Abstract
Intracellular and extracellular interactions with proteins enables the functional and mechanistic diversity of lipids. Fatty acid-binding proteins (FABPs) were originally described as intracellular proteins that can affect lipid fluxes, metabolism and signalling within cells. As the functions of this protein family have been further elucidated, it has become evident that they are critical mediators of metabolism and inflammatory processes, both locally and systemically, and therefore are potential therapeutic targets for immunometabolic diseases. In particular, genetic deficiency and small molecule-mediated inhibition of FABP4 (also known as aP2) and FABP5 can potently improve glucose homeostasis and reduce atherosclerosis in mouse models. Further research has shown that in addition to their intracellular roles, some FABPs are found outside the cells, and FABP4 undergoes regulated, vesicular secretion. The circulating form of FABP4 has crucial hormonal functions in systemic metabolism. In this Review we discuss the roles and regulation of both intracellular and extracellular FABP actions, highlighting new insights that might direct drug discovery efforts and opportunities for management of chronic metabolic diseases.
Introduction
Lipids have wide-ranging roles in many different aspects of biology, functioning as structural building blocks or fuel sources, and as intracellular and extracellular signalling molecules. For example, lipids can modify the action or location of proteins, such as kinases or ion channels, signal via proteins such as cell surface G-protein coupled receptors and can serve as ligands for transcription factors.1–3 Free fatty acids can also regulate hormone action, for example by inhibiting the insulin-stimulated phosphoinositide 3-kinase pathway4,5 and activating inflammatory molecules such as inhibitor of nuclear factor κB kinase subunit β (IKK-β)6 and c-jun N-terminal kinase (JNK),7,8 or engage pattern recognition receptors which can contribute to metabolic regulation and disease.9
However, given the relative insolubility of these fatty acids and their potential toxicity in free forms,10,11 the need for buffering entities has led to the search for noncatalytic binding proteins, including the fatty acid binding proteins (FABPs). The first FABP was originally described as a small molecular weight intracellular protein of ~12 kDa detected in the rat jejunum, owing to its ability to noncovalently bind to long chain fatty acids.12 Subsequently, other proteins that also bind to long-chain fatty acids were identified in the liver, myocardium, adipose tissue and kidney.12,13 However, as the functions of FABPs have been elucidated, these proteins do not seem to simply buffer lipids, but are in fact crucial mediators of metabolic and other biological activities.14 As we describe in this Review, we now understand that the functional diversity of FABPs is generated via lipid interactions with these chaperone proteins to support systemic homeostatic networks of immunometabolism by facilitating signalling within and between cells and communication between organs. Consequently, interest has grown in the research community for therapeutically targeting this class of proteins in metabolic and immunometabolic diseases.14 In this Review we discuss the roles and regulation of FABP4 (fatty acid-binding protein, adipocyte; which is often referred to in the literature as aP2) and the closely related FABP5 (fatty acid binding protein, epidermal; also known as mal1), the main FABPs present in adipose tissue. We will also describe the emerging biological framework, and highlight new insights into the functions and mechanisms that might directly influence drug discovery for metabolic diseases.
FABP gene expression
Understanding of the regulated expression of genes encoding FABPs and their potential regulatory and metabolic effects began to emerge after their initial discovery. For example, levels of intestinal FABP were found to increase in abundance in response to fasting and a high fat diet,13 and this protein can enhance fatty-acid CoA ligase activity.15 Fatty-acid CoA ligase is involved in the generation of substrates for β-oxidation, complex lipids and signalling molecules.16 FABPs were subsequently isolated and cloned from other tissues including the brain, heart and lung.17–25 FABP4 was identified as a cytosolic protein strongly upregulated during differentiation of preadipocytes into adipocytes. 26,27 In fact, this protein is one the most abundant proteins ever found in mature adipocytes and adipose tissue.26 Protein preparations from rat and human adipose tissue suggest that FABP4 might be involved in the esterification of long-chain fatty acids,28 and after cloning and sequencing this protein was found to be highly similar to FABP1 (fatty acid-binding protein, liver), FABP2 (fatty acid binding protein, intestinal) and the myelin P2 protein.27 We now know that FABP4 is a member of a large gene family of mammalian FABPs each containing an evolutionarily conserved four exon and three intron organization.29 Mammalian FABPs are part of a large class of intracellular and extracellular lipid proteins that bind a variety of hydrophobic ligands, including fatty acids, haeme, retinoids and different vitamins.30 Moreover, FABPs have sequence and structural similarity to lipocalins from insects, nematodes and fish.29
In initial expression analyses, FABP4 mRNA was detected at low levels in multiple tissues such as the heart and kidney,31 but the protein itself was only detectable in adipose tissue.32 Indeed, the major site of FABP4 expression is white and brown adipose tissue, and in differentiated adipocytes, this FABP is estimated to account for between 0.5% and 6% of total protein.26,33 As a marker for adipocyte differentiation, FABP4 expression is specifically induced by insulin and/or IGF-1,34 dexamethasone and fatty acids,35–37 and occurs as a downstream target of peroxisome proliferator-activated receptor γ (PPARγ) activation.38 Notably, FABP4 expression is also readily detected in other cell types such as macrophages, particularly upon inflammatory activation,39 and, under special circumstances, can be found elsewhere, such as the bronchial epithelium where it occurs independently of PPARγ.40
FABP intracellular functions
Studies in cellular and biochemical systems
Substantial effort in determining the role of FABPs has focused on defining their binding affinities and lipid cargos. Using radiolabelled or fluorescent fatty acid analogues, it was shown that FABP4 and FABP3 (fatty acid-binding protein, heart) each have one binding site per molecule, and that the binding site of FABP3 might be more hydrophobic than that of FABP4, which suggests that their intracellular functions might differ.41 Analysis of the FABP4 crystal structure has revealed that the protein binds to saturated and unsaturated fatty acids in the same conformation.42 Furthermore, in a comparison of FABP binding affinities to different ligands, FABP4 was unique among FABPs for its high affinity for both saturated and unsaturated fatty acids.43 The ability of FABP4 to transfer fatty acids to membranes increases with fatty acid chain length but is not altered by relative saturation, which suggests that FABP4-mediated transport might not be dependent on fatty acid solubility and might occur via collision with membranes.44 Interaction between FABP4 and phospholipid membranes was confirmed with the use of fourier transform infrared spectroscopy,45 and shown to be strongly regulated by positively charged lysine residues.46
The basic structure of the FABP fold is a 10-stranded antiparallel β-barrel, in which each strand forms hydrogen bonds with the following strand in an up-and-down manner (Figure 1).29 The first five and the second five strands each form sheets that are orthogonal to each other. The two sheets form a flattened barrel closed at one end with a helix-turn-helix domain between β-strands 1 and 2 closing the opposite end. The interior cavity of FABP4 is ~650 Å3, which is large enough to accommodate more than one fatty acid.47 Indeed, the cavity within FABPs is ~2–3 times the volume required for fatty acid binding and at least one FABP (FABP1) can bind multiple lipids simultaneously. 47 Interestingly, the charge distribution of side chains within the FABP4 cavity is not appreciably different from that on the surface of the protein, implying that the cavity is not hydrophobic but highly charged and polar.48 Evaluation of the binding characteristics using titration microcalorimetry indicates that the binding energy is divided relatively equally between enthalpic and entropic terms,49 which indicates that interactions at both the head-group and along the acyl chain determine binding affinity and specificity. Indeed, in both NMR and X-ray crystallographic analyses of natural and pharmacologic ligands, ligand binding seems to occur on one face of the interior cavity and is dominated by polar and non-polar interactions. 48 The interior cavity of FABP4 contains ~12 crystallographically ordered water molecules in addition to ~100 disordered waters. Ligand binding displaces some, but not all, of the ordered waters that are in close contact to the bound fatty acids, which suggests that water interactions might be critical for fatty acid association.50 Notably, other molecules might also occupy the ligand binding pocket of FABPs in the presence or absence of lipids. For example, FABP1 has been shown to bind to haeme,51 although the physiological consequences of these interactions are not well understood.
Figure 1.
Ribbon and domain structure of FABP4. The structure of FABP4 is depicted as a ribbon with bound oleic acid in space-filling spheres (carboxylate oxygen atoms in red). Also shown are four domains of FABP4: portal region for ligand entry and exit; charge quartet used for protein–protein interactions (Asp17, Asp18, Lys21 and Arg30); the salt bridge where the fatty acid carboxylate forms ion pairs with basic residues within the cavity (Arg106, Arg126 and Tyr128); the hinge where the helix-turn-helix region rotates to enable access of ligands to the cavity (Glu14, Asn15 and Phe16). Amino acid numbering system is based on the mouse Fabp4 protein (Genbank: CAJ18597.1).175
Ligand access to the interior cavity of FABP4 is likely to occur via a domain referred to as the portal region (Figure 1). The portal region is bounded by amino acids found in the loops that connect β-strand 3 to β-strand 4, β-strand 5 to β-strand 6 and α-helix II.29,48 Importantly, the volume of most ligands exceeds that of the opening from the external environment into the cavity,47 which suggests that dynamic flexibility on or around the portal region must accompany ligand binding. One plausible consideration is that the entire helix-turn-helix domain dynamically rotates at the hinge region of the connecting β-strands to allow ligand entry and exit.52 Bound fatty acids form a salt bridge with Arg106, Arg126 and Tyr128, and mutations in each of these amino acids results in proteins with undetectable fatty acid binding.53 Interestingly, within the cavity, Cys117 is covalently modified by oxidized lipids such as 4-hydroxynonenal in a process known as protein carbonylation. 50 Indeed, in 3T3-L1 adipocytes as well as in adipose tissue from mice and humans, FABP4 is the major carbonylated cytoplasmic protein,54 suggesting that FABP4 might function as an antioxidant, scavenging reactive lipids from the cellular environment.
At the plasma membrane, uptake of fatty acids is mainly regulated by transport proteins such as CD36, plasma membrane-associated FABP, and the fatty acid transport protein family.55,56 However, FABPs might also be involved in this uptake, and some evidence suggests that FABPs can interact directly with CD36.57 By enabling the uptake of sterol, FABP1 alters the lipid composition of the plasma membrane, which results in increased fluidity. 58 Further investigations have revealed that FABPs have redundant and overlapping roles in mediating uptake of fatty acids in various tissues, for example, both FABP4 and FABP5 can contribute to the import of fatty acids into the heart.59 However, FABP-promotion of fatty acid uptake might also be context or cell-type-specific. For example, in L-cell fibroblasts, overexpression of either FABP1 or FABP2 results in increased synthesis of phospholipids, but only FABP1 can increase fatty acid uptake.60 Importantly, genetic deficiency of FABP4 seems to alter adipose tissue fatty acid uptake in a lipid specific manner,61 has a profound influence on fatty acid efflux,62 and regulates de novo lipogenesis,63–65 which results in increased intracellular levels of fatty acids in FABP4-deficient cells.62 Although FABP4 binds and donates its fatty acid ligand via collisional interactions with membranes, it does not form thermodynamically stable complexes with membranes in vivo or in vitro.66 This finding suggests that intracellular fatty acid trafficking between membranes in vivo occurs via transient interactions with membranes and/or proteins.
FABPs have also been implicated in lipid sensing and response mechanisms by delivering lipids to nuclear receptors such as PPARs to mediate transcriptional programmes,67 although this effect might be context-dependent and specific to particular FABPs and PPAR isoforms. Using fluorescently labelled fatty acids, FABP1 expression was shown to promote the uptake of free fatty acids into the cytoplasm and mediate their delivery to the nucleus, with an apparent preference for longer chain fatty acids.68 Similarly, FABP5 has been implicated in the delivery of retinoic acid to PPARβ/δ with important consequences for neuronal development,69 and can also promote the hydrolysis of the endocannabinoid anandamide into a form that functions as a PPARβ/δ ligand and, therefore, regulates hippocampal function.70 In some studies that use cell culture systems, FABP4 can translocate to the nucleus and interact with, and potentially deliver ligands to PPARγ,71–73 but the functional consequences of this interaction are unknown. Alternatively, apo–FABP4 might transiently interact with PPARγ in the nucleus to extract a ligand from the protein and attenuate nuclear receptor action. As discussed further later in the text, evidence from genetic mouse models suggests that although PPARγ can directly increase FABP4 gene expression, FABP4 has a net negative effect on PPARγ activity74 and represents a negative feedback loop to control signalling from this nuclear hormone receptor.
Studies in mouse models
An increasingly comprehensive understanding of FABP functions has emerged with the establishment of genetic mouse models, the first of which was the Fabp4−/− mouse.75 The initial experience with this mouse model was quite disappointing due to a lack of any phenotype under standard laboratory conditions. This result was, at least in part, attributable to a compensatory upregulation of FABP5 in adipose tissue.75,76 However, a number of fascinating phenotypes have emerged as these models have been examined in the context of metabolic stress. In the setting of both diet-induced and genetic obesity, compensation by FABP5 is not sufficient to completely mask the biology of FABP4-deficiency, and Fabp4−/− mice were protected from obesity-induced insulin resistance and hyperglycaemia.75,77 This protection was thought be the result of changes in the composition and metabolism of lipids and resistance to chronic inflammation in the setting of obesity.75
Despite apparently normal glucose homeostasis, lean Fabp4−/− mice have reduced lipolysis and increased lipogenesis, as well as a blunted insulin secretion response to β-adrenergic stimulation,64,65 which suggests that FABP4 or an FABP4-regulated lipid signal might be involved in modulating insulin secretion. Interestingly, transgenic overexpression of FABP5 increases lipolysis and decreases cellular free fatty acid levels.65 To understand the role of FABP4 in the absence of compensatory FABP5 expression, Fabp4−/− mice were crossed with FABP5-deficient mice.61 Fabp5−/− mice are themselves moderately protected from diet-induced obesity and insulin resistance, and FABP5 overexpression is sufficient to impair glucose tolerance.78 The Fabp4–Fabp5 double mutant (Fabp4–5−/−) mice were protected from high fat diet-induced weight gain and had a more dramatic improvement in insulin sensitivity and glucose homeostasis than either of the single FABP4 or FABP5 deficiency models.61 Furthermore, Fabp4–5−/− mice were protected from high-fat diet-induced hepatic steatosis, with increased muscle AMP kinase activity and reduced hepatic expression of the key fatty acid metabolism enzyme Scd1 (encoding acyl-CoA desaturase 1), which could in part underlie the reduced lipid accumulation in this model.61 In the leptin-deficient ob/ob model, combined deficiency of FABP4 and FABP5 did not reduce obesity but did improve glucose tolerance and insulin sensitivity, resulting in reduced hepatic Scd1 expression, and protection against the development of fatty liver.79 In further studies using Fabp4–5−/− mice, a robust increase in de novo lipogenesis in adipose tissue results in increased levels of circulating palmitoleate (C16:1n7), which suppresses Scd1 expression in liver and increases insulin sensitivity.80 Increased de novo lipogenesis has also been seen in FABP4-deficient macrophages, resulting in increased production of palmitoleate, which mediates protection from lipid-induced endoplasmic reticulum stress in these cells.81 The effect of palmitoleate is in part mediated through free fatty acid receptor 4 (also known as GPR120);82 and in contrast to Fabp4–5−/− mice, in the setting of GPR120 deficiency palmitoleate levels are reduced, which leads to increased Scd1 expression, insulin resistance and glucose intolerance.83 The results of these studies demonstrate that in adipose tissue, FABPs are essential for dietary intake to dominate the lipid composition of the tissue. In their absence, adipose tissue is unaffected by the diet, which results in activation of de novo lipogenesis. Furthermore, these findings also illustrate the potential importance of adipose tissue de novo lipogenesis in controlling systemic metabolism, which under most conditions remains limited.
Surprisingly, although FABP4 is highly upregulated during preadipocyte differentiation, adipogenesis and adipose tissue formation proceed normally or are enhanced in Fabp4−/− and Fabp4–5−/− cells and mice.61,75 This finding demonstrates that FABP function is not required for specification of the adipocyte lineage and correlates with FABP4 having a potential negative feedback regulatory effect on PPARγ. However, in the context of FABP4 deficiency, the conversion of glucose to fatty acids is elevated twofold,63 which suggests that FABP4 might suppress lipogenesis in adipocytes, thereby directly contributing to the observed improvements in fasting glucose and insulin in obese FABP4-deficient mice.63 The resulting elevation of circulating levels of fatty acids leads to suppression of the 1c isoform of Srebp1 expression in the liver,61 which might contribute to the protection from development of fatty liver seen in obese FABP4-deficient mice. In addition, basal and stimulated levels of lipolysis are significantly decreased in adipocytes from Fabp4−/− mice, despite the compensatory increase in FABP5 expression.62,64
Decreased efficiency of lipolysis in FABP4-deficient mice probably occurs via modulation of the important lipolytic enzyme hormone-sensitive lipase (HSL), which binds to and is activated by FABP4 in the presence of fatty acids.49,84,85 Whether other adipose tissue lipases also interact with FABP4 or FABP5 is unknown. Using a combination of yeast-two hybrid and glutathione S-transferase-pull down assays, FABP4 was shown to interact with HSL via a domain contained on the helix-turn-helix motif defined by Asp17, Asp18, Lys21 and Arg30.84 Asp17–Arg30 and Asp18–Lys21 in FABP4 form two ion pairs that interact with similarly charged residues on HSL (for example, Asp18 of FABP4 interacts with Lys196 of HSL) to form a complex on the lipid droplet surface.85 This interaction facilitates fatty acid transfer and is consistent with the model by which FABP4 facilitates lipolysis. Given this function, it would be interesting to evaluate the role of FABP4 in the adipose tissue of hibernating mammals, which exhibits a dramatic seasonal cycling between lipolysis and lipogenesis.86
FABPs in immunity and inflammation
Lipids have a central role in immune cell signalling and, accordingly, FABPs have been implicated in immune cell biology. The major regulator of adipocyte differentiation, PPARγ, is expressed in monocytes in response to oxidized LDL, and accumulates at high levels in atherosclerotic lesions.87 PPARγ promotes FABP4 expression in macrophages,39 and indeed FABP4 is highly upregulated during foam cell formation in response to lipid loading.88 Rapamycin, an immunosuppressant that can induce hyperlipidaemia, also induces FABP4 expression in macrophages.89 Furthermore, liver X receptor agonists, which can increase expression of genes involved in cholesterol transport and triglyceride synthesis such as apolipoprotein E and cholesteryl ester transfer protein, also induce FABP4 expression in macrophages, and the FABP4 promoter contains a conserved liver X receptor response element.90
The relationship between PPARs and FABP activity is complex and might be context-dependent. Although PPARγ can initiate FABP4 expression and much evidence has been presented that FABPs can mediate ligand delivery to PPARs,67 the metabolic effects of FABP4 action (specifically the activation of lipolysis and suppression of lipogenesis) seem to be in opposition to those of PPARγ. Indeed, PPARγ activity is increased in FABP4-deficient macrophages, resulting in accelerated cholesterol efflux and decreased cholesterol content.74 Similarly, PPARγ activity is elevated in FABP5-deficient macrophages.91 These data support a model in which FABP4 (and possibly FABP5) can regulate PPARγ activity by controlling ligand availability. Furthermore, FABP4 can increase proteasomal degradation of PPARγ,92 and consequently the net effect of FABP4 seems to be to suppress PPARγ activity (Figure 2).
Figure 2.
Summary of FABP4 intracellular functions. In studies using cultured cells and mouse models, FABP4 carries out a number of intracellular roles. FABP4 suppresses adipose tissue lipogenesis and promotes lipolysis, with direct effects on the composition of the local and circulating free fatty acid pool. FABP4 is also implicated in modulating eicosanoid balance by affecting both COX2 activity and LTA4 stability, and upregulates UCP2; all these processes influence macrophage function and adipose tissue inflammation. FABPs might mediate PPAR activity by regulating ligand availability, and genetic models suggest that FABP4 opposes PPARγ action. FABP4 can also interact with HSL, JAK2 and CD36. Consequently, FABP4 acts on multiple integrated pathways to regulate lipid metabolism and inflammation, impair insulin action, promote glucose production, and contribute to the pathogenesis of immunometabolic diseases such as diabetes mellitus and atherosclerosis. Abbreviations: COX2, cyclooxygenase 2; ER, endoplasmic reticulum; FABP4, fatty acid binding protein 4; HSL, hormone-sensitive lipase; JAK2, janus kinase 2; JNK, c-jun N-terminal kinase; LTA4, leukotriene A4; LXR, liver X receptor; PGE2, prostaglandin E2; PPAR, peroxisome proliferator-activated receptor; STAT3, signal transducer and activator of transcription 3; UCP2, uncoupling protein 2.
In addition, FABP4 and FABP5 bind and stabilize leukotriene A4,93,94 and FABP4-deficient and FABP5-deficient macrophages have impaired activation of prostaglandin G/H synthase 2 expression and production of prostaglandin E2,74,91 which suggests that these FABPs can help to determine macrophage eicosanoid balance. Eicosanoids mediate adipose tissue inflammation in obesity; for example, leukotriene B4 binding to its cognate receptor on macrophages induces the expression of C-C motif chemokine 2 (commonly known as monocyte chemoattractant protein 1 [MCP-1]), which leads to potentiated inflammation, and Blt1−/− mice (which lack leukotriene B4 receptor 1) are protected from obesity-linked macrophage recruitment to adipose depots and exhibit attenuated inflammation.95
FABPs, and FABP4 in particular, have pronounced effects on cellular and systemic lipid and signalling mediator composition, with important consequences for inflammatory processes. For example, FABP4-deficient macrophages have reduced basal and stimulated expression of proinflammatory cytokines including MCP-1 and TNF,96 as well as decreased IKK-β/NF-κB activity and deficient activation-induced expression of inducible nitric oxide synthase.74 One of the intriguing results of FABP-deficiency in adipose tissue is the activation of de novo lipogenesis and the resulting production of the related products from the target cells. For example, Fabp4–5−/− mice have increased levels of palmitoleate, particularly in adipocytes, macrophages, and the fatty acid fraction of serum, but not liver.80 In subsequent studies this lipokine was shown to act on the liver as well as other cells and tissues such as macrophages to regulate lipid metabolism and inflammatory responses.80,97 In macrophages, FABP-deficiency provides strong protection against lipotoxicity. The addition of monounsaturated fatty acids such as palmitoleate to macrophages induces expression of UCP2, which encodes mitochondrial uncoupling protein 2.98 Moreover, macrophages isolated from Fabp4−/− mice have increased palmitoleate levels and increased expression of UCP2, and silencing of UCP2 in FABP4-deficient macrophages attenuates NF-κB signalling and the inflammatory response to lipopolysaccharide.98 Correspondingly, increased expression of UCP2 in macrophages is sufficient to polarize cells towards the M2 phenotype, reduce endoplasmic reticulum stress and diminish oxidative stress.98 Furthermore, in protein–protein interaction studies, FABP4 interacts directly with tyrosine-protein kinase JAK2 and might attenuate cytokine signalling within macrophages.99 Finally, the lipotoxicity that occurs in the context of atherogenesis induces the unfolded protein response within macrophages; FABP4 seems to be critical for this process and, as a result, FABP4-deficient macrophages are protected from cholesterol or palmitate-induced endoplasmic reticulum stress and cell death in vitro and in vivo.81
Given the expression of FABP4 in both macrophages and adipocytes, and the close association between insulin resistance, inflammation and atherogenesis,100 a role for FABP4 in the pathogenesis of atherosclerosis is perhaps unsurprising. FABP4 is expressed in atherosclerotic lesions and foam cells and, in the atherogenic ApoE−/− mouse model, FABP4 deficiency results in reduced macrophage accumulation in plaques and reduced plaque area in early and late stages of disease development.101–103 In further support for this crucial role, a small molecule FABP4 inhibitor, BMS309403, potently reduced lesion area in a mouse model of atherosclerosis.104 Indeed, both in genetic and chemical models of FABP4 inhibition, the anti-atherogenic phenotype seems to be the most robust.104,105 In bone marrow transplant experiments, the protection from atherosclerosis occurs largely via FABP actions in haematopoietic cells,96,102 and the findings from these studies suggest that FABP5 has a similar but smaller role in this process.91 Although FABP5 does not seem to be upregulated in FABP4−/− macrophages,96,102 combined FABP4–FABP5 deficiency provides moderate additional benefit in the ApoE−/− background by improving glucose homeostasis, resulting in reduced plaque formation and prolonged survival.105
In addition to macrophages, FABP4 is also expressed in myeloid dendritic cells, and is upregulated during their differentiation.106 Dendritic cells that are FABP4-deficient produce lower levels of cytokines including IL-12 and TNF, and have reduced capacity to activate T cells.106 These data, and the finding that FABP-deficient mice are protected against experimental autoimmune encephalomyelitis, 107 suggest that in addition to its immunometabolic roles, FABP4 might have wide-ranging effects on adaptive immunity, autoimmunity and susceptibility to infection. This is probably the result of the FABP4 effect on metabolic programming of its target cells, and needs to be further explored in mechanistic and functional studies.
Other intracellular FABP functions
Although the first expression profiles of FABP4 suggested this protein had a very limited tissue distribution, detailed analysis has revealed additional sites of expression and function in specific conditions. For example, both FABP4 and FABP5 are expressed in bronchial epithelial cells upon exposure to IL-4 and IL-13, which suggests a potential role for these FABPs in the pathogenesis of allergic asthma.40 Indeed, FABP4 expression is induced in the airway in the ovalbumin challenge model of allergic inflammation, and FABP4-deficient mice are protected from inflammatory cell infiltration in this model.40 Other investigators have described potential roles for FABP4 in endothelial cell growth and blood vessel branching,108–113 and FABP4 has also been implicated in cancer cell growth and metastasis. 114–116 The mechanisms that underly these effects are not fully understood and indeed might be pleiotropic; for example, whether the protection from asthma in Fabp4−/− mice40 is due to changes in immune responses, alterations in bronchoepithelial cells, other stromal cells or both, is unclear. Finally, conditions of ectopic lipid accumulation in tissues, especially in the presence of inflammatory and stress responses, might produce a permissive milieu that activates aberrant FABP expression in cells such as hepatocytes, or trigger expression from resident immune cells such as Kupffer cells. Although our understanding of FABP function in different cell types has increased, the development of tissue specific gain-of-function and loss-of-function models might help to fully define the targets and related functions under different conditions.
Extracellular FABPs
FABPs have been primarily studied as intracellular proteins. However, after the development of highly sensitive detection systems and serum proteomics, multiple FABP isoforms have been detected in the circulation.117 In general, these circulating FABPs are seen as markers of cell injury or death. For example, extracellular FABP3 was first detected in mice during experimental ischaemia– reperfusion injury owing to leakage of the protein through the plasma membrane, which might impair the capacity of cardiac myocytes to handle their lipid load.118 FABP3 can also be detected in the plasma and urine of patients following muscle injury or acute myocardial infarction.119,120 In healthy individuals, the plasma level of FABP2 is near or below the lower limit of detection, but is increased by approximately fourfold in patients with ischaemic bowel disease.121 Furthermore, in a human surgical model of intestinal ischaemia, FABP2 levels in venous blood correlate with the level of tissue damage.122 Similarly, elevated plasma levels of FABP1 have been associated with liver transplant rejection117 and are a biomarker for chronic hepatitis C infection.123
The lack of signal peptide sequence has led to the assumption that all FABPs detected extracellularly might result from cell leakage or death. However, evidence has been presented supporting regulated secretion of some of these proteins. For example, FABP1 is detectable in bile in the absence of cellular injury,124 and mammary-derived growth inhibitor, which is thought to represent a combination of FABP4 and FABP3,125 is present in milk.126,127 Consequently, at least some FABPs might undergo regulated release and, in addition to their usefulness as biomarkers, might have active, physiologically relevant extracellular activities. Studies from our laboratory have shown that FABP4 is secreted from cultured adipocytes and adipose tissue explants in a regulated manner in the absence of cell death, and in mice plasma FABP4 levels are elevated in response to fasting-related and lipolytic signals.128 In addition, FABP4 plasma levels are elevated in experimental models of obesity, as well as in patients with obesity, and correlate with aspects of metabolic syndrome such as insulin resistance.129 Interestingly, regulated secretion of FABP4 has also been demonstrated in cultured macrophages,130 although bone marrow transplant experiments in mice suggest that the majority of FABP4 in the circulation is generated from the stromal compartment rather than bone marrow-derived cells.128
Circulating FABP4 and metabolic pathology
Nearly 100 articles have been published regarding links between plasma levels of FABP4 and different pathologies in multiple populations, and almost half of these papers identify a positive correlation between levels of plasma FABP4, obesity and type 2 diabetes mellitus. One of the largest of these studies was a cross-sectional analysis of the 3,658 individuals within the Framingham Heart Study, in which increased plasma FABP4 levels correlated with increased BMI and insulin resistance, as well as dyslipidaemia. 131 Examination of the 1,847 participants in the Hong Kong Cardiovascular Risk Factor Prevalence Study revealed that elevated FABP4 correlated with increased BMI, insulin resistance, elevated LDL cholesterol, and even reduced survival from cardiovascular disease.132 In a follow-up study of >1,000 patients with cardiovascular disease in Germany, those patients who experienced secondary cardiovascular disease events had significantly higher plasma FABP4, and FABP4 levels were also higher in patients with type 2 diabetes mellitus, hypertension and elevated triglyceride levels than in patients without those complications.133 In other small studies, plasma FABP4 can predict the development of type 2 diabetes mellitus independent of the presence of obesity,134 is an independent biomarker of insulin resistance,135 predicts early death in patients with stroke,136 and is elevated in patients with severe obstructive sleep apnoea syndrome.137 Independently of obesity, circulating FABP4 is also higher in patients with breast cancer than in healthy individuals,138 is associated with vascular inflammation,139 and is predictive of the development of insulin resistance in recipients of liver transplants.140 Consequently, a large and growing body of evidence supports a strong link between circulating levels of FABP4, obesity and metabolic disease (Figure 3, Supplementary file 1).
Figure 3.
The association of circulating levels of FABP4 with different human diseases. A summary of studies in humans showing the association of different immunometabolic diseases with circulating levels of FABP4 organized by number of patients. Numbers in the outer circle represent the number of studies in each category. For full list of references included in this figure please see reference list in Supplementary file 1 online. Abbreviations: FABP4, fatty acid binding protein 4; NAFLD, nonalcoholic fatty-liver disease.
Although in many studies a link between weight, adiposity and circulating levels of FABP4 has been observed, in other analyses the relationship seems to be more complicated. This is particularly true in diseases with severe or rapid loss of adiposity such as lipodystrophies and anorexia nervosa, or uncontrolled lipolysis and lack of insulin such as in type 1 diabetes mellitus,141–143 which might introduce massive lipid breakdown or adipose tissue injury. This effect could result in increased levels of circulating FABP4 despite reducing the rate of secretion from adipocytes and is likely to introduce complex patterns of regulation and disease correlations.
The correlation between metabolic disease and circulating levels of FABP4 is also supported by a number of genetic studies in humans. A polymorphism in the promoter of FABP4 (–87T>C; rs77878271) that results in reduced expression of this gene is associated with reduced risk of coronary heart disease and with reduced risk of developing type 2 diabetes mellitus among patients with obesity.144 In an independent study, the same variant in the FABP4 promoter resulted in reduced FABP4 expression and was associated with decreased total cholesterol levels and reduced risk of myocardial infarction in patients with advanced atherosclerosis. 145 Moreover, in other small studies an association between FABP4 polymorphisms, plasma levels of FABP4 and metabolic outcome in polycystic ovary syndrome,146 and obstructive sleep apnoea147 have been found. Consequently, overwhelming biochemical and genetic evidence demonstrates an important correlation between circulating levels of FABP4 and metabolic disease, which raises the possibility that targeting this form of the molecule might be a useful therapeutic intervention.
Extracellular function of FABP4
Given the wide range of pathophysiological associations of high levels of circulating FABP4, the functions of this form of FABP4 are likely to be pleiotropic (Figure 4). The findings in Fabp4−/− mice, which have preserved glucose homeostasis during obesity,75 and the correlation between plasma FABP4 levels and type 2 diabetes mellitus in humans (Figure 3, Supplementary file 1) suggest that circulating FABP4 might function as an adipokine in metabolism. Indeed, in vitro, addition of exogenous FABP4 to primary hepatocytes can induce a moderate increase in expression of gluconeogenic genes such as glucose-6-phosphatase, and glucose production, and infusion of FABP4 into Fabp4−/− mice in the clamp setting increases gluconeogenic enzyme activity and hepatic glucose production.128 As dysregulated hepatic glucose production is a major contributor to the diabetic phenotype,148 this finding suggests that FABP4 is a critical factor connecting adipose tissue status to liver function and systemic metabolism. In addition, neutralization of the FABP4 protein with a polyclonal antibody significantly improved glucose tolerance and insulin sensitivity in obese mice, providing strong support for the concept that circulating FABP4 is a major contributor to metabolic decline in obesity.128 In one study, FABP4 modestly increased insulin secretion from β cells in vitro when delivered with the fatty acid linoleate. 149 However, whether this action is due to the lipid cargo alone or related to FABP4 is unclear. Furthermore, reduced insulin secretion in the setting of adrenergic stimulation has been previously described in FABP4-deficient mice, which was attributed to alteration of free fatty acid profile.64 Indeed, delivery of exogenous FABP4 can induce insulin resistance without altering β cell responsiveness,150 consequently FABP4 might initiate hyperinsulinaemia downstream of insulin resistance.
Figure 4.
Pleiotropic functions of circulating FABP4. FABP4 secretion from adipose tissue is enhanced in the setting of obesity and insulin resistance. The circulating form of FABP4 can function as a hormone and regulate hepatic glucose production. This form of the protein, and lipids regulated by FABP4 such as palmitoleate, also affect insulin action, hepatic lipid metabolism, and cardiac function. Other potential roles of circulating FABP4 include regulation of tumour growth, immune cell functions, endothelial migration, cardiomyocyte contraction and insulin secretion. Abbreviations: FABP4, fatty acid binding protein 4; FFA, free fatty acid. Modified with permission from Elsevier © Cao, H. et al. Adipocyte lipid chaperone aP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 17, 768–778 (2013).
The established link between circulating levels of FABP4, atherosclerosis and cardiovascular disease (Figure 3, Supplementary file 1) has led some researchers to investigate the potential function of circulating FABP4 in endothelial cell biology and cardiac function. Exogenous FABP4 can induce replication and migration of human coronary artery smooth muscle cells151 and inhibit expression of NOS3 in human umbilical vein endothelial cells.152 Exogenous FABP4 also suppresses contraction in cultured cardiomyocytes in vitro, although whether this effect is a specific FABP4 function is unclear.153 This activity of FABP4 on cardiomyocyte function is potentiated in the presence of epoxyeicosatrienoic acids, which supports the concept that fatty acid ligands can modulate the function of FABP4.154 In addition, FABPs themselves regulate the production of lipids that have effects on cardiomyocytes, therefore FABPs are likely to exert both direct and indirect effects on cardiac function. For example, palmitoleate, a lipid produced at high levels in adipocytes lacking FABP4, is required to sustain cardiac function in the python.155
In mice, the most dramatic effect of genetic deficiency in FABP4 has been observed in models of atherosclerosis, 101–103 but the contribution of circulating levels of FABP4 to inflammatory and cardiovascular function has not yet been defined. In addition, determining the extracellular roles of other FABP family members, and the degree to which each can functionally compensate for the others, will be a crucial question for future research. Continuing investigation of the relative affinities of FABP isoforms for different ligands will also help to determine their roles in delivering and modulating extracellular lipid.
Regulation of FABP4 secretion
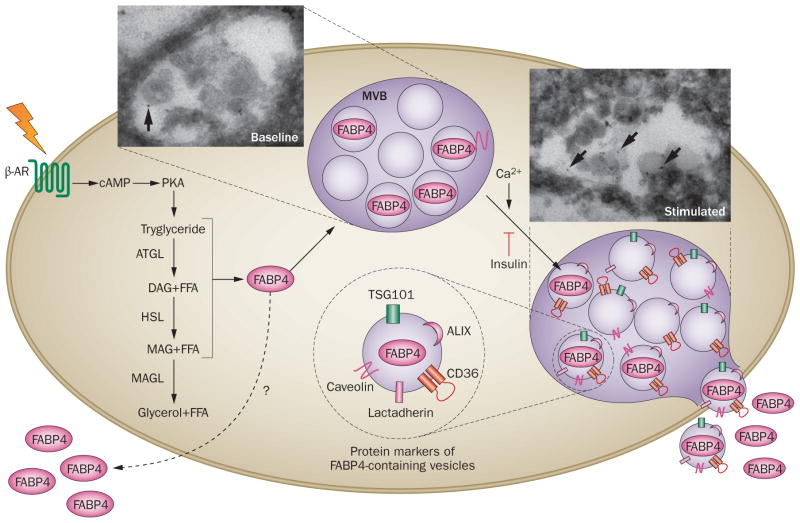
Given the lack of a signal peptide, one can reason that regulated FABP4 secretion might occur via a non-classical pathway. Indeed, inhibitors of classical secretion do not alter FABP4 release from cultured adipocytes.128 As described previously in the text, circulating FABP4 functions by enhancing hepatic glucose production; plasma FABP4 levels are elevated during fasting, and FABP4 secretion is suppressed by insulin.128 In addition, in clamp studies, administration of exogenous FABP4 impairs insulin sensitivity while FABP4 neutralization improves glucose disposal.128 Taken together, these findings indicate that FABP4 is an adipose-derived factor that integrates with components of the fasting response to maintain glucose homeostasis. Secretion of FABP4 is responsive to signals that induce lipolysis, including β-adrenergic receptor agonists, forskolin and isobutyl-methylxanthine, 128 and chemical inhibition or genetic deficiency of HSL and patatin-like phospholipase domain-containing protein 2 (also known as adipose triglyceride lipase) abrogate β-adrenergic-induced FABP4 secretion.156 Interestingly, this effect might be linked to the lipolysis-induced increase in free fatty acids, and indeed lipid infusion in vivo and lipid treatment of adipocytes in vitro is sufficient to increase FABP4 secretion.156 Increased FABP4 release has also been seen in adipocytes exposed to hypoxia,149 which might in part explain the increased level of FABP4 in circulation observed in obese rodent models and humans with obesity, in whom adipose tissue is poorly oxygenated.157 In adipocytes, FABP4 localizes to multivesicular bodies and its presence in these structures is enhanced upon stimulation of lipolysis.156 Fractionation of the media of cultured adipocytes suggests that FABP4 is present in caveolin-1 positive microvesicles,158 and in electron microscopy and cell fractionation experiments FABP4 is localized to vesicles that are ~100 nm in diameter and positive for the exosome markers CD36 and programmed cell death 6-interacting protein (also known as ALIX).156 Remarkably, FABP4 localization to these vesicles also increases in response to lipolytic signals.156 In addition, increasing the concentration of intracellular calcium increases FABP4 secretion from both macrophages and adipocytes (Figure 5).130,158 Interestingly, FABP4 secretion via an exosome-like vesicular pathway in adipocytes is not entirely dependent on its lipid-binding activity, as mutants in the lipid binding domain of FABP4 can still be secreted, albeit at a lower efficiency than the wild-type protein.156 Although this vesicular secretory pathway has been clearly demonstrated in experimental studies, the vast majority of FABP4 present in the serum is in a free form.156 Whether multiple routes of FABP4 secretion exist that perhaps support different forms of the molecule is unclear. Improved understanding of the molecular mechanisms that drive FABP4 secretion has the potential to lead to novel therapeutic interventions that modulate level of this hormone and its subsequent metabolic effects.
Figure 5.
Regulated vesicular secretion of FABP4. The secretion of FABP4 from adipocytes has been demonstrated in vivo in response to fasting and signals that induce lipolysis such as β-AR stimulation. Furthermore, the actions of lipolytic enzymes ATGL and HSL (but not MAGL) are required for the induction of FABP4 secretion, and an influx in FFAs is also sufficient for increased secretion. Following lipolytic stimulation, FABP4 is recruited to multivesicular bodies, and secreted in vesicles that have exosome markers including CD36, calveolin, lactadherin (also known as milk fat globule factor 8 protein), TSG101, and ALIX, as shown on the right. The secretion of FABP4 has also been shown to be responsive to Ca2+ signalling, and to be inhibited by insulin. Whether alternative paths exist that support FABP4 secretion is currently unknown. Visualization of multivesicular bodies containing labelled FABP4 (arrows) before and after β-AR stimulation are shown in insets. Abbreviations: β-AR, β-adrenergic receptor; ALIX, programmed cell death 6 interacting protein; ATGL, adipose triglyceride lipase; DAG, diacyl glycerol; FFA, free fatty acid; HSL, hormone-sensitive lipase; MAG, monoacylglycerol; MAGL, monoacylglycerol lipase; MVB, multivesicular body; PKA, protein kinase A; TSG101, tumour susceptibility gene 101 protein.
Therapeutic implications of FABPs
Much of the therapeutic research surrounding FABP activity has been focused on FABP4. These studies have been based on animal models of obesity, diabetes mellitus and cardiovascular disease, as well as genetic association studies, and population and longitudinal studies in humans in which plasma FABP4 levels are associated with disease risk and adverse prognosis. The similarities in cellular, molecular and biochemical studies between humans and model systems all suggest that targeting FABP4 in these chronic immunometabolic diseases might be a useful therapeutic approach. For example, treating genetically obese mice with BMS309403 improves glucose homeostasis and insulin sensitivity, reduces adipose tissue inflammation, and reduces lipid accumulation in the liver.104 This synthetic small molecule FABP4 inhibitor also significantly limited atherosclerotic lesion formation and improved endothelial function in ApoE−/− mice,104,159 and reduced acute liver injury and high fat high cholesterol diet-induced non-alcoholic fatty liver disease.160 At the level of the macrophage, another small molecule FABP4 inhibitor, HTS01037, induces UCP2 expression and attenuates proinflammatory NF-κB signalling.98 Other FABP4 inhibitors have been developed,161–165 although their in vivo efficacy remains incompletely characterized. However, some have been shown to improve glucose homeostasis and hepatic lipid accumulation in mice with diet-induced obesity.165
In addition to novel synthetic small molecule FABP4 inhibitors, a screen of FDA-approved drugs resulted in the discovery of several FABP4-binding molecules.166 Among these, a broad-spectrum antibiotic, levofloxacin, was identified as a high-affinity inhibitor of FABP4.166 In addition, benzbromarone, a drug with uricosuric properties, was also identified as an inhibitor of FABP4 and can significantly reduce blood glucose in the db/db mouse model of obesity and type 2 diabetes mellitus.167 The metabolic phenotypes generated by these molecules or their derivatives will be interesting to study, although in general the small molecule approach might be challenging due to issues of nonspecificity and off-target effects inherent with this therapeutic strategy. In addition, whether small molecule-mediated inhibition of both intracellular and extracellular FABP4 actions will be desirable therapeutically is unknown, as some intracellular actions might be important for normal adipocyte function.
Major advances have been made in targeting molecules in specific cell types using modified carriers for small interfering RNAs. For example, targeting TNF or osteopontin using β1,3-D-glucan-encapsulated siRNAs in adipose tissue or macrophages of obese animals improved insulin sensitivity and glucose tolerance.168,169 Another elegant strategy to target FABP4 in adipose tissue has been developed using short-hairpin RNAs (shRNAs) coupled to a peptide sequence that facilitates specific delivery to adipose tissue in vivo, which resulted in a marked suppression of FABP4 expression.170 Targeting FABP4 in adipose tissue using this method increased insulin sensitivity and glucose tolerance and reduced blood glucose levels, which recapitulates the effects of FABP4 genetic deficiency and demonstrates the feasibility of therapeutically targeting FABP4 in vivo using interfering RNA.170
Owing to the emerging insights related to the biological function of secreted FABP4, especially in the context of metabolic diseases, it was interesting to consider the possibility of targeting FABP4 using antibody-mediated neutralization. Surprisingly, treatment of obese diabetic mice with a polyclonal antibody targeting FABP4 significantly improved insulin sensitivity and glucose homeostasis.128 Translation of this strategy into clinical efficacy will require the development of potent monoclonal antibodies, and to date only one group of investigators has reported the application of an FABP4 monoclonal antibody to obese diabetic mice, which modestly improved glucose tolerance with no effect on insulin sensitivity.171 In addition, the extent to which FABP4 biology in metabolic disease is dictated by the secreted versus the cellular forms must be defined, and how other FABP proteins might be capable of functional compensation also needs to be understood.
Interesting possibilities also exist for the use of FABP4 as a target in cancer, including ovarian, breast and prostate malignancies. Although the data are less prevalent for this use, the role for FABP4 in stroma–tumour interactions and the energy extraction of cancer cells from adipose depots or sites of metastasis (for example, in ovarian tumours114 and prostate cancer172) justify further study and testing of the advanced therapeutic molecules targeting FABP4.
Conclusions
Studies conducted in the past 20 years have clearly demonstrated the exciting biological functions carried out by the simple proteins that bind to fatty acids. In this Review we discussed the biology of FABPs, primarily FABP4 (also known as aP2), which is the best characterized member of this family with important roles in metabolic homeostasis and immunometabolic diseases. However, ongoing studies are identifying interesting biological functions of the other isoforms, and in the coming years, we should have further exciting insights into their functions in physiology and pathophysiology.
Two developments have marked critical paradigm shifts in our understanding of FABPs. The first relates to their mechanistic complexity and vast biological diversity, which illustrates that these proteins are far more than simple reservoirs or buffers for excess fatty acids. The second is the finding that these proteins do not act only as intracellular mediators but also have extracellular functions. The discovery that some FABPs undergo regulated secretion and exert specific biological functions in tissues other than their origin reveals that this family of proteins are perhaps the most versatile endocrine hormones and potent integrators of tissue status, especially lipid homeostasis, with systemic metabolic homeostasis.
However, with progress comes complexity and, as the fields of FABP and lipid biology research have advanced, the puzzle of how these molecules interact has grown exponentially. In our opinion, one of the most important areas of future investigation involves resolution of how different FABPs interact with various ligands, and the biological implications of these interactions, as well as an understanding of the physiological, dietary and pathophysiological conditions under which FABP biology can be altered by the lipid milieu and composition. New research has revealed the presence of metabolically-active lipids produced in state-specific settings.80,155,173,174 In combination with the numerous lipid binding proteins that also show hormonal activity, there seem to be infinite permutations by which these lipids might integrate metabolic and inflammatory status and transmit signals. Unraveling these details will require a concerted effort, but also provide an exciting opportunity for the development of novel therapeutic approaches.
The second area of future investigation relates to the mechanisms of FABP4 action, especially the most proximal steps that initiate the signalling cascades that are characterized as downstream of FABP action. Specifically ,the determination of the molecular organization of FABP4 within an extracellular vesicle, and what determinants on FABP4 define packaging and release from these vesicular compartments is an important consideration. Finally, do cell surface molecules exist that interact with FABPs to propagate their signal, and what protein complexes and lipid ligands, inside or outside of the cell, assemble as signalling nodes for these molecules? Resolution of these critical questions will greatly enhance our current understanding of not only FABPs but also the diverse functions of lipids as signalling intermediates and endocrine hormones.
Supplementary Material
Key points.
Fatty acid-binding proteins (FABPs) are versatile proteins that can modulate lipid fluxes, trafficking, signalling and metabolism
Fatty acid-binding protein, adipocyte (FABP4) regulates metabolic and inflammatory pathways, and in mouse models its inhibition can improve type 2 diabetes mellitus and atherosclerosis
FABP4 is actively secreted by adipocytes and its levels are increased in obesity; in humans, elevated circulating FABP4 levels are associated with obesity, metabolic disease and cardiac dysfunction
Circulating FABP4 is secreted through a vesicular pathway and has pleiotropic roles that include the stimulation of hepatic glucose production
Targeting FABP4 offers a novel therapeutic approach for the treatment of many metabolic diseases
The signalling components of hormonal FABP4 and determinants of FABP-mediated functions in the context of specific lipid or other cargo are issues that must be addressed in future research
Acknowledgments
The authors thank members of the Hotamisligil and Bernlohr laboratories for helpful discussions. We thank A. P. Arruda for assistance in generating the initial figures, and K. Claiborn for critical reading and editing of the manuscript. The Hotamisligil laboratory is supported in this area by research funding from the NIH (grant number DK064360) and a sponsored research agreement with Union Chimique Belge. The Bernlohr laboratory is supported by the NIH (grant number DK053189).
Footnotes
Author contributions
Both authors researched data for the article, discussed the content, and wrote, reviewed and edited the manuscript before submission.
Supplementary information is linked to the online version of the paper at www.nature.com/nrendo.
Competing interests
G.S.H. receives research funding under a sponsored agreement with Union Chimique Belge. D.A.B. declares no competing interests.
Contributor Information
Gökhan S. Hotamisligil, Department of Genetics and Complex Diseases and Sabri Ülker Center, Harvard T.H. Chan School of Public Health, 677 Huntington Avenue, Boston, MA 02115, USA
David A. Bernlohr, Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, 321 Church Street SE, Minneapolis, MN 55455, USA
References
- 1.Poveda JA, et al. Lipid modulation of ion channels through specific binding sites. Biochim Biophys Acta. 2014;1838:1560–1567. doi: 10.1016/j.bbamem.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 4.Dresner A, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 6.Kim JK, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci USA. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen MT, et al. JNK and tumor necrosis factor-α mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Severeid L, Connor WE, Long JP. The depressant effect of fatty acids on the isolated rabbit heart. Proc Soc Exp Biol Med. 1969;131:1239–1243. doi: 10.3181/00379727-131-34078. [DOI] [PubMed] [Google Scholar]
- 11.Gordon GB. Saturated free fatty acid toxicity. II. Lipid accumulation, ultrastructural alterations, and toxicity in mammalian cells in culture. Exp Mol Pathol. 1977;27:262–276. doi: 10.1016/0014-4800(77)90035-1. [DOI] [PubMed] [Google Scholar]
- 12.Ockner RK, Manning JA, Poppenhausen RB, Ho WK. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972;177:56–58. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- 13.Ockner RK, Manning JA. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974;54:326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ockner RK, Manning JA. Fatty acid binding protein. Role in esterification of absorbed long chain fatty acid in rat intestine. J Clin Invest. 1976;58:632–641. doi: 10.1172/JCI108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta. 2010;1801:246–251. doi: 10.1016/j.bbalip.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ockner RK, Manning JA, Kane JP. Fatty acid binding protein. Isolation from rat liver, characterization, and immunochemical quantification. J Biol Chem. 1982;257:7872–7878. [PubMed] [Google Scholar]
- 18.Gordon JI, Alpers DH, Ockner RK, Strauss AW. The nucleotide sequence of rat liver fatty acid binding protein mRNA. J Biol Chem. 1983;258:3356–3363. [PubMed] [Google Scholar]
- 19.Bass NM, Raghupathy E, Rhoads DE, Manning JA, Ockner RK. Partial purification of molecular weight 12,000 fatty acid binding proteins from rat brain and their effect on synaptosomal Na+-dependent amino acid uptake. Biochemistry. 1984;23:6539–6544. doi: 10.1021/bi00321a040. [DOI] [PubMed] [Google Scholar]
- 20.Haq RU, Shrago E, Christodoulides L, Ketterer B. Purification and characterization of fatty acid binding protein in mammalian lung. Exp Lung Res. 1985;9:43–55. doi: 10.3109/01902148509061527. [DOI] [PubMed] [Google Scholar]
- 21.Sweetser DA, Lowe JB, Gordon JI. The nucleotide sequence of the rat liver fatty acid-binding protein gene. Evidence that exon 1 encodes an oligopeptide domain shared by a family of proteins which bind hydrophobic ligands. J Biol Chem. 1986;261:5553–5561. [PubMed] [Google Scholar]
- 22.Sacchettini JC, Said B, Schulz H, Gordon JI. Rat heart fatty acid-binding protein is highly homologous to the murine adipocyte 422 protein and the P2 protein of peripheral nerve myelin. J Biol Chem. 1986;261:8218–8223. [PubMed] [Google Scholar]
- 23.Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99:299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- 24.Kurtz A, et al. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe R, et al. Molecular cloning of a cDNA encoding a novel fatty acid-binding protein from rat skin. Biochem Biophys Res Commun. 1994;200:253–259. doi: 10.1006/bbrc.1994.1442. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman BM, Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980;255:8811–8818. [PubMed] [Google Scholar]
- 27.Bernlohr DA, Angus CW, Lane MD, Bolanowski MA, Kelly TJ., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci USA. 1984;81:5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haq RU, Christodoulides L, Ketterer B, Shrago E. Characterization and purification of fatty acid-binding protein in rat and human adipose tissue. Biochim Biophys Acta. 1982;713:193–198. doi: 10.1016/0005-2760(82)90236-3. [DOI] [PubMed] [Google Scholar]
- 29.LaLonde JM, Bernlohr DA, Banaszak LJ. The up-and-down β-barrel proteins. FASEB J. 1994;8:1240–1247. doi: 10.1096/fasebj.8.15.8001736. [DOI] [PubMed] [Google Scholar]
- 30.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285:32679–32683. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zezulak KM, Green H. Specificity of gene expression in adipocytes. Mol Cell Biol. 1985;5:419–421. doi: 10.1128/mcb.5.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernlohr DA, Doering TL, Kelly TJ, Jr, Lane MD. Tissue specific expression of p422 protein, a putative lipid carrier, in mouse adipocytes. Biochem Biophys Res Commun. 1985;132:850–855. doi: 10.1016/0006-291x(85)91209-4. [DOI] [PubMed] [Google Scholar]
- 33.Matarese V, Bernlohr DA. Purification of murine adipocyte lipid-binding protein. Characterization as a fatty acid- and retinoic acid-binding protein. J Biol Chem. 1988;263:14544–14551. [PubMed] [Google Scholar]
- 34.Blake WL, Clarke SD. Induction of adipose fatty acid binding protein (a-FABP) by insulin-like growth factor-1 (IGF-1) in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 1990;173:87–91. doi: 10.1016/s0006-291x(05)81025-3. [DOI] [PubMed] [Google Scholar]
- 35.Amri EZ, Ailhaud G, Grimaldi P. Regulation of adipose cell differentiation. II. Kinetics of induction of the aP2 gene by fatty acids and modulation by dexamethasone. J Lipid Res. 1991;32:1457–1463. [PubMed] [Google Scholar]
- 36.Amri EZ, Bertrand B, Ailhaud G, Grimaldi P. Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res. 1991;32:1449–1456. [PubMed] [Google Scholar]
- 37.Distel RJ, Robinson GS, Spiegelman BM. Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992;267:5937–5941. [PubMed] [Google Scholar]
- 38.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 39.Pelton PD, Zhou L, Demarest KT, Burris TP. PPARγ activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem Biophys Res Commun. 1999;261:456–458. doi: 10.1006/bbrc.1999.1071. [DOI] [PubMed] [Google Scholar]
- 40.Shum BO, et al. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116:2183–2192. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wootan MG, Bass NM, Bernlohr DA, Storch J. Fatty acid binding sites of rodent adipocyte and heart fatty acid binding proteins: characterization using fluorescent fatty acids. Biochemistry. 1990;29:9305–9311. doi: 10.1021/bi00492a001. [DOI] [PubMed] [Google Scholar]
- 42.Xu Z, Bernlohr DA, Banaszak LJ. The adipocyte lipid-binding protein at 1.6-A resolution. Crystal structures of the apoprotein and with bound saturated and unsaturated fatty acids. J Biol Chem. 1993;268:7874–7884. [PubMed] [Google Scholar]
- 43.Richieri GV, Ogata RT, Kleinfeld AM. Equilibrium constants for the binding of fatty acids with fatty acid-binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J Biol Chem. 1994;269:23918–23930. [PubMed] [Google Scholar]
- 44.Wootan MG, Bernlohr DA, Storch J. Mechanism of fluorescent fatty acid transfer from adipocyte fatty acid binding protein to membranes. Biochemistry. 1993;32:8622–8627. doi: 10.1021/bi00084a033. [DOI] [PubMed] [Google Scholar]
- 45.Gericke A, Smith ER, Moore DJ, Mendelsohn R, Storch J. Adipocyte fatty acid-binding protein: interaction with phospholipid membranes and thermal stability studied by FTIR spectroscopy. Biochemistry. 1997;36:8311–8317. doi: 10.1021/bi970679s. [DOI] [PubMed] [Google Scholar]
- 46.Herr FM, Matarese V, Bernlohr DA, Storch J. Surface lysine residues modulate the collisional transfer of fatty acid from adipocyte fatty acid binding protein to membranes. Biochemistry. 1995;34:11840–11845. doi: 10.1021/bi00037a023. [DOI] [PubMed] [Google Scholar]
- 47.LiCata VJ, Bernlohr DA. Surface properties of adipocyte lipid-binding protein: response to lipid binding, and comparison with homologous proteins. Proteins. 1998;33:577–589. doi: 10.1002/(sici)1097-0134(19981201)33:4<577::aid-prot10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 48.Banaszak L, et al. Lipid-binding proteins: a family of fatty acid and retinoid transport proteins. Adv Protein Chem. 1994;45:89–151. doi: 10.1016/s0065-3233(08)60639-7. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins-Kruchten AE, et al. Fatty acid-binding protein-hormone-sensitive lipase interaction. Fatty acid dependence on binding. J Biol Chem. 2003;278:47636–47643. doi: 10.1074/jbc.M307680200. [DOI] [PubMed] [Google Scholar]
- 50.Hellberg K, et al. X-ray crystallographic analysis of adipocyte fatty acid binding protein (aP2) modified with 4-hydroxy-2-nonenal. Protein Sci. 2010;19:1480–1489. doi: 10.1002/pro.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borchers T, Spener F. Involvement of arginine in the binding of heme and fatty acids to fatty acid-binding protein from bovine liver. Mol Cell Biochem. 1993;123:23–27. doi: 10.1007/BF01076471. [DOI] [PubMed] [Google Scholar]
- 52.Jenkins AE, Hockenberry JA, Nguyen T, Bernlohr DA. Testing of the portal hypothesis: analysis of a V32G, F57G, K58G mutant of the fatty acid binding protein of the murine adipocyte. Biochemistry. 2002;41:2022–2027. doi: 10.1021/bi015769i. [DOI] [PubMed] [Google Scholar]
- 53.Sha RS, Kane CD, Xu Z, Banaszak LJ, Bernlohr DA. Modulation of ligand binding affinity of the adipocyte lipid-binding protein by selective mutation. Analysis in vitro and in situ. J Biol Chem. 1993;268:7885–7892. [PubMed] [Google Scholar]
- 54.Grimsrud PA, Picklo MJ, Sr, Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- 55.Chabowski A, Gorski J, Luiken JJ, Glatz JF, Bonen A. Evidence for concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane. Prostaglandins Leukot Essent Fatty Acids. 2007;77:345–353. doi: 10.1016/j.plefa.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 57.Spitsberg VL, Matitashvili E, Gorewit RC. Association and coexpression of fatty-acid-binding protein and glycoprotein CD36 in the bovine mammary gland. Eur J Biochem. 1995;230:872–878. doi: 10.1111/j.1432-1033.1995.tb20630.x. [DOI] [PubMed] [Google Scholar]
- 58.Woodford JK, Jefferson JR, Wood WG, Hubbell T, Schroeder F. Expression of liver fatty acid binding protein alters plasma membrane lipid composition and structure in transfected L-cell fibroblasts. Biochim Biophys Acta. 1993;1145:257–265. doi: 10.1016/0005-2736(93)90297-d. [DOI] [PubMed] [Google Scholar]
- 59.Iso T, et al. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler Thromb Vasc Biol. 2013;33:2549–2557. doi: 10.1161/ATVBAHA.113.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy EJ, Prows DR, Stiles T, Schroeder F. Liver and intestinal fatty acid-binding protein expression increases phospholipid content and alters phospholipid fatty acid composition in L-cell fibroblasts. Lipids. 2000;35:729–738. doi: 10.1007/s11745-000-0579-x. [DOI] [PubMed] [Google Scholar]
- 61.Maeda K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Coe NR, Simpson MA, Bernlohr DA. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J Lipid Res. 1999;40:967–972. [PubMed] [Google Scholar]
- 63.Shaughnessy S, Smith ER, Kodukula S, Storch J, Fried SK. Adipocyte metabolism in adipocyte fatty acid binding protein knockout mice (aP2−/−) after short-term high-fat feeding: functional compensation by the keratinocyte [correction of keritinocyte] fatty acid binding protein. Diabetes. 2000;49:904–911. doi: 10.2337/diabetes.49.6.904. [DOI] [PubMed] [Google Scholar]
- 64.Scheja L, et al. Altered insulin secretion associated with reduced lipolytic efficiency in aP2−/− mice. Diabetes. 1999;48:1987–1994. doi: 10.2337/diabetes.48.10.1987. [DOI] [PubMed] [Google Scholar]
- 65.Hertzel AV, et al. Lipid metabolism and adipokine levels in fatty acid-binding protein null and transgenic mice. Am J Physiol Endocrinol Metab. 2006;290:E814–E823. doi: 10.1152/ajpendo.00465.2005. [DOI] [PubMed] [Google Scholar]
- 66.Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta. 2000;1486:28–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 67.Schroeder F, et al. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- 68.Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F. Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. J Biol Chem. 2002;277:29139–29151. doi: 10.1074/jbc.M202923200. [DOI] [PubMed] [Google Scholar]
- 69.Yu S, Levi L, Siegel R, Noy N. Retinoic acid induces neurogenesis by activating both retinoic acid receptors (RARs) and peroxisome proliferator-activated receptor β/δ (PPARβ/δ) J Biol Chem. 2012;287:42195–42205. doi: 10.1074/jbc.M112.410381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu S, Levi L, Casadesus G, Kunos G, Noy N. Fatty acid-binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator-activated receptor β/δ (PPARβ/δ) in the brain. J Biol Chem. 2014;289:12748–12758. doi: 10.1074/jbc.M114.559062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan NS, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22:5114–5127. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adida A, Spener F. Adipocyte-type fatty acid-binding protein as inter-compartmental shuttle for peroxisome proliferator activated receptor γagonists in cultured cell. Biochim Biophys Acta. 2006;1761:172–181. doi: 10.1016/j.bbalip.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 73.Ayers SD, Nedrow KL, Gillilan RE, Noy N. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARγby FABP4. Biochemistry. 2007;46:6744–6752. doi: 10.1021/bi700047a. [DOI] [PubMed] [Google Scholar]
- 74.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γand IκB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hotamisligil GS, et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 76.Bernlohr DA, Coe NR, Simpson MA, Hertzel AV. Regulation of gene expression in adipose cells by polyunsaturated fatty acids. Adv Exp Med Biol. 1997;422:145–156. doi: 10.1007/978-1-4757-2670-1_12. [DOI] [PubMed] [Google Scholar]
- 77.Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141:3388–3396. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 78.Maeda K, et al. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes. 2003;52:300–307. doi: 10.2337/diabetes.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao H, et al. Regulation of metabolic responses by adipocyte/macrophage fatty acid-binding proteins in leptin-deficient mice. Diabetes. 2006;55:1915–1922. doi: 10.2337/db05-1496. [DOI] [PubMed] [Google Scholar]
- 80.Cao H, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Erbay E, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh DY, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ichimura A, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 84.Shen WJ, Sridhar K, Bernlohr DA, Kraemer FB. Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc Natl Acad Sci USA. 1999;96:5528–5532. doi: 10.1073/pnas.96.10.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith AJ, et al. Physical association between the adipocyte fatty acid-binding protein and hormone-sensitive lipase: a fluorescence resonance energy transfer analysis. J Biol Chem. 2004;279:52399–52405. doi: 10.1074/jbc.M410301200. [DOI] [PubMed] [Google Scholar]
- 86.Hampton M, et al. Deep sequencing the transcriptome reveals seasonal adaptive mechanisms in a hibernating mammal. PLoS ONE. 2011;6:e27021. doi: 10.1371/journal.pone.0027021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 88.Fu Y, Luo N, Lopes-Virella MF, Garvey WT. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165:259–269. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 89.Liu QY, Nambi P. Sirolimus upregulates aP2 expression in human monocytes and macrophages. Transplant Proc. 2004;36:3229–3231. doi: 10.1016/j.transproceed.2004.10.086. [DOI] [PubMed] [Google Scholar]
- 90.Liu QY, Quinet E, Nambi P. Adipocyte fatty acid-binding protein (aP2), a newly identified LXR target gene, is induced by LXR agonists in human THP-1 cells. Mol Cell Biochem. 2007;302:203–213. doi: 10.1007/s11010-007-9442-5. [DOI] [PubMed] [Google Scholar]
- 91.Babaev VR, et al. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-γ-regulated genes. Arterioscler Thromb Vasc Biol. 2011;31:1283–1290. doi: 10.1161/ATVBAHA.111.225839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garin-Shkolnik T, Rudich A, Hotamisligil GS, Rubinstein M. FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes. 2014;63:900–911. doi: 10.2337/db13-0436. [DOI] [PubMed] [Google Scholar]
- 93.Zimmer JS, Dyckes DF, Bernlohr DA, Murphy RC. Fatty acid binding proteins stabilize leukotriene A4: competition with arachidonic acid but not other lipoxygenase products. J Lipid Res. 2004;45:2138–2144. doi: 10.1194/jlr.M400240-JLR200. [DOI] [PubMed] [Google Scholar]
- 94.Dickinson Zimmer JS, Voelker DR, Bernlohr DA, Murphy RC. Stabilization of leukotriene A4 by epithelial fatty acid-binding protein in the rat basophilic leukemia cell. J Biol Chem. 2004;279:7420–7426. doi: 10.1074/jbc.M311404200. [DOI] [PubMed] [Google Scholar]
- 95.Spite M, et al. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol. 2011;187:1942–1949. doi: 10.4049/jimmunol.1100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Layne MD, et al. Role of macrophage-expressed adipocyte fatty acid binding protein in the development of accelerated atherosclerosis in hypercholesterolemic mice. FASEB J. 2001;15:2733–2735. doi: 10.1096/fj.01-0374fje. [DOI] [PubMed] [Google Scholar]
- 97.Chan KL, et al. Palmitoleate reverses high fat-induced pro-inflammatory macrophage polarization via AMPK. J Biol Chem. doi: 10.1074/jbc.M115.646992. http://dx.doi.org/10.1074/jbc.M115.646992. [DOI] [PMC free article] [PubMed]
- 98.Xu H, et al. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Mol Cell Biol. 2015;35:1055–1065. doi: 10.1128/MCB.01122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thompson BR, Mazurkiewicz-Munoz AM, Suttles J, Carter-Su C, Bernlohr DA. Interaction of adipocyte fatty acid-binding protein (AFABP) and JAK2: AFABP/aP2 as a regulator of JAK2 signaling. J Biol Chem. 2009;284:13473–13480. doi: 10.1074/jbc.M900075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perrella MA, et al. Absence of adipocyte fatty acid binding protein prevents the development of accelerated atherosclerosis in hypercholesterolemic mice. FASEB J. 2001;15:1774–1776. doi: 10.1096/fj.01-0017fje. [DOI] [PubMed] [Google Scholar]
- 102.Makowski L, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boord JB, et al. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22:1686–1691. doi: 10.1161/01.atv.0000033090.81345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Furuhashi M, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boord JB, et al. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110:1492–1498. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rolph MS, et al. Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein AP2. J Immunol. 2006;177:7794–7801. doi: 10.4049/jimmunol.177.11.7794. [DOI] [PubMed] [Google Scholar]
- 107.Reynolds JM, et al. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:313–321. doi: 10.4049/jimmunol.179.1.313. [DOI] [PubMed] [Google Scholar]
- 108.Elmasri H, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harjes U, Bridges E, McIntyre A, Fielding BA, Harris AL. Fatty acid-binding protein 4, a point of convergence for angiogenic and metabolic signaling pathways in endothelial cells. J Biol Chem. 2014;289:23168–23176. doi: 10.1074/jbc.M114.576512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Daly C, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elmasri H, et al. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis. 2012;15:457–468. doi: 10.1007/s10456-012-9274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ghelfi E, et al. Fatty acid binding protein 4 regulates VEGF-induced airway angiogenesis and inflammation in a transgenic mouse model: implications for asthma. Am J Pathol. 2013;182:1425–1433. doi: 10.1016/j.ajpath.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saint-Geniez M, et al. Fatty acid binding protein 4 deficiency protects against oxygen-induced retinopathy in mice. PLoS ONE. 2014;9:e96253. doi: 10.1371/journal.pone.0096253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cataltepe O, et al. Fatty acid binding protein 4 is expressed in distinct endothelial and non-endothelial cell populations in glioblastoma. Neuropathol Appl Neurobiol. 2012;38:400–410. doi: 10.1111/j.1365-2990.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 116.Lee D, et al. Expression of fatty acid binding protein 4 is involved in the cell growth of oral squamous cell carcinoma. Oncol Rep. 2014;31:1116–1120. doi: 10.3892/or.2014.2975. [DOI] [PubMed] [Google Scholar]
- 117.Pelsers MM, et al. Liver fatty acid-binding protein as a sensitive serum marker of acute hepatocellular damage in liver transplant recipients. Clin Chem. 2002;48:2055–2057. [PubMed] [Google Scholar]
- 118.Knowlton AA, Apstein CS, Saouf R, Brecher P. Leakage of heart fatty acid binding protein with ischemia and reperfusion in the rat. J Mol Cell Cardiol. 1989;21:577–583. doi: 10.1016/0022-2828(89)90823-7. [DOI] [PubMed] [Google Scholar]
- 119.Tanaka T, Hirota Y, Sohmiya K, Nishimura S, Kawamura K. Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clin Biochem. 1991;24:195–201. doi: 10.1016/0009-9120(91)90571-u. [DOI] [PubMed] [Google Scholar]
- 120.Kleine AH, Glatz JF, Van Nieuwenhoven FA, Van der Vusse GJ. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol Cell Biochem. 1992;116:155–162. doi: 10.1007/BF01270583. [DOI] [PubMed] [Google Scholar]
- 121.Kanda T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339–343. doi: 10.1053/gast.1996.v110.pm8566578. [DOI] [PubMed] [Google Scholar]
- 122.Schellekens DH, et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol. 2014;48:253–260. doi: 10.1097/MCG.0b013e3182a87e3e. [DOI] [PubMed] [Google Scholar]
- 123.Akbal E, et al. Liver fatty acid-binding protein is a diagnostic marker to detect liver injury due to chronic hepatitis C infection. Arch Med Res. 2013;44:34–38. doi: 10.1016/j.arcmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 124.Foucaud L, et al. Output of liver fatty acid-binding protein (L-FABP) in bile. Biochim Biophys Acta. 1999;1436:593–599. doi: 10.1016/s0005-2760(98)00171-4. [DOI] [PubMed] [Google Scholar]
- 125.Specht B, et al. Mammary derived growth inhibitor is not a distinct protein but a mix of heart-type and adipocyte-type fatty acid-binding protein. J Biol Chem. 1996;271:19943–19949. doi: 10.1074/jbc.271.33.19943. [DOI] [PubMed] [Google Scholar]
- 126.Brandt R, et al. A 13-kilodalton protein purified from milk fat globule membranes is closely related to a mammary-derived growth inhibitor. Biochemistry. 1988;27:1420–1425. doi: 10.1021/bi00405a005. [DOI] [PubMed] [Google Scholar]
- 127.Bronsky J, et al. Adiponectin, adipocyte fatty acid binding protein, and epidermal fatty acid binding protein: proteins newly identified in human breast milk. Clin Chem. 2006;52:1763–1770. doi: 10.1373/clinchem.2005.063032. [DOI] [PubMed] [Google Scholar]
- 128.Cao H, et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013;17:768–778. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu A, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52:405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 130.Schlottmann I, Ehrhart-Bornstein M, Wabitsch M, Bornstein SR, Lamounier-Zepter V. Calcium-dependent release of adipocyte fatty acid binding protein from human adipocytes. Int J Obes (Lond ) 2014;38:1221–1227. doi: 10.1038/ijo.2013.241. [DOI] [PubMed] [Google Scholar]
- 131.Kaess BM, et al. Cardiometabolic correlates and heritability of fetuin-A, retinol-binding protein 4, and fatty-acid binding protein 4 in the Framingham Heart Study. J Clin Endocrinol Metab. 2012;97:E1943–E1947. doi: 10.1210/jc.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chow WS, et al. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc. 2013;2:e004176. doi: 10.1161/JAHA.112.004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.von Eynatten M, et al. Circulating adipocyte-fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol. 2012;32:2327–2335. doi: 10.1161/ATVBAHA.112.248609. [DOI] [PubMed] [Google Scholar]
- 134.Tso AW, et al. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30:2667–2672. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- 135.Ishimura S, et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS ONE. 2013;8:e81318. doi: 10.1371/journal.pone.0081318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tso AW, et al. Serum adipocyte fatty acid-binding protein associated with ischemic stroke and early death. Neurology. 2011;76:1968–1975. doi: 10.1212/WNL.0b013e31821e54b3. [DOI] [PubMed] [Google Scholar]
- 137.Balci MM, et al. Serum levels of adipocyte fatty acid-binding protein are independently associated with left ventricular mass and myocardial performance index in obstructive sleep apnea syndrome. J Investig Med. 2012;60:1020–1026. doi: 10.2310/JIM.0b013e31826868f2. [DOI] [PubMed] [Google Scholar]
- 138.Hancke K, Grubeck D, Hauser N, Kreienberg R, Weiss JM. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat. 2010;119:367–367. doi: 10.1007/s10549-009-0577-9. [DOI] [PubMed] [Google Scholar]
- 139.Yoo HJ, et al. Serum adipocyte fatty acid-binding protein is associated independently with vascular inflammation: analysis with 18F-fluorodeoxyglucose positron emission tomography. J Clin Endocrinol Metab. 2011;96:E488–E492. doi: 10.1210/jc.2010-1473. [DOI] [PubMed] [Google Scholar]
- 140.Schmilovitz-Weiss H, et al. Serum adipocyte fatty acid binding protein in liver transplant recipients and the metabolic syndrome. Ann Hepatol. 2012;11:343–349. [PubMed] [Google Scholar]
- 141.Haluzikova D, et al. Serum concentrations of adipocyte fatty acid binding protein in patients with anorexia nervosa. Physiol Res. 2009;58:577–581. doi: 10.33549/physiolres.931575. [DOI] [PubMed] [Google Scholar]
- 142.Comerford KB, Buchan W, Karakas SE. The effects of weight loss on FABP4 and RBP4 in obese women with metabolic syndrome. Horm Metab Res. 2014;46:224–231. doi: 10.1055/s-0033-1353204. [DOI] [PubMed] [Google Scholar]
- 143.Stejskal D, Karpisek M, Bronsky J. Serum adipocyte-fatty acid binding protein discriminates patients with permanent and temporary body weight loss. J Clin Lab Anal. 2008;22:380–382. doi: 10.1002/jcla.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tuncman G, et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci USA. 2006;103:6970–6975. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saksi J, et al. Low-expression variant of fatty acid-binding protein 4 favors reduced manifestations of atherosclerotic disease and increased plaque stability. Circ Cardiovasc Genet. 2014;7:588–598. doi: 10.1161/CIRCGENETICS.113.000499. [DOI] [PubMed] [Google Scholar]
- 146.Wang J, et al. FABP4: a novel candidate gene for polycystic ovary syndrome. Endocrine. 2009;36:392–396. doi: 10.1007/s12020-009-9228-5. [DOI] [PubMed] [Google Scholar]
- 147.Bhushan B, et al. Fatty-acid binding protein 4 gene polymorphisms and plasma levels in children with obstructive sleep apnea. Sleep Med. 2011;12:666–671. doi: 10.1016/j.sleep.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu LE, et al. Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol Metab. 2014;3:465–473. doi: 10.1016/j.molmet.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kralisch S, et al. Circulating adipocyte fatty acid-binding protein induces insulin resistance in mice in vivo. Obesity (Silver Spring) 2015;23:1007–1013. doi: 10.1002/oby.21057. [DOI] [PubMed] [Google Scholar]
- 151.Girona J, et al. FABP4 induces vascular smooth muscle cell proliferation and migration through a MAPK-dependent pathway. PLoS ONE. 2013;8:e81914. doi: 10.1371/journal.pone.0081914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aragones G, et al. Fatty acid-binding protein 4 impairs the insulin-dependent nitric oxide pathway in vascular endothelial cells. Cardiovasc Diabetol. 2012;11:72. doi: 10.1186/1475-2840-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lamounier-Zepter V, et al. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res. 2009;105:326–334. doi: 10.1161/CIRCRESAHA.109.200501. [DOI] [PubMed] [Google Scholar]
- 154.Lamounier-Zepter V, et al. Interaction of epoxyeicosatrienoic acids and adipocyte fatty acid-binding protein in the modulation of cardiomyocyte contractility. Int J Obes (Lond ) 2015;39:755–761. doi: 10.1038/ijo.2014.193. [DOI] [PubMed] [Google Scholar]
- 155.Riquelme CA, et al. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science. 2011;334:528–531. doi: 10.1126/science.1210558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ertunc ME, et al. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res. 2015;56:423–434. doi: 10.1194/jlr.M055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kralisch S, et al. Adipocyte fatty acid-binding protein is released from adipocytes by a non-conventional mechanism. Int J Obes (Lond ) 2014;38:1251–1254. doi: 10.1038/ijo.2013.232. [DOI] [PubMed] [Google Scholar]
- 159.Lee MY, et al. Chronic administration of BMS309403 improves endothelial function in apolipoprotein E-deficient mice and in cultured human endothelial cells. Br J Pharmacol. 2011;162:1564–1576. doi: 10.1111/j.1476-5381.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hoo RL, et al. Pharmacological inhibition of adipocyte fatty acid binding protein alleviates both acute liver injury and non-alcoholic steatohepatitis in mice. J Hepatol. 2013;58:358–364. doi: 10.1016/j.jhep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 161.Lehmann F, et al. Discovery of inhibitors of human adipocyte fatty acid-binding protein, a potential type 2 diabetes target. Bioorg Med Chem Lett. 2004;14:4445–4448. doi: 10.1016/j.bmcl.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 162.Barf T, et al. N.-Benzyl-indolo carboxylic acids: design and synthesis of potent and selective adipocyte fatty-acid binding protein (A-FABP) inhibitors. Bioorg Med Chem Lett. 2009;19:1745–1748. doi: 10.1016/j.bmcl.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 163.Liu X, et al. New aromatic substituted pyrazoles as selective inhibitors of human adipocyte fatty acid-binding protein. Bioorg Med Chem Lett. 2011;21:2949–2952. doi: 10.1016/j.bmcl.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 164.Chen J, Wang J, Zhu W. Binding modes of three inhibitors 8CA, F8A and I4A to A-FABP studied based on molecular dynamics simulation. PLoS ONE. 2014;9:e99862. doi: 10.1371/journal.pone.0099862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu Q, et al. Design, synthesis and biological evaluation of thiazole- and indole-based derivatives for the treatment of type II diabetes. Eur J Med Chem. 2012;52:70–81. doi: 10.1016/j.ejmech.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 166.Wang Y, et al. Discovery of FDA-approved drugs as inhibitors of fatty acid binding protein 4 using molecular docking screening. J Chem Inf Model. 2014;54:3046–3050. doi: 10.1021/ci500503b. [DOI] [PubMed] [Google Scholar]
- 167.Cai HY, et al. Benzbromarone, an old uricosuric drug, inhibits human fatty acid binding protein 4 in vitro and lowers the blood glucose level in db/db mice. Acta Pharmacol Sin. 2013;34:1397–1402. doi: 10.1038/aps.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aouadi M, et al. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc Natl Acad Sci USA. 2013;110:8278–8283. doi: 10.1073/pnas.1300492110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Aouadi M, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Won YW, et al. Oligopeptide complex for targeted non-viral gene delivery to adipocytes. Nat Mater. 2014;13:1157–1164. doi: 10.1038/nmat4092. [DOI] [PubMed] [Google Scholar]
- 171.Miao X, et al. The mAb against adipocyte fatty acid-binding protein 2E4 attenuates the inflammation in the mouse model of high-fat diet-induced obesity via toll-like receptor 4 pathway. Mol Cell Endocrinol. 2015;403:1–9. doi: 10.1016/j.mce.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 172.Herroon MK, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–2123. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yore MM, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159:318–332. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.NCBI. Fabp4 [Mus musculus] GenBank: CAJ18597.1. 2005 [online], http://www.ncbi.nlm.nih.gov/protein/71060107.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.