Abstract
Using the thermal decomposition of organometallics method we have synthesized high-quality, iron oxide nanoparticles of tailorable size up to ~15nm and transferred them to a water phase by coating with a biocompatible polymer. The magnetic behavior of these particles was measured and fit to a log-normal distribution using the Chantrell method and their polydispersity was confirmed to be very narrow. By performing calorimetry measurements with these monodisperse particles we have unambiguously demonstrated, for the first time, that at a given frequency, heating rates of superparamagnetic particles are dependent on particle size, in agreement with earlier theoretical predictions.
Keywords: Magnetic fluid hyperthermia, Nanoparticle, Magnetic relaxation, Superparmagnetism
1. Introduction
Increased heating rates of magnetic fluids are an important challenge in order to minimize dosages of magnetic fluids needed to reach therapeutic temperatures in magnetic fluid hyperthermia (MFH). Possible approaches to increase heating rates of super-paramagnetic particles for MFH would be to increase the anisotropy of the nanoparticles (shape or magnetocrystalline) or increasing the field strength used for treatment. This work focuses on high-quality, monodisperse, biocompatible iron oxide nano-particles of very high phase purity. Even though other high-quality nanoparticles with much higher anisotropies, such as Co and Co-based alloys are readily synthesized [1,2], biological constraints prevent the use of these and other materials with higher anisotropies because of their known toxicity. In addition, maximum frequency, f and field strength, H0 are also limited to biocompatible values (typically, their product f H0<5×109 A/m s).
An alternative approach to increase the heating rates would be to increase the monodispersity of a sample of magnetite nanoparticles. In fact, for monodisperse samples it has been predicted that there would be an optimum particle size which would yield the highest heating rate for a given set of measurement conditions (frequency, field amplitude, sample viscosity, temperature, coating, etc.). At this optimum size, very high heating rates are achievable [3]. Many of the nanoparticles currently studied are made with the co-precipitation method [4], which results in particles that can be polydisperse in size and shape and tend to agglomerate. Increased polydispersity will rapidly decrease the overall heating capability of a sample and negate the conditions where an optimum size can be found.
All real ferrofluid samples are polydisperse to some degree, therefore polydispersity of size, shape, crystallinity and surface coating needs to be taken into account for accurate modeling of the heating properties of the particles. In this work, we synthesize iron oxide nanoparticles by thermal decomposition of organometallic precursors. These particles have been extensively characterized and have been shown to have extreme uniformity in size, crystallinity and shape with control of particle size up to ~15 nm. Subsequently, the nanoparticles have been transferred to the water phase with the biocompatible polymer, Pluronic F127. As crystallinity and shape are uniform for these samples, models need to only take into account polydispersity of particle size, calculated with the Chantrell method [5].
As it is technically difficult to measure heating rates as a function of frequency, we have performed calorimetric measurements at a constant frequency as a function of particle size for various field strengths.
2. Experimental details
2.1. Synthesis and characterization
Spherical iron oxide nanoparticles were synthesized in our labs with a protocol published elsewhere [6,7]. As-synthesized particles are not soluble in aqueous solutions, therefore they were coated with Pluronic F127 in order to transfer them from a non-polar organic solvent to the aqueous buffer, phosphate buffered saline [8]. Ferrofluids were concentrated by evaporating the solvent under a gentle argon stream.
A Phillips 420 transition electron microscope (TEM), operating at an accelerating voltage of 120 keV, was used to routinely characterize nanoparticles’ size and shape (Fig. 1). Their size and distribution were also routinely determined by dynamic light scattering. The 10 nm iron oxide particles were shown to be magnetite (Fe3O4) by comparing the ratios of L3 to L2 transitions in electron energy-loss spectroscopy [9]. Particle size and size distribution were determined magnetically by fits to room temperature magnetization curves, collected with a VSM, using the Chantrell method. To accommodate the possible variation in the phase of the iron oxide as a function of size, we conservatively assumed 75 emu/g at saturation in all calculations to determine the mass of the magnetic portion of the sample. Alternatively, iron concentrations of phase-transferred nanoparticles were confirmed with Jarell Ash 955 Inductively Coupled Plasma (ICP)–Atomic Emission Spectrophotometer. Mass of the iron oxide nanoparticles was determined assuming all the iron was in Fe3O4 phase resulting in good agreement with the values determined magnetically.
Fig. 1.
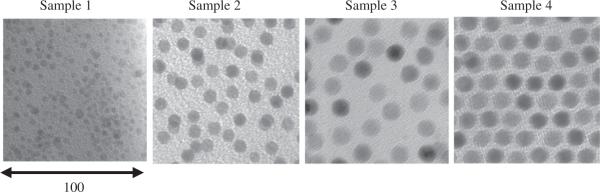
Transmission electron microscopy (Bright Field) images of the four samples used in the calorimetry measurements.
Table 1 lists the collected data for the nanoparticle samples. Note the difference between the particle size determined from TEM and from the Chantrell fitting. This difference may be due to the error in the assumption of 75 emu/g for these samples. Alternatively, this may indicate that there is a magnetic dead layer on the particles. Because of greater population sampling and the possibility of a magnetic dead layer, the values for sample diameter, polydispersity (σ) and concentration (m%) were determined through magnetic measurements.
Table 1.
Diameter measured with TEM, Chantrell method, polydispersity determined from Chantrell method, concentrations during calorimetry measurement, initial susceptibility and SLP measured at H0 = 24.5 kA/m for ferrofluid samples.
| Sample | DTEM (nm) | DChantrell (nm) | σ | Concentration (m%) | χ 0 | SLP24.5kA/m (W/g) |
|---|---|---|---|---|---|---|
| 1 | 5 | 4.6 | 0.075 | 0.202 | 0.93 | 180 |
| 2 | 10 | 8.5 | 0.15 | 0.283 | 4.96 | 130 |
| 3 | 14 | 11.2 | 0.21 | 0.0867 | 12.31 | 447 |
| 4 | 12.8 | 9.6 | 0.22 | 0.252 | 7.98 | 200 |
2.2. Calorimetric measurements
Calorimetric measurements were made on a modified induction heater with a 3 turn, water-cooled copper pipe. Calorimetric measurements were performed at 400 kHz with various ac-field amplitudes (12.4, 16.3, 21.9 and 24.5 kA/m). Approximately 0.5 mL of ferrofluid was used per measurement and placed in an insulated Falcon tube. Temperature was monitored with a Cu–Cu/Ni thermocouple with an ice bath reference. Experiments were run for 300 s with 1 s intervals. The first ~60 s were run before the field was turned on in order to collect the background temperature.
The temperature of the ferrofluid sample was measured as a function of time and the specific loss power (SLP) was calculated as
| (1) |
where c is the heat capacity of water, msample is the mass of the sample, and mironoxide is the mass of the iron oxide in the sample measured magnetically and dT/dt is the slope of the heating curve.
3. Results and discussion
3.1. Power dissipation for polydispersions
The specific loss power for a monodisperse sample of super-paramagnetic particles can be written as
| (2) |
where χ0 is the initial dc susceptibility, H0 is the field amplitude, f is the frequency of the measurement, and τ is the relaxation time. The relaxation time is a weighted average (1/τ = 1/τB+1/τN) between the Brownian, τB, and Néel, τN, relaxations which are defined as
| (3) |
and
| (4) |
where η is the viscosity of the matrix fluid, kB the Boltzmann constant, T the absolute temperature (K), VH the hydrodymanic volume of the particle which includes any non-magnetic layer, V the magnetic volume and τ0 the attempt time here assumed to be 10−9 s. However, for real ferrofluids polydispersity must be taken into account. Polydispersity of particle size can be modeled with a log-normal distribution:
| (5) |
where ln R0 is the median and σ the standard deviation of ln R. Now the volumetric heat release rate of a polydispersion is
| (6) |
3.2. Calorimetric results
The SLP measured for the samples at varying ac-field amplitudes are shown in Fig. 2. The data fit well to the square law as expected, indicating the quality of the measurements.
Fig. 2.
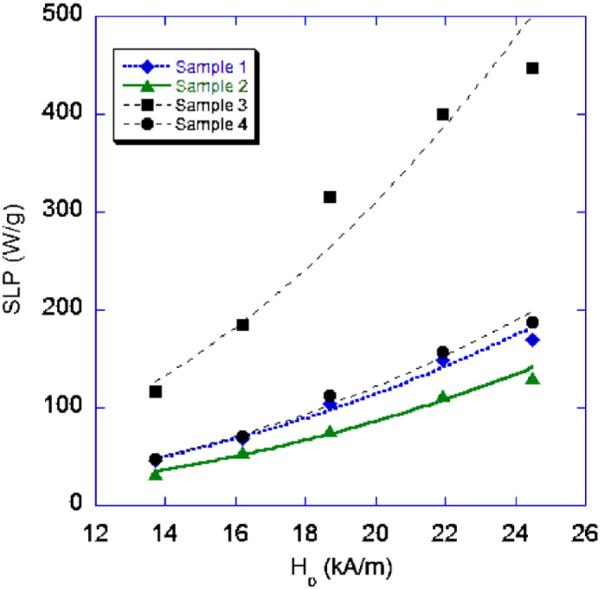
Specific loss power vs. ac-field amplitude at a frequency of 400 kHz.
Plots of the SLP for the samples as a function of size as well as calculated values with various σ values measured for the samples are shown in Fig. 3. Calculations were performed using parameters for a salt water solution with a specific heat of 4.19 J/g K, mass density 1.027 g/m3 and viscosity 0.0010 kg/m s. The hydrodynamic volume of the particle was calculated to include an approximate thickness of 12 nm accounting for the surfactant and Pluronic coating. Magnetic field conditions were set at f = 400 kHz and H0 = 24.5 kA/m The figure shows that as polydispersity increases, SLP decreases very rapidly. Additionally, there is a narrow size range which yields extremely high heating rates with a peak, for these specific experimental conditions, for particles with diameters ~12.5 nm.
Fig. 3.
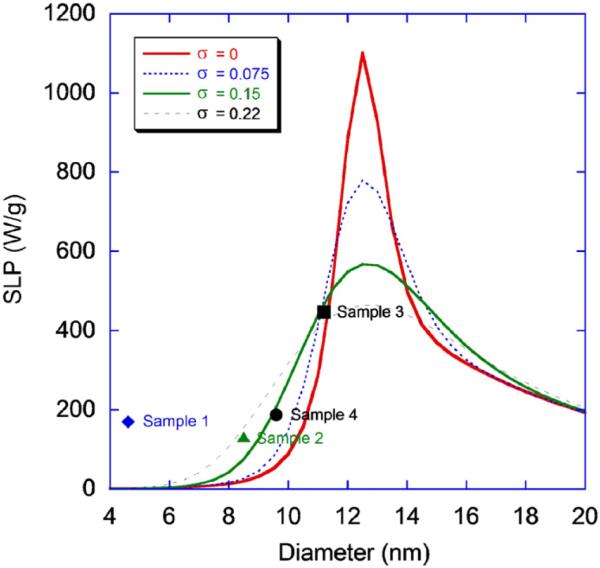
SLP as a function of particle size for H0 = 24.5 kA/m. Plots are calculated for various polydispersity indexes. SLP of samples 1–4 are plotted for comparison with theoretical values.
The data shows that the heating rate is dependent on particle size, although sample 1 has a heating rate much higher than expected. Results also indicate a broadening of SLP with sample polydispersity as predicted [3]. This is the first time a size-dependant effect has been demonstrated for nanoparticles made from a single synthesis method and uniform shape. It is possible that higher heating rates are achievable by increasing the magnetic core of the particle to approximately 12.5 and further decreasing the polydispersity of the sample. However, in practice, this is very challenging as the chemical synthesis of iron oxide involves a delayed nucleation that rapidly increases the polydispersity of samples above the maximum size measured. Lastly, the heating rates are comparable, if not better than some of the best commercial particles available today [10].
4. Summary and conclusions
Increasing heating rates of ferrofluids for MFH is necessary for successful implementation of the technique and minimize dosage for use in clinical settings. In this work, we show that substantially higher heating rates are achievable with iron oxides by decreasing polydispersity of the ferrofluid and by optimizing the size of the nanoparticles for a given set of conditions, most specifically the frequency of the ac-field. We have performed calorimetric measurements at a constant frequency of 400 kHz with various ac-field amplitudes. Measurements were performed on highly crystalline and uniform samples of various sizes. This work demonstrates that SLP does indeed vary with particle size. Highest SLP measured was 447 W/g at 24.5 kA/m for 11.2 nm particles and models indicate that higher heating rates are possible by increasing the size of the particles to ~12.5 nm.
Acknowledgements
This work was supported by NSF/DMR 050142 and the National Physical Science Consortium.
References
- 1.Bao Y, Krishnan KM. J. Magn. Magn. Mater. 2005;293:15. [Google Scholar]
- 2.Krishnan KM, Pakhomov AB, Bao Y, Blomqvist P, Chun Y, Gonzales M, Griffin K, Ji X, Roberts BK. J. Mater. Sci. 2006;41:793. [Google Scholar]
- 3.Rosensweig RE. J. Magn. Magn. Mater. 2002;252:370. [Google Scholar]
- 4.Nedkov I, Merodiiska T, Slavov L, Vandenberghe RE, Kusano Y, Takada J. J. Magn. Magn. Mater. 2006;300:358. [Google Scholar]
- 5.Chantrell RW, Popplewell J, Charles SW. IEEE Transactions on Magnetics. 1978;14:975. [Google Scholar]
- 6.Gonzales M, Krishnan Kannan M. J. Magn. Magn. Mater. 2005;293:265. [Google Scholar]
- 7.Hyeon T, Lee SS, Park J, Chung Y, Na HB. J. Am. Chem. Soc. 2001;123:12798. doi: 10.1021/ja016812s. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales M, Krishnan Kannan M. J. Magn. Magn. Mater. 2007;311:59. [Google Scholar]
- 9.Gonzales M, Calderon H. Kannan M. Krishnan. 2005 unpublished. [Google Scholar]
- 10.Hergt R, et al. J. Magn. Magn. Mater. 2004;280:358. [Google Scholar]


