Abstract
A study finds that pain hypersensitivity in male and female mice is differentially dependent on microglia and T cells, and describes a sex-specific response to microglia-targeted pain treatments.
Animal studies1,2 have spawned great interest in using microglial inhibitors such as minocycline to treat pain in humans. However, these studies were conducted largely on male rodents. Now Sorge et al.3 have evaluated several microglial inhibitors in nerve-injured mice of both sexes. The study—led by Jeffrey Mogil, who has championed the testing of males and females in pain studies4—found that microglial inhibitors did reduce allodynia, a form of pain hypersensitivity to touch, in males. Surprisingly however, these inhibitors were ineffective in female mice, despite a robust activation of spinal microglia (Fig. 1). They instead found that cells of the adaptive immune system promote pain hypersensitivity in females. While focused on pain, these findings could have implications for other neurological disorders that disproportionately affect one sex, such as autism and neurodegeneration, and where microglia and immune cells are implicated5,6.
Figure 1.
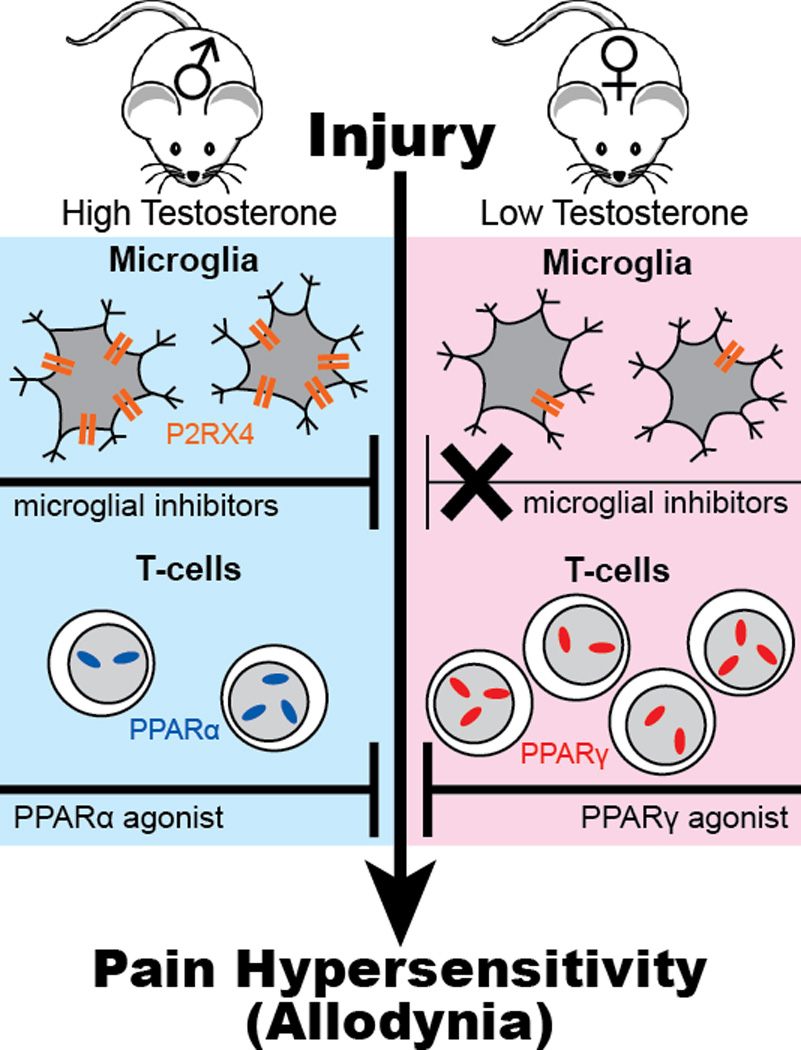
Pain mechanisms differ in male and female mice. Nerve injury activates microglial cells in the spinal cord of male and female mice, but microglial inhibitors only block allodynia in males. P2RX4 is upregulated in males only. Female mice have about twice as many T cells as males. Testosterone increases PPARα and decreases PPARγ gene expression in T cells. Compounds that activate PPARα inhibit mechanical pain hypersensitivity (allodynia) in males whereas those that activate PPARγ inhibit allodynia in females.
Chronic pain is a highly prevalent condition and occurs more often in women4. Pain-related symptoms in males and females, including pain sensitivity, response to analgesic therapies and risk for opioid-induced hyperalgesia, also differ4,7. However, male mice are frequently used to represent both sexes in pain research. As part of previous efforts to characterize pain processing in both sexes, Mogil’s group found that pain resulting from inflammation or nerve injury depends on spinal Toll-like receptor 4 (TLR4) in male but not female mice8. In their current study, they sought to better understand the mechanistic basis for this sex-specific effect by examining microglia, the spinal cell type that expresses TLR4.
As expected, Sorge et al.3 found equivalent levels of microglial activation in the spinal cords of male and female mice in response to peripheral nerve injury, accompanied by similar levels of allodynia. The surprise came when they interrupted spinal microglial activity—pharmacologically or genetically—and found that these interventions relieved allodynia in male mice only. This sex-specific response depended on testosterone levels, as minocycline did not relieve allodynia in castrated males but did relieve allodynia in testosterone-treated females. Sorge et al.3 also examined microglial gene expression. The only sex-specific difference they observed was in expression of the purinergic receptor P2RX4: it was upregulated only in nerve-injured male mice (Fig. 1).
Following neuropathic injury, the spinal cord becomes infiltrated with adaptive immune cells, including T cells, which are implicated in mechanical allodynia9. Sorge et al.3 found that, relative to males, female mice had higher basal numbers of T cells in the blood and increased T cell marker expression in the spinal cord after injury. These data hinted that T cells might promote allodynia in females, as microglia do in males.
To examine the role of the adaptive immune cells in pain hypersensitivity more directly, the authors studied T cell-deficient mice of both sexes and found they developed allodynia equivalent to their wild-type counterparts. Remarkably, minocycline relieved allodynia in T cell-deficient females, but was not effective in these females when the T cell population was restored through transplantation. These findings thus indicate that allodynia is established in females by T cells. Yet, in the absence of T cells or when testosterone levels are elevated, pain hypersensitivity can be established in females by the microglia-based system. Determining precisely how these two cell types interact and promote pain hypersensitivity differentially in each sex will require further research. Sorge et al.3 have at least made it clear that testosterone influences this sex difference.
To dissect testosterone’s role in regulating the balance between the T cell and microglia mechanisms, Sorge et al.3 examined the contribution of peroxisome proliferator activated receptors (PPARs), as previous work had indicated that testosterone upregulates PPARα and downregulates PPARγ in T cells10. PPARs are nuclear receptors that negatively regulate production of proinflammatory mediators. Sorge et al.3 found that a PPARα agonist relieved allodynia in males but not females, while a PPARγ agonist relieved allodynia in females but not males (Fig. 1). As was the case with minocycline, responsiveness to these agonists was reversed by altering testosterone levels.
Doctors are not likely to give their female chronic pain patients testosterone supplements to enable minocycline-responsiveness. Therefore, a better understanding of all the factors that mediate minocycline’s efficacy may be important for improving its clinical use. Assuming these sex differences found in rodents apply to humans, this T cell-dependent mechanism in female mice could provide new cellular and molecular clues as to why women are at greater risk for chronic pain. For example, rheumatoid arthritis, which features inflammation, pain and hyperactive T cells, is also more prevalent in women11. The higher levels of circulating T cells in women12 may make them more susceptible to developing disorders that result from increased T cell activity. And, given the molecular differences between T cells in male and female mice (Fig. 1), T cells in females might be more sensitive to chemicals that are released following tissue injury.
The pain field was excited about the prospects of using microglial inhibitors to treat pain in humans until recent clinical trials with minocycline failed to show efficacy in patients with neuropathic or persistent pain13,14. These trials lumped males and females together when evaluating outcomes. The work by Sorge et al.3 reveals the importance of evaluating pain outcomes separately in males and females. This message is timely given the ongoing clinical trials with minocycline in patients with pain and other neurological disorders. These new findings may reinvigorate interest in microglia-targeted approaches.
Given the pharmaceutical industry’s quest for blockbuster drugs that work broadly on pain in all people, this study also highlights limitations to a one-sex-fits-all drug development model. Indeed, future ‘precision pain medicines’ may need to account for whether the patient does or does not have a Y chromosome. Lastly, it is important to note that microglia and immune cells are implicated in other neurological diseases, many of which disproportionately affect one sex over the other. These include Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and autism. In time, the study by Sorge et al.3 could have ramifications that extend well beyond pain, possibly foreshadowing sex-specific pathologies and responsiveness to therapies for diverse neurological disorders.
References
- 1.Taves S, Berta T, Chen G, Ji RR. Neural Plast. 2013;2013:753656. doi: 10.1155/2013/753656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. Front Cell Neurosci. 2013;7:191. doi: 10.3389/fncel.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorge RE, et al. Nat. Neurosci. 2015;18 doi: 10.1038/nn.4053. XXX–XXX [Production: add pp] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogil JS. Nat Rev Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 5.Chen JA, Penagarikano O, Belgard TG, Swarup V, Geschwind DH. Annu Rev Pathol. 2015;10:111–144. doi: 10.1146/annurev-pathol-012414-040405. [DOI] [PubMed] [Google Scholar]
- 6.Skaper SD, Facci L, Giusti P. CNS Neurol Disord Drug Targets. 2014;13:1654–1666. doi: 10.2174/1871527313666141130224206. [DOI] [PubMed] [Google Scholar]
- 7.Fillingim RB. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 8.Sorge RE, et al. J Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costigan M, et al. J Neurosci. 2009;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang MA, et al. Proc Natl Acad Sci U S A. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kvien TK, Uhlig T, Odegard S, Heiberg MS. Ann N Y Acad Sci. 2006;1069:212–222. doi: 10.1196/annals.1351.019. [DOI] [PubMed] [Google Scholar]
- 12.Bouman A, Heineman MJ, Faas MM. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 13.Vanelderen P, et al. Anesthesiology. 2015;122:399–406. doi: 10.1097/ALN.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 14.Martinez V, et al. Pain. 2013;154:1197–1203. doi: 10.1016/j.pain.2013.03.028. [DOI] [PubMed] [Google Scholar]


