease in Crohn’s disease—a case series and review of the literature. Inflamm Bowel Dis. 2012;18:49–54. doi: 10.1002/ibd.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy S.K., Zhan M., Alexander H.R., El-Kamary S.S. Nonalcoholic fatty liver disease is associated with benign gastrointestinal disorders. World J Gastroenterol. 2013;19:8301–8311. doi: 10.3748/wjg.v19.i45.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma Y.Y., Li L., Yu C.H. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Plessis J., Vanheel H., Janssen C.E. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58:1125–1132. doi: 10.1016/j.jhep.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Seki E., Schanbl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447–458. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crespo J., Cayon A., Fernandez-Gil P. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 34.Alaaeddine N., Sidaoui J., Hilal G. TNF-α messenger ribonucleic acid (mRNA) in patients with nonalcoholic steatohepatitis. Eur Cytokine Netw. 2012;23:107–111. doi: 10.1684/ecn.2012.0313. [DOI] [PubMed] [Google Scholar]
- 35.Bahccioglu I.H., Yalniz M., Ataseven H. Levels of serum hyaluronic acid, TNF-alpha and IL-8 in patients with nonalcoholic steatohepatitis. Hepatogastroenterology. 2005;52:1549–1553. [PubMed] [Google Scholar]
- 36.Zolotarevsky Y., Hecht G., Koutsouris A. A membrane-permeant peptide that inhibits mlc kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 37.Wang F., Graham W.V., Wang Y. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Sadi R., Guo S., Ye D. Mechanism of IL-1β modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J Immunol. 2013;190:6596–6606. doi: 10.4049/jimmunol.1201876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J.K., Bettencourt R., Brenner D. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS ONE. 2012;7:e34218. doi: 10.1371/journal.pone.0034218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki T., Yoshinaga N., Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csak T., Pillai A., Ganz M. Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis. Liver Int. 2014;34:1402–1413. doi: 10.1111/liv.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pena A.D., Leclercq I., Field J. NF-κB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Tomita K., Tamiya G., Ando S. Tumour necrosis factor signalling through activation of Kupffer cells plays an essential role in liver fibrosis of nonalcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koca S.S., Bahcecioglu I.H., Poyrazoglu O.K. The treatment with antibody to TNF-alpha reduces the inflammation, necrosis, and fibrosis in the non-alcoholic steatohepatitis induced by the methionine-and choline-deficient diet. Inflammation. 2008;31:91–98. doi: 10.1007/s10753-007-9053-z. [DOI] [PubMed] [Google Scholar]
- 45.Pinto Lde F., Compri C.M., Fornari J.V. The immunosuppressant drug, thalidomide, improves hepatic alterations induced by a high-fat diet in mice. Liver Int. 2010;30:603–610. doi: 10.1111/j.1478-3231.2009.02200.x. [DOI] [PubMed] [Google Scholar]
- 46.Campanati A., Ganzetti G., Di Sario A. The effect of etanercept on hepatic fibrosis risk in patients with non-alcoholic fatty liver disease, metabolic syndrome, and psoriasis. J Gastroenterol. 2013;48:839–846. doi: 10.1007/s00535-012-0678-9. [DOI] [PubMed] [Google Scholar]
- 47.Chen P., Starkel P., Turner J.R. Dysbiosis-induced intestinal inflammation activates TNFRI and mediates alcoholic liver disease in mice. Hepatology. 2014 doi: 10.1002/hep.27489. http://dx.doi.org/10.1002/hep.27489 Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peled Y., Watz C., Gilat T. Measurement of intestinal permeability using 51Cr-EDTA. Am J Gastroenterol. 1985;80:770–773. [PubMed] [Google Scholar]