Abstract
Multiple lines of evidence link the incidence of diabetes to the development of Alzheimer’s disease (AD). Patients with diabetes have a 50 to 75% increased risk of developing AD. Cyclin dependent kinase 5 (Cdk5) is a serine/threonine protein kinase, which forms active complexes with p35 or p39, found principally in neurons and in pancreatic β cells. Recent studies suggest that Cdk5 hyperactivity is a possible link between neuropathology seen in AD and diabetes. Previously, we identified P5, a truncated 24-aa peptide derived from the Cdk5 activator p35, later modified as TFP5, so as to penetrate the blood-brain barrier after intraperitoneal injections in AD model mice. This treatment inhibited abnormal Cdk5 hyperactivity and significantly rescued AD pathology in these mice. The present study explores the potential of TFP5 peptide to rescue high glucose (HG)-mediated toxicity in rat embryonic cortical neurons. HG exposure leads to Cdk5- p25 hyperactivity and oxidative stress marked by increased reactive oxygen species production, and decreased glutathione levels and superoxide dismutase activity. It also induces hyperphosphorylation of tau, neuroinflammation as evident from the increased expression of inflammatory cytokines like TNF-α, IL-1β, and IL-6, and apoptosis. Pretreatment of cortical neurons with TFP5 before HG exposure inhibited Cdk5-p25 hyperactivity and significantly attenuated oxidative stress by decreasing reactive oxygen species levels, while increasing superoxide dismutase activity and glutathione. Tau hyperphosphorylation, inflammation, and apoptosis induced by HG were also considerably reduced by pretreatment with TFP5. These results suggest that TFP5 peptide may be a novel candidate for type 2 diabetes therapy.
Keywords: Alzheimer’s disease, cyclin dependent kinase 5, diabetes, neuroinflammation, oxidative stress
INTRODUCTION
Alzheimer’s disease (AD) affects 4.5 million Americans and the incidence is expected to reach over 13 million by 2050 [1]. At the same time, over 20 million Americans have diabetes and this incidence is increasing by 5% per year [2]. Multiple studies report that patients with diabetes have a 50 to 75% increased risk of developing AD compared to age- and gender-matched control groups [3–7]. In parallel, a recent study of the Mayo Clinic AD Patient Registry reveals that 80% of AD patients have either type 2 diabetes (T2DM) or impaired fasting glucose [8]. The main physiological link between AD and T2DM is that both conditions are associated with peripheral and central insulin signaling abnormalities [9].
The relationship between insulin and the levels of interstitial and cellular glucose differs markedly in muscles and neurons. Neuronal physiology cannot accommodate episodic glucose uptake under the influence of insulin. Hyperglycemia in diabetes causes up to a fourfold increase in neuronal glucose levels. Diabetic neuropathy is a serious consequence of long-term intracellular glucose metabolism that leads to neuronal damage [10]. One mechanism of glucose neurotoxicity in diabetic patients is oxidative stress, promoted by glucose through a combination of free radical generation and impaired free radical scavenging in mitochondria [11, 12]. Hyperglycemia, in animals and in vitro models of diabetes, induces elevated oxidative stress; it increases superoxide and results in reduced levels of key antioxidative enzymes [13].
Previously we reported that Cdk5 regulates insulin secretion in glucose stimulated pancreatic β cells [14]. Cells overexpressing p35, treated with high glucose (HG), showed induction of p25, the p35-derived truncated fragment which hyperactivates Cdk5 in neurons. As a result, insulin secretion was inhibited and cells became apoptotic. Roscovitine treatment or co-infection with dominant negative Cdk5 increased insulin secretion and inhibited apoptosis [14]. It is note-worthy that deregulation and hyperactivation of Cdk5 has been suggested as contributing to the pathology seen in AD in a manner similar to that seen above. In addition to amyloid-β and neurofibrillary tangles (the hallmarks of AD pathology), it has been reported that AD brains have increased Cdk5 activity due to higher levels of p25 [15]. Factors inducing oxidative stress in neurons result in increased intracellular calcium and calpain activation which, in turn, cleaves p35 into p25 and p10 fragments [16]. Cdk5- p25 forms a more stable and hyperactive complex, causing aberrant phosphorylation of cytoskeletal components like tau and neurofilaments, and induces cell death. Here we have shown that AD and T2DM may share a common pathway to pathology, i.e., hyperactivation of Cdk5/p25. If so, do they share responses to a common therapeutic approach?
Because of its contribution to tau pathology, Cdk5- p25 has been identified as a prime therapeutic target for AD [16]. Accordingly, compounds like aminothizole, resembling roscovitine, a kinase inhibitor targeting ATP binding sites in Cdk5 and other kinases, have been studied as potential therapeutic agents [17]. These compounds, however, lack specificity; although inhibiting Cdk5-p25 hyperactivity, they also inhibit Cdk5-p35 and other cyclin-dependent kinases, which lead to serious toxic side effects. Our approach to this problem, based on structure and kinetics of the Cdk5-p25 complex, resulted in the production of several small truncated peptides of p35, which competed with p25 binding and inhibited Cdk5 hyperactivation in vitro [18–20]. A small peptide, P5, comprising 24 aa, specifically inhibited Cdk5-p25 activity in cultured cortical neurons without affecting the normal endogenous Cdk5/p35 activity, nor the activity of several cell cycle kinases [19, 20]. It also reduced hyperphosphorylated tau and protected cortical neurons from apoptosis. The P5 peptide, modified as TFP5 with an 11-aa peptide derived from transactivator of transcription (Tat) protein was conjugated at the C terminus, and fluoresceinisothiocyanate (FITC; a green fluorescent tag) with a linker (GGG) was attached at the N terminus to facilitate passage through the blood-brain barrier, when injected intraperitoneally in AD model mice, significantly reduced Cdk5-p25 hyperactivity, hyperphosphorylated tau, and rescued behavior deficits of spatial working memory and motor deficits [21]. Based on these encouraging results, we decided to check the efficacy of the TFP5 peptide against HG-mediated toxicity in cortical neurons where hyperactivated Cdk5 is induced.
MATERIALS AND METHODS
Materials
p35 (C-19) polyclonal antibody, Cdk5 (C-8) polyclonal antibody, and Cdk5 (J-3) monoclonal antibody were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), all used at 1:300–500 dilutions. Phospho-Tau-Ser199/Ser202, PHF1, Tau1, and Tau5 monoclonal antibodies were obtained from Biosource International Inc. (Camarillo, CA) and used at 1:1000 and 1:500 dilutions. AT-8 and AT180 monoclonal antibodies were purchased from Thermo Scientific (Rockford, IL) and used at 1:500. Phospho-Tau-Ser422 was purchased from GenScript (Piscataway, NJ) and used at 1:1000. Antibodies to caspase-3 and cleaved caspase-3 were obtained from Cell Signaling Technologies (Beverly, MA). Cdk5 inhibitor roscovitine was obtained from Biomol Research Laboratories, Inc. (Plymouth, PA). TFP5, p5 conjugated with TAT peptide, and scrambled TFP5 peptide (SCB) were synthesized by peptide 2.0 (VA, USA). Sequences used were as follows: TFP5, FITCGGGKEAFWDR-CLSVINLMSSKMLQINAYARAARRAARR; Scb peptide, FITCGGGGGGFWDRCLSGKGKMSSKG GGINAYARAARRAARR; P5+TAT, KEAFWDR-CLSVINLMSSKMLQINAYARAARRAARR.
Cell culture
Primary neuron-glial cultures were prepared from E-18 rat fetuses as described previously with some modifications [22, 23]. Briefly, the whole cerebral cortex was dissected and minced in Earle’s balanced salt solution. Individual cells were first treated with papain (25 U/mL) and DNase1 (25 U/mL) in Earle’s Balanced Salt solution for 45 min at 37°C. After they were rinsed, the cells were dissociated mechanically in Earle’s balanced salt solution by using a Pasteur pipette. The cell suspension was laid on Earle’s balanced salt solution containing 4% BSA and trypsin centrifuged at 1,000 rpm for 5 min. The pellet was resuspended in Neurobasal-A medium with B-27 supplement, 0.25 mmol/L Glutamax-1, 40 U/mL penicillin, 40 mg/mL streptomycin (Neurobasal-A/B-27), and 10% dialyzed horse serum. Cells were plated on polylysine-coated 96-well plates or 12-well chamber slides at 3 × 104 cells per well. Cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. Half of the medium was replaced with Neurobasal-A/B-27 and 5% dialyzed horse serum twice a week. After 7 days of plating, the composition of major cell types was estimated by counting cells immunostained with antibodies against cell-type-specific markers. Neurons were stained with an antibody against MAP-2. Astrocytes were stained with an antibody against GFAP. Microglia were stained by using Mac-1 antibodies.
Primary cultures of rat embryonic cortical neurons were prepared from E-18 rat fetuses as described previously [22, 23]. Neuron-enriched cultures were prepared by growing dissociated cells in Neurobasal A/B-27 without serum. The density of plated cells in neuron-enriched cultures was not different from that of neuron/glial mixed cultures. For glial-enriched cultures, dissociated cells were grown in DMEM containing10% heat-inactivated horse serum. After 7 days, neurons were killed by exposure to 1 mmol/L glutamate, and the medium was changed. Glial enrichment was verified by immunostain for GFAP and MAP-2. The treatment protocol for most experiments was as follows: after 7 days in culture (DIC) neurons were pretreated with TFP5 (100 nM) or scrambled peptide for 12 h. Pretreated cultures were then exposed to different concentrations of glucose [3mM (LG) and 30mM (HG)]. The cells were fixed for immunocytochemistry analyses or lysed with lysis buffer immunoprecipitation and western blot analyses.
Western blot analysis
Western blot analysis was performed as described previously [20]. In brief, an equal amount of total protein (20µg/lane) was resolved on a 4–20% SDS-polyacrylamide gel and blotted onto a PVDF membrane. This membrane was incubated in blocking buffer containing 20mM Tris-HCI (pH 7.4), 150mM NaCl, and 0.1% (v/v) Tween 20 (TTBS) plus 5% dry milk (w/v) for 1 h at room temperature followed by incubation overnight at 4°C in primary antibodies. The membranes were then washed four times in TTBS (5 min/each), followed by incubation in secondary antibody (goat anti-mouse or goat anti-rabbit IgG (HL)- HRP conjugate at a dilution of 1:3,000) for 1 h at room temperature. Western blots were analyzed using the Amersham Biosciences ECL kit following the manufacturer’s instructions (GE Healthcare).
Immunoprecipitation and kinase assays
Kinase assays were performed as described previously with modification [21]. Briefly, 7 DIC primary rat cortical neurons, pretreated with TFP5 for 12 h, were incubated in LG or HG at different times, then lysed. Cdk5 was immunoprecipitated with the polyclonal C8 antibody for 2 h at 4°C, and immunoglobulin was isolated using Protein A-Sepharose beads for 2 h at 4°C. Immunoprecipitates were washed three times with lysis buffer and then once with 1X kinase buffer containing 20mMTris-Cl, pH 7.4, 1mMEDTA, 1mM EGTA, 10mM MgCl2, 10mM sodium fluoride, and 1mMsodium orthovanadate. The samples were added to the reaction mix containing kinase buffer, 50 µM ATP, 20 µg of histone H1, and 0.5 µCi of 32P-ATP and incubated at 30°C for one hour. Reactions were halted by the addition of loading buffer, and samples were then electrophoresed on 12% SDS-PAGE gels. Histone bands were visualized by Coomassie blue staining, and gels were dried, then autoradiograph scanned on a Phosphor imager. Radioactive band density was analyzed using Image J software, and statistical analysis was performed. In pad assays, 25 µl aliquots of the incubation mixture were placed on a What man p81 paper square, air-dried, and washed five times for 15 min each in 75mM phosphoric acid and once in 95% ethanol. After air drying, squares were transferred to vials containing Bio-Safe II scintillation fluid for counting.
Immunocytochemistry
Immunocytochemistry analysis was performed as described previously [22]. Briefly, cells were fixed on coverslips for 30 min at room temperature in 4% paraformaldehyde in PBS and then permeabilized and blocked in 5% FBS with 0.1% Triton X-100 in 1X PBS for 1 h. The cover slips were incubated overnight at 4°C with primary antibodies diluted in blocking buffer. After a wash in PBS (three times, 15 min each), the cover slips were incubated with Texas Red goat anti-rabbit IgG secondary antibody for 1 h at room temperature. This was followed by washing three times with PBS and mounting in an aqueous medium. Fluorescent images were obtained with a Zeiss LSM-510 laser scanning confocal microscope. Images were processed and merged by Adobe Photoshop software.
Reactive oxygen species (ROS) measurement
In the presence of ROS, intracellular esterases cleave acetate groups from CMH2DCFDA, trapping nonfluorescent 2,7-dichlorofluorescein (DCF) in the neuron. Subsequent oxidation yields fluorescent DCF, which detects transient rises in ROS, including H2O2 and, in particular, peroxynitrites. To confirm that DCF was able to detect increased oxidative stress within this culture system, cortical neurons in control medium were treated with 1.0mM H2O2. Fluorescence was measured at 480 nm/530nm at each time period. Having established that DCF accurately and sensitively measures ROS generation, ROS activity for these cells was quantified using an Oxidelect ROS assay kit (Cell Biolabs, San Diego, CA). Briefly, cortical neurons were cultured in 96-well plates.for 7 days. Cells were then pretreated with p5 conjugated with TAT for 12 h (here we used p5 peptide tagged with TAT and no FITC labeling), then treated with DCFH-DA in the presence of different concentrations of glucose in culture medium at 37°C for different times. Cells were washed twice with PBS buffer. The reactions were terminated with the addition of 100 µL of 2X cell lysis buffer. Fluorescence was measured at 480 nm/530 nm. DCFH-DA-treated cells incubated with or without H2O2 were used as positive controls.
Total glutathione and superoxide dismutase (SOD) activity
Seven DIC cortical neurons were pretreated with TFP5 for 12 h, then incubated in different concentration of glucose and assayed for total glutathione and SOD activity using an OxiSelect™assays kit (STA 341, STA 340, CellBiolabs, San Diego, CA).
Cytokine assays: TNF-α, IL-1β, and IL-6
The concentration of TNF-α, IL-1β, and IL-6 in the mixed culture medium was measured with ELISA kit (Thermoscientific), according to the manufacturer’s instructions. Absolute concentrations were derived by comparison with a standard curve.
Statistical analysis
Data were expressed as the mean ± standard error (S.E.) of the means. For a statistical analysis of the data, group means were compared by one-way analysis of variance (ANOVA) with post hoc analysis. The Tukey–Karmer test post hoc was applied to identify significance among groups. p < 0.05 was considered to be statistically significant. GraphPad software, Inc. (version 3.06) was used for statistical analysis.
RESULTS
High glucose induces p25 expression and hyperactivates Cdk5 in cortical neurons, a time course study
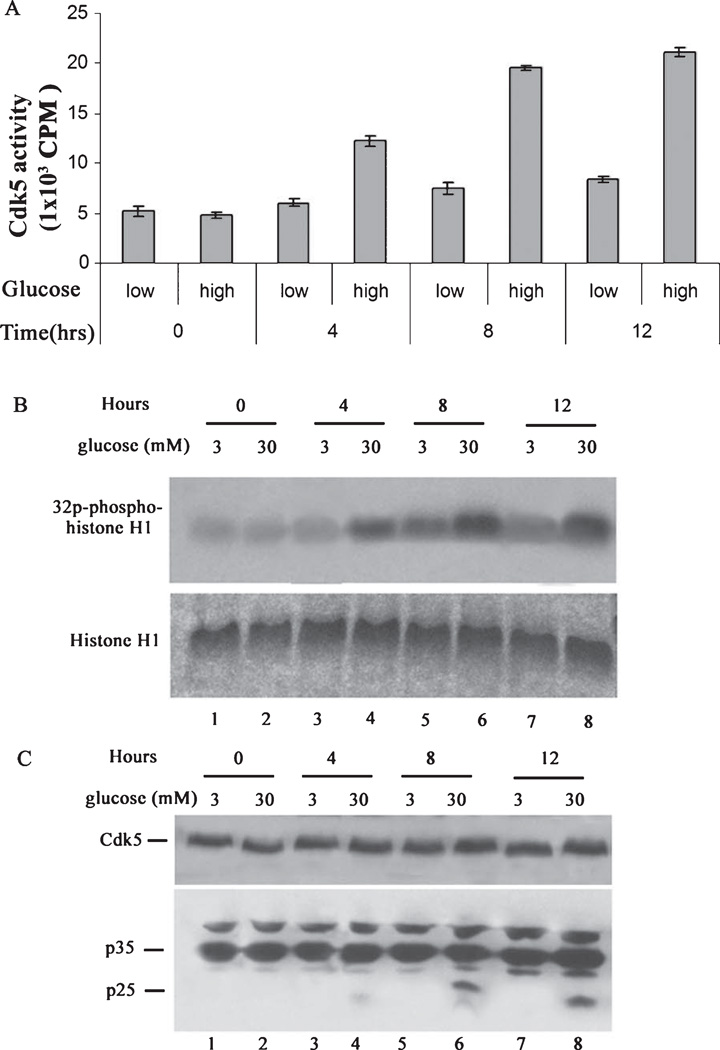
In a recent study, AD-type CNS neuropathology was instigated in rabbit by alloxan-induced diabetes model [24], a compelling result linking AD and diabetes. Accordingly in our study, we first determined whether HG treatment induces Cdk5 hyperactivation in cortical neurons. To test this hypothesis, 7 DIC cortical neurons were treated with high (30 mM, HG) and low (3 mM, LG) concentrations of glucose andCdk5 activity and expression were assayed. A time dependent increase in the activity of Cdk5 was observed in the presence of HG conditions for the period of 12 h. After 4 h, Cdk5 activity increased reaching a maximum at 8 h which persisted to 12 h (Fig. 1A, B). Glucose treatment does not affect the expression of the Cdk5 but it induces increased Cdk5 activity (Fig. 1C). Though P35 expression increased slightly over time, p25 expression was most evident at 8–12 h (Fig. 1D). We suggest that the hyperactivity of the Cdk5 at 8 h is due to the generation of p25. Based upon this result, the 8 h time point was selected for further study as representative of hyperglycemic conditions.
Fig. 1.
High glucose induces p25 expression and overactivates Cdk5 activity in cortical neurons, a time course study. Primary cortical neurons were treated with low (3 mM) and high (30 mM) glucose for various times. Cells were lysed and Cdk5 was immunoprecipitated using C-8 antibody from equal amounts of lysates. Immunoprecipitates were then subjected to kinase assay with histone H1 as substrate. The activity was quantified from three separate experiments as summarized in the bar graphs (A). Samples of the reactions were electrophoresed and prepared as autoradiographs as shown in (B). In separate experiments, cortical neurons were treated as above followed by SDS-PAGE and western analysis with the Cdk5 and p35 antibodies (C).
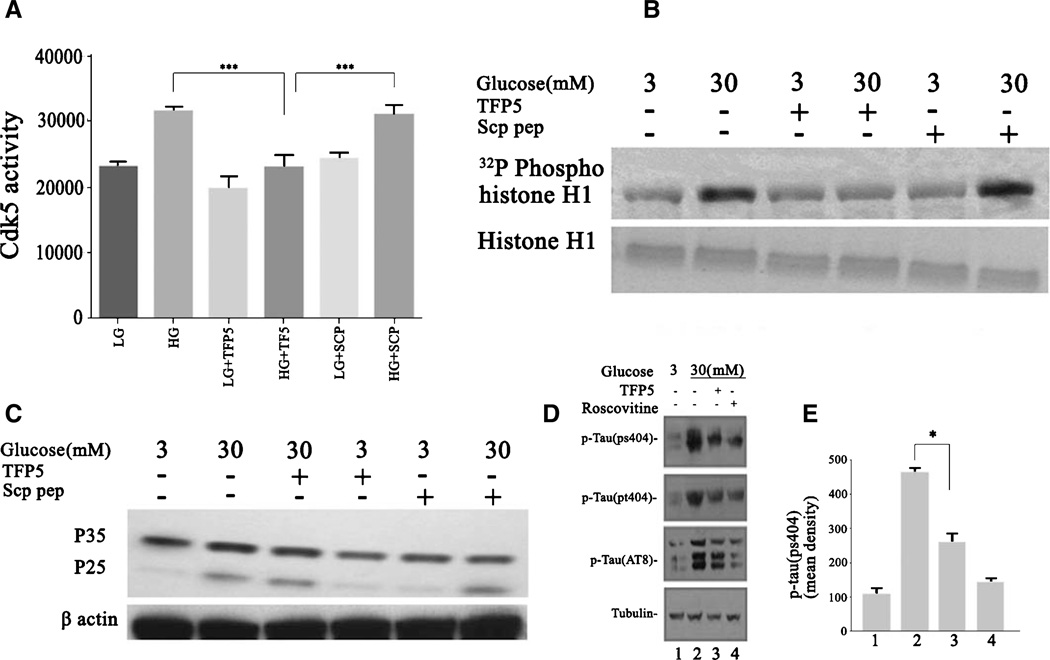
TFP5 inhibits HG-induced Cdk5-p25 hyperactivity and tau hyperphosphorylation
Since HG induces hyperactivation of Cdk5 and increased expression of the p25 in cortical neurons, does pretreatment with TFP5 reduces these effects in these cells? Neurons pretreated for 12 h with TFP5 (100 nM) were subjected to HG for 8 h, lysed, and prepared for Cdk5 immunoprecipitation and kinase assay. The data are shown in Fig. 2A and B. HG treatment causes increases in endogenous Cdk5 activity as visualized in the radioautograph by phospho-histone H1 and in the pad assay. Pretreatment with TFP5 dramatically reduced Cdk5 activity in the HG-treated neurons to basal levels. The scrambled peptide, however, had no effect on the Cdk5 activity. Western blot assays of whole cell lysates from similarly treated cortical neurons exposed with HG revealed an upregulation of the expression of p25 (Fig. 2C). The increased p25 expression was correlated with an increased level of endogenous tau phosphorylation at residues Ser202 and Thr205 (Fig. 2D, E). Pretreatment with TFP5 inhibited the HG-mediated tau hyperphosphorylation, restoring it to basal levels (Fig. 2D). In summary, HG stimulation cause hyperphosphorylation of tau in cortical neurons and pretreatment with TFP5 effectively inhibits Cdk5 phosphorylation of tau sites.
Fig. 2.
Cdk5 hyperactivity and tau hyperphosphorylation are inhibited by TFP5 pretreatment in cortical neurons exposed to HG for 8 h. Primary cortical neurons were pretreated with/without TFP5 (100 nM) or scrambled peptide for 12 h then incubated in low and high glucose, respectively for 8 h. Cdk5 was immunoprecipitated using C-8 antibody from equal amounts of lysates. Immunoprecipitates were then subjected to in vitro kinase assays with histone H1 as substrate. Activity, as counts/min, was quantified from three separate experiments and summarized in the bar graphs (A) (***p < 0.001), while SDS-PAGE and autoradiographs were prepared as shown in (B). C) Cortical neurons were treated as in (A) after which SDS-PAGE and western blots were prepared with the p35 antibody. Note p25 expression in all high glucose lanes. D) Cortical neurons pretreated with/without TFP5 for 12 h then with low and high glucose for 8 h. Lanes 1–3; roscovitine in lane 4 as a control SDS-PAGE and western blots prepared with phospho-tau antibodies (anti-pS404, anti-Ps205, and AT8). E) The bar graphs show the mean optical density of phospho-tau (ps404). The results are expressed as mean ± S.E.M. of three independent experiments (*p < 0.01).
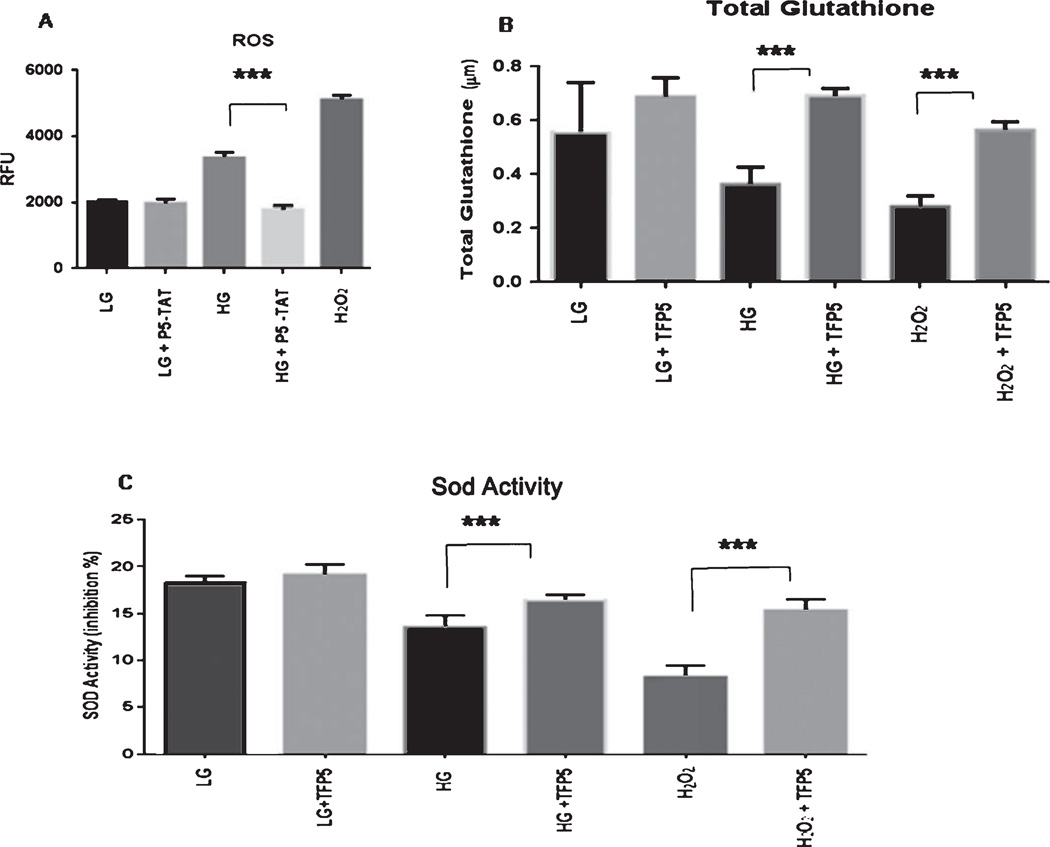
TFP5 pretreatment reduces oxidative stress induced by high glucose
To study the mechanism underlying the neuroprotective effects of TFP5 against HG-induced oxidative stress, we measured the production of ROS, in the presence of HG (30 mM) for 8 h with or without TFP5 (here we used p5 peptide that contains only p5 conjugated to TAT peptide without the FITC tag). DCF levels were measured in neurons exposed to different conditions. In the presence of HG, there was a significant increase in the mean level of DCF compared with the corresponding low glucose (Fig. 3A), indicating increased production of ROS. P5 pretreated neurons exposed to HG showed a significant decrease of ROS. Here, we used H2O2 as a positive control (Fig. 3A). We further examined the levels of antioxidant in the presence of HG in cortical neurons. We compared the antioxidant enzyme (SOD activity) and total glutathione levels in the TFP5 treated group with the corresponding control groups. SOD activity decreased significantly after 8 h in HG-treated cells whereas, SOD activity was virtually restored after TFP5 pretreatment (p < 0.001, Fig. 3B). At the same time, the decreased total glutathione content was significantly restored after TFP5 pretreatment (Fig. 3C).
Fig. 3.
Pre-treatment with TFP5 rescues glucotoxic cortical neurons from oxidative stress due to increased reactive oxygen species (ROS) production and decreased antioxidant capacity. A). Cortical neurons were pretreated with p5-TAT (instead of fluorescent TFP5) for 12 h then incubated with DCFH-DA in the presence of different concentrations of glucose in culture medium for 8 h. DCF fluorescence was measured at 480 nm/530 nm using an OxiSelect™ assay kit for ROS. The results are expressed as mean ± S.E.M. of four independent experiments, ***p < 0.001. B, C) Primary cortical neurons were pretreated with/without TFP5 for 12 h then exposed to low (LG) and high glucose (HG) for 8 h before assaying for total glutathione and superoxide dismutase (SOD) activity measured using an OxiSelect™ assays kit. H2O2 used as positive control. The results are expressed as mean ± S.E.M. of four independent experiments (***p < 0.001).
These results showed that TFP5 pretreatment increased the defenses against oxidative stress by rescuing SOD activity and total glutathione levels which succeed in reducing ROS production.
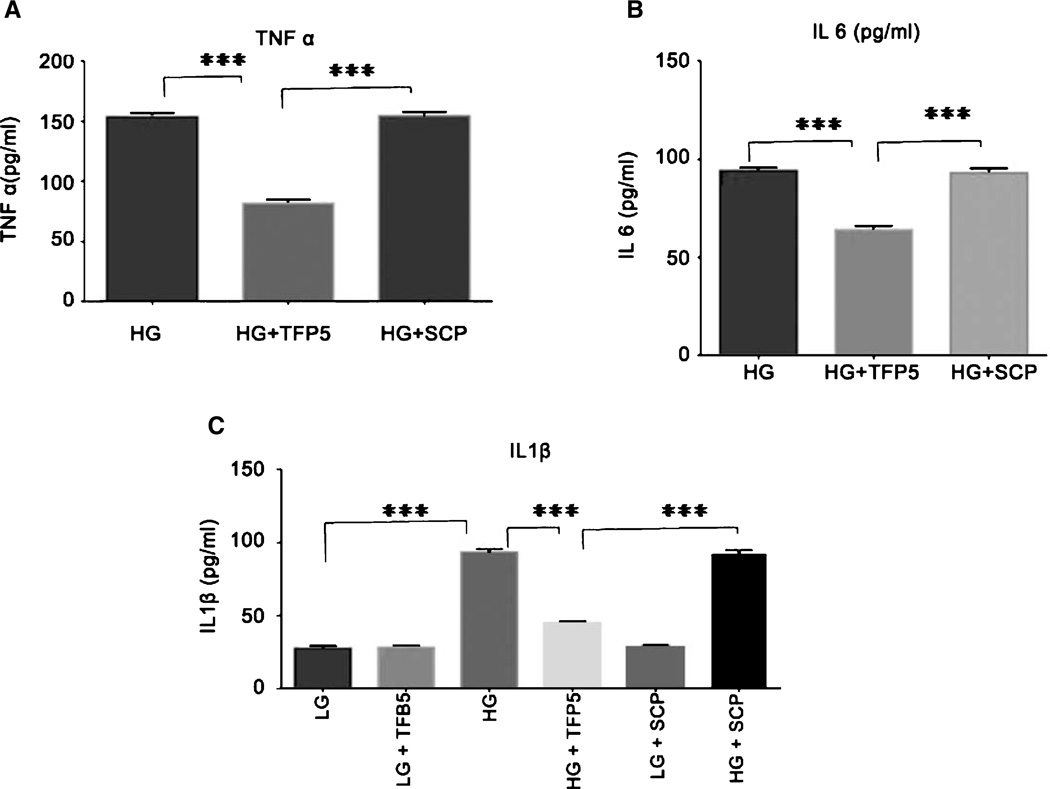
TFP5 pretreatment rescues inflammation caused by high glucose
Using the ELSIA kit we measured the concentrations of TNF-α, IL-1β, and IL-6 in supernatants from glial enriched cultures. In control cultures, TNF-α and IL-6 concentrations were below the detection limit of the assay. HG incubation for 8 h resulted in elevated TNF-α, IL-1β, and IL-6 concentrations that were attenuated by pretreatment with TFP5 (Fig. 4A–C, p < 0.05). The scrambled peptide had no effect on increased levels of inflammatory cytokines due to HG. This result shows that TFP5 inhibited neuronal inflammation in glucotoxic neurons.
Fig. 4.
TFP5 reduces increased production of inflammatory cytokines induced by high glucose (HG) in cultures of primary cortical neurons; scrambled peptide has no effect. A) Increased levels of TNF-α induced by HG in primary cortical neurons for 8 h are reduced by 12 h pretreatment with TFP5. B) Effect of TFP5 on production of IL-6. C) Effect of TFP5 on production of IL-1β. The results are expressed as mean ± S.E.M. of three independent experiments (***p < 0.001). The level of TNF-α and IL-6 were not detected at low glucose (LG, 3mM) concentration.
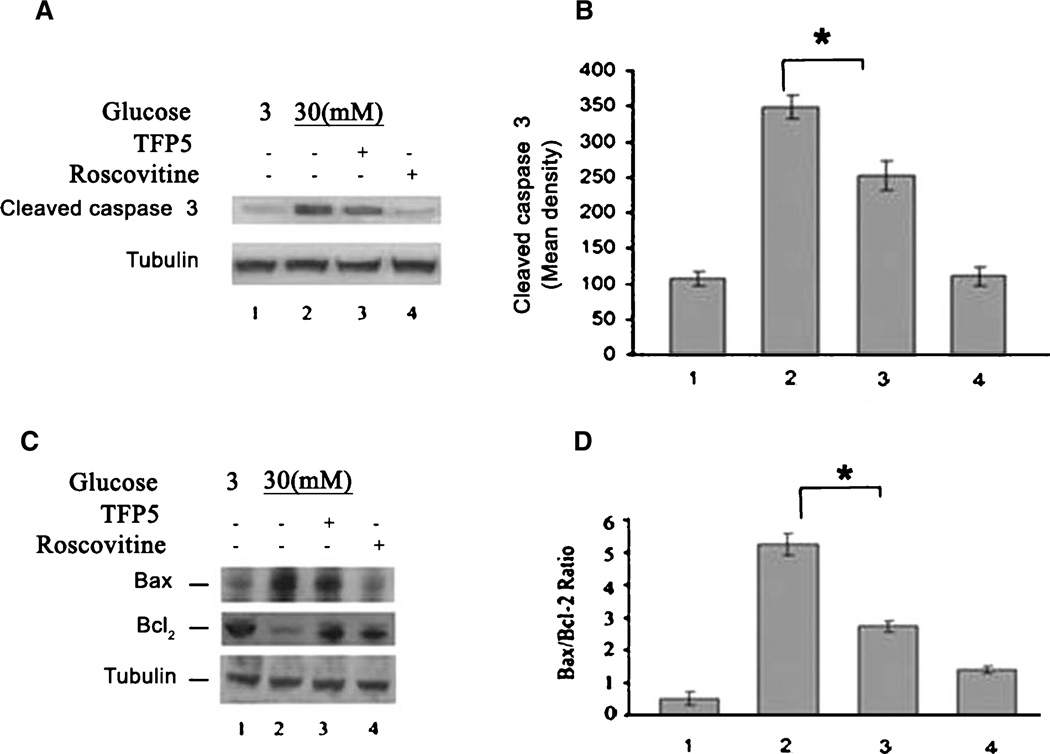
TFP5 attenuates glucotoxic-induced neuronal apoptosis
Cortical neurons exposed to glucotoxic conditions undergo extensive cell death. Accordingly, we determined the effect of TFP5 on neuronal apoptosis caused by HG. Cortical neurons pre-treated with or without TFP5 were incubated with HG for 8 h, lysed and prepared for western blots, using cleaved caspase-3 antibody as an assay for apoptosis. As seen in Fig. 5A, HG caused an increase in cleaved caspase-3 levels (Fig. 5A, lane 2). This was prevented in neurons pretreated with TFP5 (Fig. 5A, B, lane 3). Roscovitine was used as a positive control. As an additional confirmation of the anti-apoptotic effects of TFP5, we measured the expression of Bax and Bcl2. As shown in Fig. 5C and D, the Bax protein expression increased with HG, while the Bcl-2 protein markedly decreased compared to the LG group. TFP5 pretreatment rescues these effects. The ratio of Bax to Bcl-2 in the HG group was significantly higher than that in the LG group (Fig. 5D, p < 0.01). When the cells were pretreated with TFP5, the ratio of Bax to Bcl-2 significantly decreased (Fig. 5D, p < 0.05).
Fig. 5.
TFP5 can ameliorate apoptosis induced by high glucose. A)Western blot of the expression of cleaved caspase 3 in cortical neurons treated with high and low glucose for 8 h with or without pretreatment with TFP5. B) Quantification of mean density from three separate experiments (*p < 0.01). C) Western blots of Bax and Bcl-2 expression in cortical neurons treated with high and low glucose for 8 h with or without TFP5 pre-treatment. D) Quantification of the ratios of mean densities of Bax/Bcl (*p < 0.01).
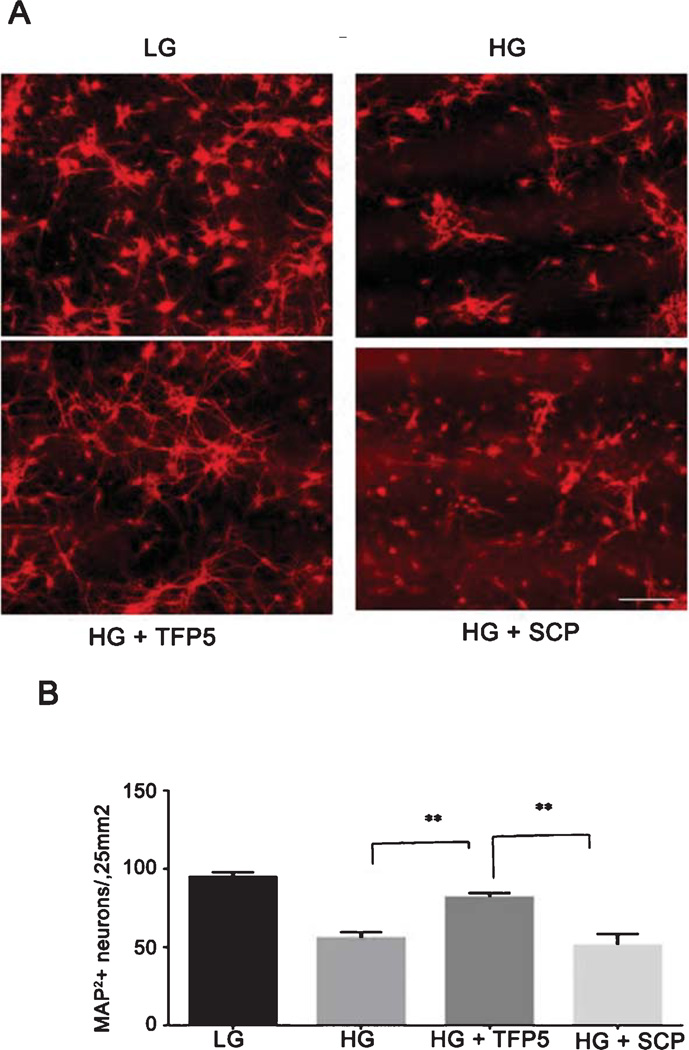
To confirm that apoptosis reflected a reduction in neuronal death, mixed cultures were immunostained with the neuronal marker MAP-2. After HG treatment, there was approximately a 50% reduction of neuritic networks and neuronal perikarya (Fig. 6A, compare LG with HG). TFP5 pretreatment prevented the loss of MAP-2-positive neurons in HG-treated cultures, whereas scrambled peptide had no effect. Counts of MAP-2-positive neurons showed that TFP5 prevented the loss of neurons in HG-treated cultures (Fig. 6B, p < 0.001), a finding consistent with the results obtained with the apoptotic marker.
Fig. 6.
TFP5 rescues glucotoxic neuronal cell death. A) Primary cortical neurons, pre-treated with or without TFP5 or scrambled peptide, were incubated in low (LG) and high glucose (HG) for 8 h then prepared for immunocytochemistry. MAP-2 antibody was used as a neuronal marker; Texas red was used as the secondary antibody. Pretreatment with TFP5 protected MAP-2–positive neurons against the HG-induced cell loss (Bar = 100 µm). B) Quantification of the results. Cell counts were performed as follows: 10 independent fields were analyzed using NIH Image J software. The bar graph shows the number of MAP-2 labeled neurons as mean ± S.E.M. from four separate experiments (**p < 0.01).
DISCUSSION
Stress-induced formation of a hyperactive Cdk5/p25 stable complex is one of several mechanisms of neuronal dysfunction and cell death which may link diabetes to AD [8–10, 24]. Accordingly, this enzyme complex has initially been identified as a target for AD therapy [15, 16]. Previously, we identified and characterized P5, a small peptide derived from p35, the Cdk5 activator, which specifically inhibited Cdk5/p25 hyperactivity at low doses in situ. It did not affect endogenous Cdk5-p35 essential for neuronal development and function, nor did it inhibit related Cdks in proliferating cells [18–20]. Hence, in a study of its efficacy as a therapeutic candidate for AD, we modified P5 as TFP5, with an FITC tag and a TAT peptide to penetrate the blood-brain barrier. TFP5, delivered as a series of intraperitoneal injections into an AD model mouse restored the normal behavioral and biological phenotype [21]. To link this study to diabetes, here, we successfully demonstrated that TFP5 rescued cortical neurons in vitro stressed by HG-induced toxicity.
Initially, we demonstrated that cortical neurons exposed to HG undergo a sustained elevation of Cdk5/p25 hyperactivity, mimicking similar effects of amyloid-β treatment in vitro [18]. The effect also shares some features with the glucotoxic phenotype of pancreatic β cells which shut down insulin secretion in response to hyperactive Cdk5 [14, 25]. HG also evokes oxidative stress in neurons (i.e., increased ROS, decreased total glutathione and SOD activity) as well as inflammation and cell death, phenotypes consistent with those reported for patients and experimental animals [2, 9–13]. We have also shown that TFP5 inhibition of Cdk5-p25 hyperactivity protects cortical neurons from glucose toxicity. Here, too, the rescue effects of TFP5 on HG-stressed neurons resemble those produced by related peptides on neurons expressing AD-like phenotypes [18–20]. Moreover, the control scrambled peptide, which does not affect Cdk5 activity, fails to rescue neurons from glucose toxicity. What is most striking is that the rescue from glucotoxicity shares the same features as the rescue of AD phenotypes in mutant mice, i.e., reduction of Cdk5 hyperactivity, tau hyperphosphorylation, neuroinflammation, and neuronal apoptosis. TFP5 rescues these abnormal phenotypes without affecting the normal Cdk5/p35 activity, nor related cell cycle Cdks. These findings suggest that hyperactivation of Cdk5- p25 is common to the T2D phenotype in β cells with the AD phenotype in neurons.
The relationship between Cdk5 hyperactivity, oxidative stress, and inflammation at the biochemical level is not well understood, neither in diabetes nor in AD. One commonly cited factor inducing pathology in neurons and pancreatic β cells is oxidative stress (e.g., production of ROS) that may induce Cdk5/p25 activation and inflammation [25–29]. In favor of this possibility is the demonstration that ROS-induced human neuroblastoma-derived cells results in an elevation of p35/Cdk5 activity [29]. Based on these findings, we suggest that Cdk5-p25 hyperactivation during HG exposure may be linked to the generation of ROS. In fact, it has been shown that HG stimulates ROS production and expression of proinflammatory cytokines in diabetic vascular, renal, and brain diseases [30–34]. A recent report indicates that patients with diabetes show inflammation in the CNS, early brain aging, and cognitive impairments [35]. Accordingly, HG has been recognized as a significant factor in CNS disorders.
The data reviewed above suggest that stress activation (ROS, glucotoxicity, or glutamate excitotoxicity) of Cdk5/p25 is an early stage in the cellular events leading to pathology and cell death. This seems to be the case in p25 transgenic mice which respond initially to p25 overexpression with Cdk5 hyperactivation, neuroinflammation (astrogliosis microgliosis), tau hyperphosphorylation, amyloidgenesis, and an AD behavioral phenotype [36, 37]. A novel biochemical pathway for cdk5/p25-induced neuroinflammation has been proposed to explain the results of p25 overexpression in these transgenic mice [37]. The key to this model is the activation of cytosolic phospholipaseA2 (cPLA2) by Cdk5/25 and the induction of lysophosphatidylcholine which, in turn, induces inflammation (i.e., cytokine release from astrocytes and microglia), cell death, and cognitive decline. A parallel pathway of Cdk5 hyperphosphorylation of tau and amyloidogenesis is coupled to the neuroinflammatory pathway. The model is supported by data showing the early induction of GFAP, a marker for astrogliosis followed by microglial-derived cytokines [37]. Application of this model to neuronal-induced glucotoxicity evokes, as a first step, oxidative stress via production of ROS and down regulated endogenous anti-oxidative mechanisms through the glycation of scavenging enzymes and depletion of antioxidants. The successful reversal of oxidative stress of HG-treated neurons by TFP5 is consistent with the model of a prior hyperactivation of Cdk5/p25. Moreover, the reduction of glucotoxic-induced neuronal apoptosis by TFP5 treatment also supports the suggestion that apoptosis reflects a hyperactive Cdk5/p25. TFP5 pretreatment significantly decreased caspase-3 expression and the ratio of Bax to Bcl-2 expression, both critical markers of increased apoptosis. The data suggest that TFP5, by virtue of its specific inhibition of glucotoxic-induced hyperactive Cdk5/p25, has a potent neuroprotective action. It may act therapeutically in diabetes as well as in those neurodegenerative disorders in which hyperactive Cdk5/p25 has been identified as a factor contributing to the pathology.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Programs of the NIH, National Institute of Neurological Disorders and Stroke.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=2002).
REFERENCES
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Feldman EL. Oxidative stress and diabetic neuropathy: A new understanding of an old problem. J Clin Invest. 2003;111:431–433. doi: 10.1172/JCI17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 4.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOEgene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, Kappelle LJ. Increased risk of Alzheimer’s disease in Type II diabetes: Insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans. 2005;33:1041–1044. doi: 10.1042/BST0331041. [DOI] [PubMed] [Google Scholar]
- 6.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28:71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 7.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ. The effects of type 1 diabetes on cognitive performance: A meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 8.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 13.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng YL, Hu YF, Zhang A, Wang W, Li B. Overexpression of p35 in Min6 pancreatic beta cells induces a stressed neuron-like apoptosis. J Neurol Sci. 2010;299:101–107. doi: 10.1016/j.jns.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 15.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 16.Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2004;10:452–458. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Meijer L, Kim SH. Chemical inhibitors of cyclin-dependent kinases. Methods Enzymol. 1997;283:113–128. doi: 10.1016/s0076-6879(97)83011-x. [DOI] [PubMed] [Google Scholar]
- 18.Zheng YL, Kesavapany S, Gravell M, Hamilton RS, Schubert M, Pant HC. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 2005;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng YL, Amin ND, Hu YF, Rudrabhatla P, Shukla V, Pant HC. A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J Biol Chem. 2010;285:34202–34212. doi: 10.1074/jbc.M110.134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng YL, Li BS, Amin ND, Albers W, Pant HC. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur J Biochem. 2002;269:4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
- 21.Shukla V, Zheng YL, Mishra SK, Amin ND, Steiner J, Pant HC. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. FASEB J. 2013;27:174–186. doi: 10.1096/fj.12-217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Pant HC. Mitogen-activated protein kinases (Erk1,2) phosphorylateLys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci. 1998;18:4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kesavapany S, Amin N, Zheng YL, Nijhara R, Jaffe H, Pant HC. p35/cyclin-dependent kinase 5 phosphorylation of ras guanine nucleotide releasing factor 2 (RasGRF2) mediates Rac-dependent extracellular signal-regulated kinase 1/2 activity, altering RasGRF2 and microtubule-associated protein 1b distribution in neurons. J Neurosci. 2004;24:4421–4431. doi: 10.1523/JNEUROSCI.0690-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitel CL, Kasinathan C, Kaswala RH, Klein WL, Frederikse PH. Amyloid-β and tau pathology of Alzheimer’s disease induced by diabetes in a rabbit animal model. J Alzheimers Dis. 2012;32:291–305. doi: 10.3233/JAD-2012-120571. [DOI] [PubMed] [Google Scholar]
- 25.Ubeda M, Kemp DM, Habener JF. Glucose-induced expression of the cyclin-dependent protein kinase 5 activator p35 involved in Alzheimer’s disease regulates insulin gene transcription in pancreatic beta-cells. Endocrinology. 2004;145:3023–3031. doi: 10.1210/en.2003-1522. [DOI] [PubMed] [Google Scholar]
- 26.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, Watada H, Leibiger IB, Yamasaki Y, Hori M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes. 2003;52:2896–2904. doi: 10.2337/diabetes.52.12.2896. [DOI] [PubMed] [Google Scholar]
- 28.Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005;280:11107–11113. doi: 10.1074/jbc.M410345200. [DOI] [PubMed] [Google Scholar]
- 29.Strocchi P, Pession A, Dozza B. Up-regulation of cDK5/p35 by oxidative stress in human neuroblastoma IMR-32 cells. J Cell Biochem. 2003;88:758–765. doi: 10.1002/jcb.10391. [DOI] [PubMed] [Google Scholar]
- 30.Bellenger J, Bellenger S, Bataille A, Massey KA, Nicolaou A. High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: Inflammatory pathway inhibition. Diabetes. 2011;60:1090–1099. doi: 10.2337/db10-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan Y, Jiang CT, Xue B, Zhu SG, Wang X. High glucose stimulates TNFα and MCP-1 expression in rat microglia via ROS and NF-κB pathways. Acta Pharmacol Sin. 2011;32:188–193. doi: 10.1038/aps.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Li G, Wang Z, Zhang X, Yao L. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience. 2012;202:58–68. doi: 10.1016/j.neuroscience.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 33.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Cui X, Zacharek A, Cui Y, Roberts C. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llewelyn JG. The diabetic neuropathies: Types, diagnosis and management. J Neurol Neurosurg Psychiatry. 2003;74:ii15–ii19. doi: 10.1136/jnnp.74.suppl_2.ii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muyllaert D, Terwel D, Kremer A, Sennvik K, Borghgraef P. Neurodegeneration and neuroinflammation in cdk5/p25-inducible mice: A model for hippocampal sclerosis and neocortical degeneration. Am J Pathol. 2008;172:470–485. doi: 10.2353/ajpath.2008.070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundaram JR, Chan ES, Poore CP, Pareek TK, Cheong WF, Kesavapany S. Cdk5/p25-induced cytosolic PLA2-mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci. 2012;32:1020–1034. doi: 10.1523/JNEUROSCI.5177-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]