Abstract
Objective
To investigate the association between otolith function and age-related gait impairment.
Study design
Cross-sectional analysis within the Baltimore Longitudinal Study of Aging (BLSA), a longitudinal prospective cohort study.
Setting
Vestibular testing and gait laboratory within an acute care teaching hospital.
Patients
Community-dwelling participants, who did not use assistive devices.
Intervention(s)
Cervical and ocular vestibular-evoked myogenic potentials (VEMPs) as measures of saccular and utricular function respectively, and gait speed as a measure of age-related gait impairment.
Main outcome measure(s)
Cervical and ocular VEMP latency and amplitude responses, and usual, rapid, and narrow walk gait speed assessment over a 6 meter course.
Results
We analyzed 246 subjects (mean age 73.2, range 26–98). Significant decreases in gait speed with age were observed for all three gait types (p=0.000). Age-related vestibular losses were noted for both men and women. After age adjustment, cervical VEMP latencies were associated with lower usual (p=0.029), rapid (p=0.005), and narrow (p=0.012) gait speeds in women. In men, increases in cVEMP latency were associated with increased rapid gait speed (p=0.009). Ocular VEMPs were not associated with gait speed in men or women.
Conclusions
These findings suggest that age-related declines in saccular function are associated with changes in gait in a cohort of community-dwelling individuals. Additionally, these data suggest that males and females with age-related saccular loss make opposite adjustments to their gait: males increase gait speed while women decrease gait speed.
Introduction
The vestibular system is responsible for maintaining the sense of head orientation and acceleration, both at rest and in motion(1,2). Vestibular inputs are thought to play a greater role during locomotion by controlling gaze(3) and head(4,5) stabilization through vestibulo-ocular connections and trunk stabilization through vestibulo-spinal connections. As gait speed increases, vertical displacement of both the body center of mass(6) and the head(7) increase. This leads to an increased role for vestibular information, and particularly the otolith organs (the saccule and the utricle), in the coordination of balance and body movements. As expected, disorders of the vestibular system have been associated with gait abnormalities including decreased stride length(8), decreased gait speeds(9), and increased variation of stance, swing, and double support duration(10). Reduced gait speed and increased gait variability have been associated with increased fall risk and survival, particularly in aging populations(11,12).
While several cross-sectional(13–22) and longitudinal(23) studies have reported age-related declines in vestibular function, the impact of vestibular loss due to age on gait outcomes has not been fully addressed. Previous studies have shown that head impulse testing, when used to screen for semi-circular canal function in older individuals, predicted slower gait speeds and increased fall risk(24). Yet the impact of the otolith organs in age-related vestibular loss and functional gait parameters has not been fully investigated.
The identification of vestibular evoked myogenic potentials (VEMP) and the characterization of different stimulus and response parameters have made possible the independent assessment of saccular versus utricular function. Air-conducted cervical VEMPs (cVEMP) and bone vibration conducted ocular VEMPs (oVEMP) have been described as measures of saccular and utricular function respectively(25). In this study, we investigated the association between age-related changes in saccular and utricular function and gait in a cohort of community-dwelling individuals across the age range. We considered three different types of gait: usual, rapid, and narrow gait. This allowed us to assess whether age-related vestibular loss had a greater impact during gait with increased head displacement (rapid gait) or decreased base of support (narrow gait). Thus, the aim of this study was to measure VEMPs and gait characteristics in an older population in order to determine the impact of age-related otolithic vestibular loss on gait.
Materials and Methods
This study was conducted as part of the Baltimore Longitudinal Study of Aging (BLSA), a longitudinal observational study initiated in 1958. All testing was conducted at an inpatient facility with onsite gait and vestibular laboratories.
Participants
From February of 2013 to September of 2013, community-dwelling participants underwent both cVEMP and oVEMP testing in conjunction with gait measurements as part of a three-day inpatient stay at the BLSA facility. Participants were excluded from oVEMP testing if they could not participate in the calibration procedures due to blindness. Exclusion criteria for cVEMP testing included conductive hearing loss or the inability to maintain a flexed sternocleidomastoid (SCM) muscle during the testing interval. Gait measures were recorded only for participants able to ambulate without the use of an assistive device. Information on participant age and gender were also collected.
VEMP Testing
VEMP testing was conducted using a commercial electromyographic (EMG) system (Medelec Synergy, software version 14.1, Care Fusion, Dublin, Ohio). Cervical VEMPs were elicited using monoaural air-conducted 500 Hz (125 dB SPL) tone bursts at a rate of 5 pulses per second delivered for 20 seconds through over-the-ear headphones (Viasys Healthcare, Madison, Wisconsin). Recording electrodes were placed along the ipsilateral SCM belly and the sternoclavicular junction as well a ground electrode on the sternum. Participants were placed in the supine position with their upper bodies elevated 30 degrees above the horizontal and were asked to actively flex their neck by raising their heads just prior to testing. The beginning of the cVEMP response was identified as the maximal positive deflection between 11 and 15 ms after the onset of the stimulus, with the subsequent maximal negative deflection indicating the end of the response. Peak-to-peak amplitudes were corrected for the background EMG activity (greater than 40 microvolts) recorded just prior to stimulus onset.
Ocular VEMPS were elicited using manual taps delivered to the hairline in the midline using a reflex hammer with an inertial trigger (VIASYS Healthcare). The first recording electrode was centered 2 cm below the pupil with the second located 0.5 cm below the edge of the first. Electrode placement and symmetry was checked with 20-degree vertical saccades (<25% asymmetry) prior to testing. During administration of the stimulus the participant was instruction to fix their gaze at a target 30 degrees above their normal gaze position. The beginning of the oVEMP response was identified as the maximal negative peak occurring 5–10 ms after the onset of the stimulus, with the subsequent maximal positive deflection indicating the end of the response.
Gait Testing
Gait data was collected as part of the extended Short Physical Performance Battery(26). All gait speeds were hand-timed over a 6-meter course. Participants were asked to complete the course at their usual walking speed before being asked to attempt the course at their fastest possible speed. Narrow walk pace was also measured over a 6-meter course, with participants instructed to walk only within a marked 20 cm wide path. Touching or crossing the marked boundaries two or more times resulted in a failed attempt. Participants unable to complete the gait courses without assistance were not considered for analysis.
Statistical Analysis
Multiple regression was used to compare gait and vestibular parameters. Left and right-sided responses were correlated within each patient, with correlation coefficients as follows: oVEMP latency (r=0.666, p=0.000); oVEMP amplitude (r=0.986, p=0.000); cVEMP latency (r=0.432, p=0.000); and cVEMP amplitude (r=0.665, p=0.000). Thus bilateral responses were averaged for analysis. In the absence of a VEMP response the amplitude was assigned a value of zero, whereas for latency analysis it was treated as missing data. Patients with unilateral VEMP losses were excluded from analysis. All results were considered significant at the level of p<0.05. Stata/SE version 12.0 (StataCorp, College Station, Texas) was used for statistical analysis.
Results
Demographics
A sample of 314 participants from the BLSA underwent VEMP testing and was available for analysis. Of the 314 participants 246 also had gait data. The mean age of this subset was 73.2 (sd 11.6, range 26–98). Men comprised 43.5% of the sample. Participants with gait data were not significantly different from participants without gait data with respect to mean age and gender distribution.
Vestibular Parameters and Aging
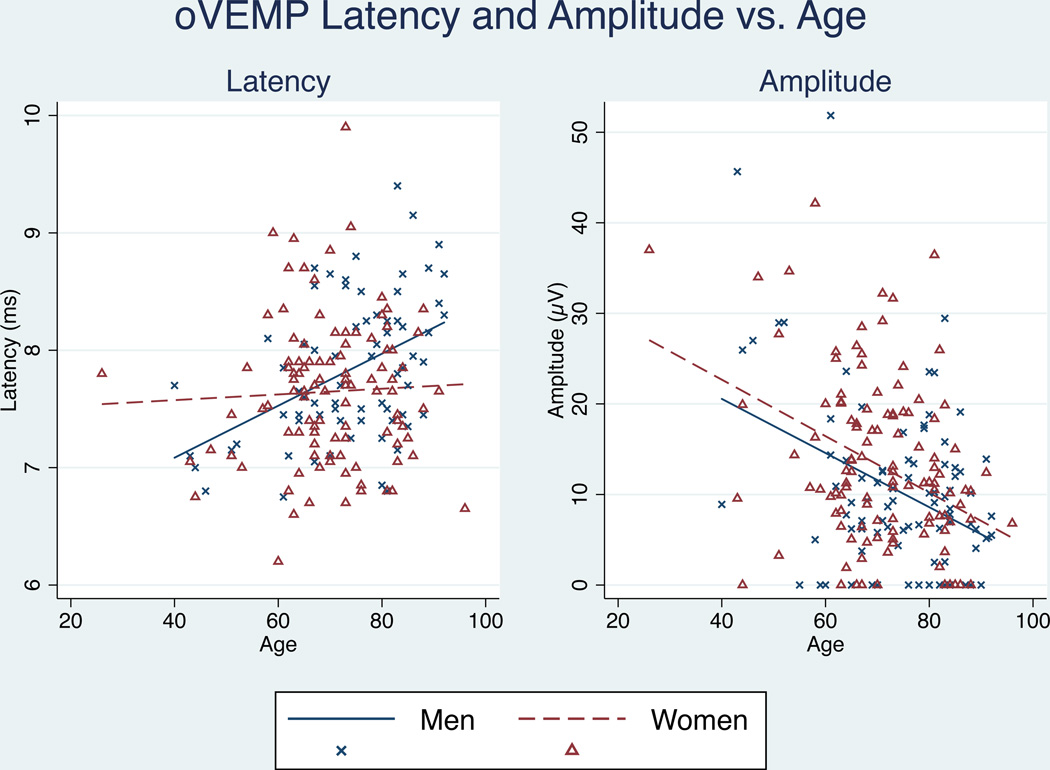
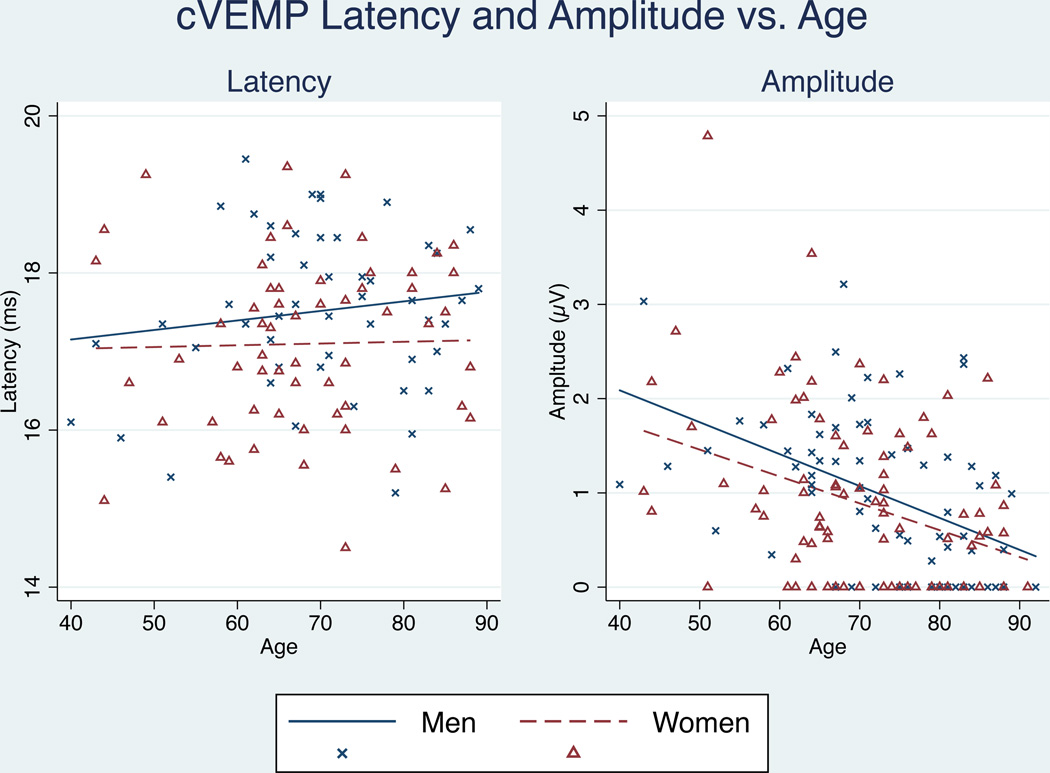
Consistent with prior studies, we observed significant trends in VEMP amplitude and latency associated with increasing age. Trends varied between men and women, therefore analyses are presented separately by gender. Ocular VEMP latency increased with age only in men (p=0.000), while oVEMP peak-to-peak amplitude decreased for both men and women with age. (Figure 1) Cervical VEMP latency failed to show any change with age for either men (p=0.322) or women (p=0.180), while cVEMP peak-to-peak amplitude showed significant decreases with age for both men (p=0.000) and women (p=0.001). (Figure 2)
Figure 1.
Effect of aging on oVEMP latency and amplitude. oVEMP latencies significantly increased only in men (p < 0.05). cVEMP amplitudes decreased significantly for both men and women with age (p < 0.05)
Figure 2.
Effect of aging on cVEMP amplitude and latency. cVEMP latency was not significant for either men or women. CVEMP amplitude significantly decreased with age for both men and women (p < 0.05).
Gait Parameters and Aging
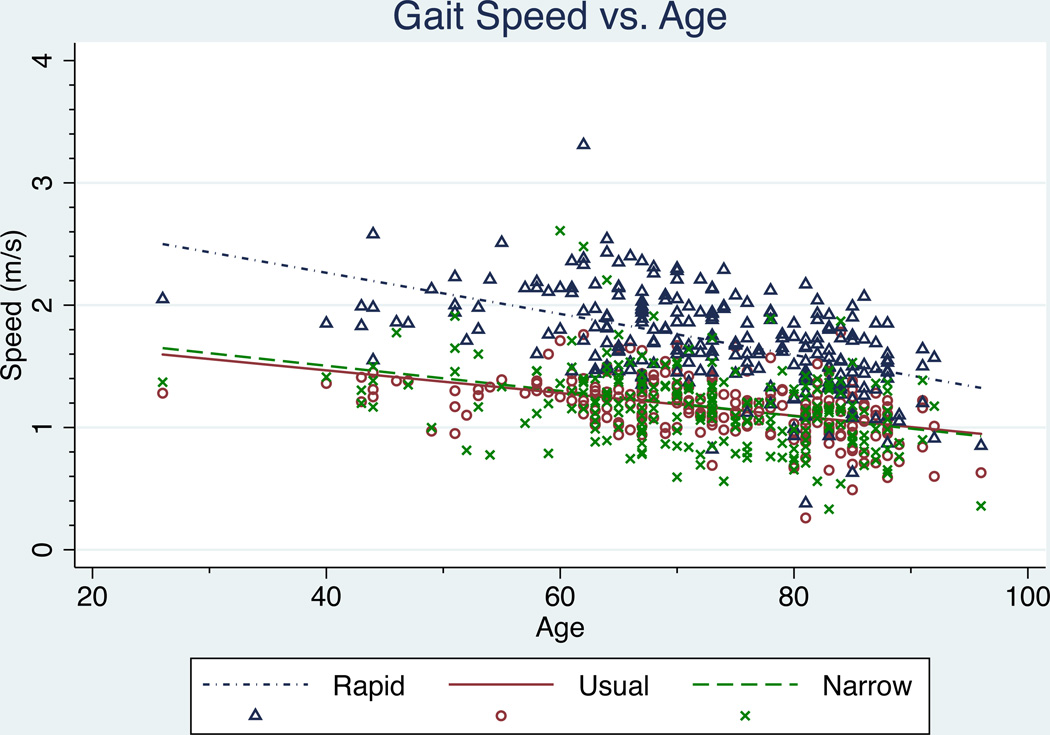
As expected, significant declines in gait speed with age were observed for all three gait types (p=0.000). No significant differences in rate of decline were observed between men and women for usual gait speed (p=0.247), rapid gait speed (p=0.153), or narrow walk gait speed (p=0.838), therefore aggregate data across gender are depicted. (Figure 3)
Figure 3.
Effect of aging on gait speed for three different gait types. Significant associations between age and gait speed were observed for all three gait types (p<0.05).
Associations between Vestibular Function and Gait Speed
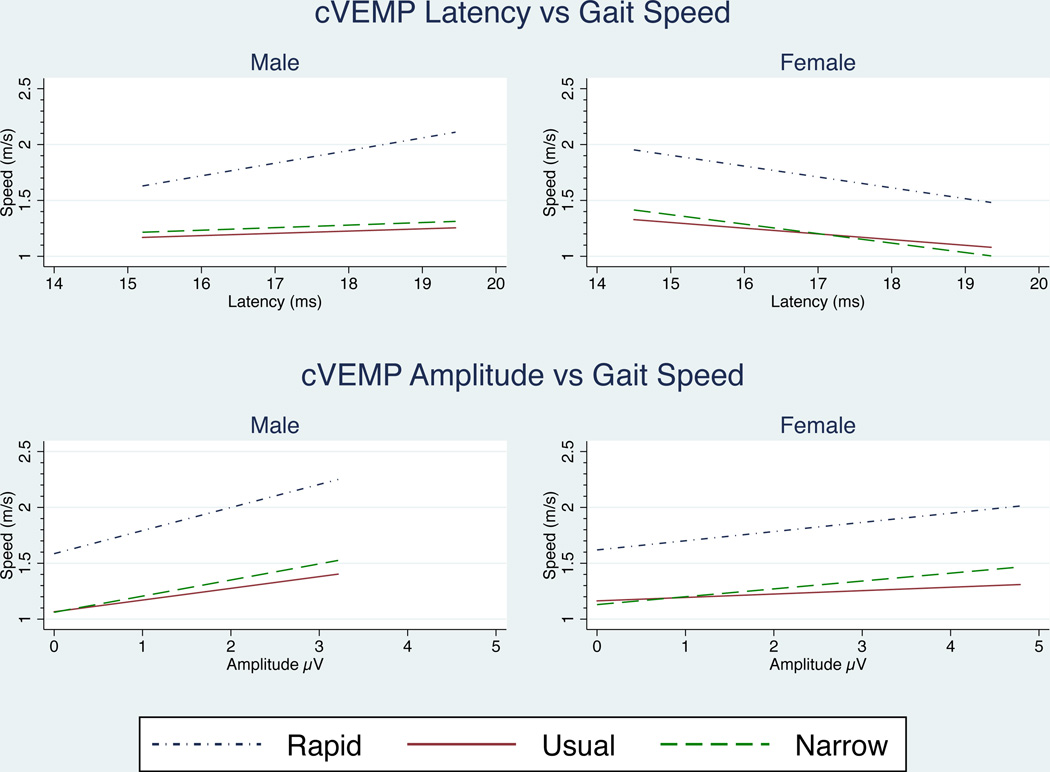
We next evaluated associations between VEMP and gait speed in age-adjusted analyses. (Table 1) We observed that increased cVEMP latencies in women were associated with lower usual (p=0.029), rapid (p=0.005), and narrow walk (p=0.012) gait speeds. In men, increasing cVEMP latency predicted increasing rapid gait speed (p=0.009). Increasing cVEMP latency was also associated with faster usual gait speed and faster narrow walk gait speed in men, but these associations were not statistically significant. Cervical VEMP peak-to-peak amplitudes were not significantly associated with gait speeds for either men or women. However, in men, positive trends between cVEMP amplitudes and usual (p=0.141), rapid (p=0.062), and narrow walk (p=0.090) support the association of saccular function with gait speed. (Figure 4)
Table 1.
Age-adjusted multiple regression of VEMPs vs. Gait Parameters (α = 0.05).
| Usual Gait Speed | Rapid Gate Speed | Narrow Walk Pace | |||||
|---|---|---|---|---|---|---|---|
| Coeff | p | Coeff | p | Coeff | p | ||
| CVEMP | Latency | ||||||
| Men | 0.035 | 0.244 | 0.139 | 0.009 | 0.04 | 0.433 | |
| Women | −0.050 | 0.029 | −0.094 | 0.005 | −0.081 | 0.012 | |
| Amplitude | |||||||
| Men | 0.049 | 0.141 | 0.108 | 0.062 | 0.049 | 0.090 | |
| Women | 0.003 | 0.890 | 0.034 | 0.324 | 0.047 | 0.124 | |
| OVEMP | Latency | ||||||
| Men | 0.030 | 0.498 | −0.091 | 0.221 | 0.039 | 0.583 | |
| Women | −0.032 | 0.342 | 0.019 | 0.697 | −0.017 | 0.707 | |
| Amplitude | |||||||
| Men | −0.002 | 0.468 | −0.004 | 0.322 | −0.003 | 0.450 | |
| Women | −0.002 | 0.374 | 0.000 | 0.888 | −0.002 | 0.372 | |
Figure 4.
Effect of cVEMP amplitude and latency on gait speed. cVEMP latency was significant for women across all three gait functions after age-adjustment. In men, only rapid gate was significant. No significant relationships existed for cVEMP amplitude (α = 0.05). Regression lines were fitted over the amplitude and latency ranges measured for each gender.
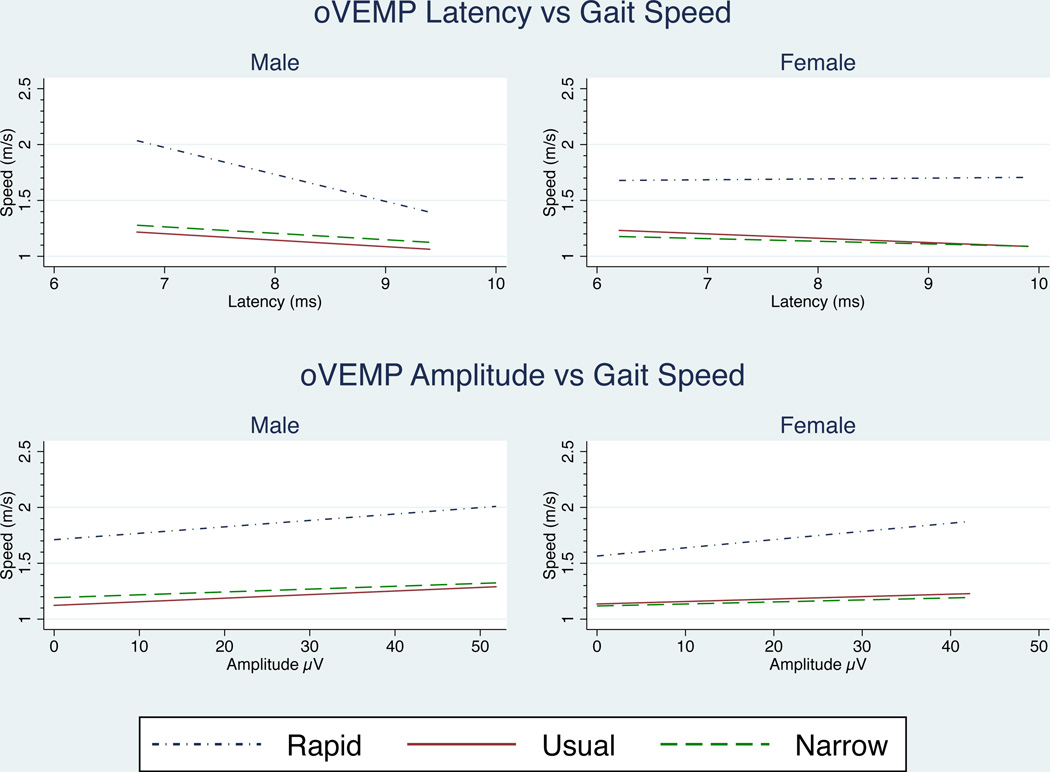
After adjusting for age, neither oVEMP amplitudes nor latencies had significant relationships with gait function for men or women, suggesting a lesser role for utricular inputs during locomotion. (Figure 5)
Figure 5.
Effect of oVEMP amplitude and latency on gait speed. No significant relationships existed after adjusting for age (α = 0.05). Regression lines were fitted over the amplitude and latency ranges measured for each gender.
Discussion
This study is among the first to evaluate the association between age-related otolith vestibular loss and gait speed. While previous studies have collected VEMP data in aging populations(22), these data have not been linked to gait function. Studies that have evaluated the influence of vestibular function on gait have largely focused on tests of semicircular function such as head-impulse testing(24) or head-shake tests(8). Our analysis suggests that age-related changes in otolith function influence gait speed, and that these associations differ between men and women.
The finding of significant associations between cVEMPs and gait speed is consistent with prior studies demonstrating a greater role for the saccule during locomotion(4,5). Vertical linear accelerations in the sagittal plane during locomotion generate shearing forces within the vertically oriented saccular macula, resulting in increased saccular signaling. Thus, dysfunction of the saccule in the form of longer saccular latencies, could delay information needed for multisensory integration and coordinated gait. The lack of utricular influence, as evidenced by non-significant oVEMP correlations with gait speed in these data, may also reflect the anatomy and physiology of the utricle. The utricular maculi are horizontally oriented, and are most responsive to head tilt and horizontal translation. Thus utricular dysfunction would not be likely to affect gait as markedly as a dysfunction of the saccule.
Interestingly, the changes in gait speed associated with saccular dysfunction appear to differ between men and women. In the presence of increased saccular latencies, men have higher gait speeds, whereas women have lower gait speeds. Studies suggest that the adoption of a rapid gait confers greater stability in patients with a vestibulopathy, as automated spinal locomotion overrides the vestibular inputs(27,28). However, the advantages associated with a more rapid gait may not be the same for both men and women due to differences in hip geometry, body composition (e.g. muscle mass), or center of mass. The different strategies in men and women may alternatively be attributable to a difference in the sensitivity of the saccule to vertical linear displacement between men and women. While absolute vertical head displacement is larger in men, when corrected for leg length, men and women experience the same relative head bob during gait(29). If the size of the saccule is conserved between men and women, the smaller absolute displacement experienced by women may lead to decreased saccular signaling compared to men. The adoption of gender-specific gait strategies in response to a physiologic impairment has been observed previously. For example, studies have shown that men and women adopt different gait strategies in response to osteoarthritis, with faster and longer leg swings and decreased single support time being observed in men(30).
Several limitations of this study should be noted. The participants of this study represent a generally healthy population, who are available to travel and are committed to recurrent visits over several decades. Thus, our findings may not be fully generalizable to the population at large. Moreover, we only considered gait speed in this study. Ongoing analyses are currently examining kinematic data from a 3D motion capture system to more fully characterize the walking characteristics (e.g. joint movements, center of mass sway) of individuals with decreased otolith and semicircular canal function. However, such measurements may not be readily available to the clinician, making them less practicable as a diagnostic or evaluative measure. Finally our analyses adjusted for age, as a surrogate for age-related losses of other sensory and motor functions that influence gait function. There is a possibility of residual confounding by sensory or motor impairment not captured by the age variable alone.
This study confirms both age-related declines in gait speed and age-related loss of otolith function. These findings further suggest that age-related declines in saccular function are associated with changes in gait in a cohort of community-dwelling individuals. Additionally, these data suggest that males and females with age-related saccular loss make opposite adjustments to their gait: males increase gait speed while women decrease gait speed.
Acknowledgments
Support/Funding disclosure:
NIH, American Otological Society Clinician Scientist Award, and the NIA Older Americans Independence Center Research Career Development Core grant
References
- 1.Horak FB, Shupert CL, Dietz V, et al. Vestibular and somatosensory contributions to responses to head and body displacements in stance. Exp Brain Res. 1994;100:93–106. doi: 10.1007/BF00227282. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg JM. The vestibular system : a sixth sense. Oxford; New York: Oxford University Press; 2012. pp. xiii–541. [Google Scholar]
- 3.Whitney SL, Marchetti GF, Pritcher M, et al. Gaze stabilization and gait performance in vestibular dysfunction. Gait Posture. 2009;29:194–198. doi: 10.1016/j.gaitpost.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pozzo T, Berthoz A, Lefort L, et al. Head stabilization during various locomotor tasks in humans. II. Patients with bilateral peripheral vestibular deficits. Exp Brain Res. 1991;85:208–217. doi: 10.1007/BF00230002. [DOI] [PubMed] [Google Scholar]
- 5.Pozzo T, Berthoz A, Vitte E, et al. Head stabilization during locomotion. Perturbations induced by vestibular disorders. Acta Otolaryngol Suppl. 1991;481:322–327. doi: 10.3109/00016489109131413. [DOI] [PubMed] [Google Scholar]
- 6.Massaad F, Lejeune TM, Detrembleur C. The up and down bobbing of human walking: a compromise between muscle work and efficiency. J Physiol. 2007;582:789–799. doi: 10.1113/jphysiol.2007.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirasaki E, Moore ST, Raphan T, et al. Effects of walking velocity on vertical head and body movements during locomotion. Exp Brain Res. 1999;127:117–130. doi: 10.1007/s002210050781. [DOI] [PubMed] [Google Scholar]
- 8.Lang J, Ishikawa K, Hatakeyama K, et al. 3D body segment oscillation and gait analysis for vestibular disorders. Auris Nasus Larynx. 2013;40:18–24. doi: 10.1016/j.anl.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JC, Cohen HS, Sangi-Haghpeykar H. Vestibular disorders and dual task performance: impairment when walking a straight path. J Vestib Res. 2011;21:167–174. doi: 10.3233/VES-2011-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angunsri N, Ishikawa K, Yin M, et al. Gait instability caused by vestibular disorders - analysis by tactile sensor. Auris Nasus Larynx. 2011;38:462–468. doi: 10.1016/j.anl.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrager MA, Kelly VE, Price R, et al. The effects of age on medio-lateral stability during normal and narrow base walking. Gait Posture. 2008;28:466–471. doi: 10.1016/j.gaitpost.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterka RJ, Black FO. Age-related changes in human posture control: sensory organization tests. J Vestib Res. 1990;1:73–85. [PubMed] [Google Scholar]
- 14.Peterka RJ, Black FO, Schoenhoff MB. Age-related changes in human vestibulo-ocular and optokinetic reflexes: pseudorandom rotation tests. J Vestib Res. 1990;1:61–71. [PubMed] [Google Scholar]
- 15.Peterka RJ, Black FO, Schoenhoff MB. Age-related changes in human vestibulo-ocular reflexes: sinusoidal rotation and caloric tests. J Vestib Res. 1990;1:49–59. [PubMed] [Google Scholar]
- 16.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31:793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol. 2001;112:1971–1979. doi: 10.1016/s1388-2457(01)00645-9. [DOI] [PubMed] [Google Scholar]
- 18.Welgampola MS, Colebatch JG. Selective effects of ageing on vestibular-dependent lower limb responses following galvanic stimulation. Clin Neurophysiol. 2002;113:528–534. doi: 10.1016/s1388-2457(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 19.Brantberg K, Granath K, Schart N. Age-related changes in vestibular evoked myogenic potentials. Audiol Neurootol. 2007;12:247–253. doi: 10.1159/000101332. [DOI] [PubMed] [Google Scholar]
- 20.Paige GD. Senescence of human visual-vestibular interactions. 1. Vestibulo-ocular reflex and adaptive plasticity with aging. J Vestib Res. 1992;2:133–151. [PubMed] [Google Scholar]
- 21.Paige GD. Senescence of human visual-vestibular interactions: smooth pursuit, optokinetic, and vestibular control of eye movements with aging. Exp Brain Res. 1994;98:355–372. doi: 10.1007/BF00228423. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal Y, Zuniga MG, Davalos-Bichara M, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33:832–839. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baloh RW, Enrietto J, Jacobson KM, et al. Age-related changes in vestibular function: a longitudinal study. Ann N Y Acad Sci. 2001;942:210–219. doi: 10.1111/j.1749-6632.2001.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal Y, Davalos-Bichara M, Zuniga MG, et al. Head impulse test abnormalities and influence on gait speed and falls in older individuals. Otol Neurotol. 2013;34:1729–1735. doi: 10.1097/MAO.0b013e318295313c. [DOI] [PubMed] [Google Scholar]
- 25.Welgampola MS, Carey JP. Waiting for the evidence: VEMP testing and the ability to differentiate utricular versus saccular function. Otolaryngol Head Neck Surg. 2010;143:281–283. doi: 10.1016/j.otohns.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–m649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 27.Jahn K, Strupp M, Schneider E, et al. Differential effects of vestibular stimulation on walking and running. Neuroreport. 2000;11:1745–1748. doi: 10.1097/00001756-200006050-00029. [DOI] [PubMed] [Google Scholar]
- 28.Brandt T, Strupp M, Benson J. You are better off running than walking with acute vestibulopathy. Lancet. 1999;354:746. doi: 10.1016/S0140-6736(99)03179-7. [DOI] [PubMed] [Google Scholar]
- 29.Senden R, Grimm B, Heyligers IC, et al. Acceleration-based gait test for healthy subjects: reliability and reference data. Gait Posture. 2009;30:192–196. doi: 10.1016/j.gaitpost.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Debi R, Mor A, Segal O, et al. Differences in gait patterns, pain, function and quality of life between males and females with knee osteoarthritis: a clinical trial. BMC Musculoskelet Disord. 2009;10:127. doi: 10.1186/1471-2474-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]