Abstract
Background
The prevalence of severe obesity is rising in the US. Although mild to moderately elevated Body Mass Index (BMI) is associated with reduced mortality after acute ischemic stroke, less is known about severe obesity.
Methods and Results
Acute ischemic stroke patients (n=1,791) aged ≥45 years were identified from the bi-ethnic population-based Brain Attack Surveillance in Corpus Christi (BASIC) study from June 1, 2005 to December 31, 2010. Median follow-up was 660 days. BMI was abstracted from the medical record. Survival was estimated by BMI category (underweight normal-weight, overweight, class 1 obesity, class 2 obesity, and severe obesity) using Kaplan-Meier methods. Hazard ratios for the relationship between BMI modeled continuously and mortality were estimated from Cox regression models after adjusting for patient factors. The median BMI was 27.1 kg/m2 (interquartile range, 23.7–31.2) and 56% were Mexican American. A total of 625 (35%) patients died during the study period. Persons with higher baseline BMI had longer survival in unadjusted analysis (P<0.01). After adjusting for demographics, stroke severity, stroke and mortality risk factors, the relationship between BMI and mortality was U-shaped. The lowest mortality risk was observed among patients with an approximate BMI of 35 kg/m2, whereas those with lower or higher BMI had higher mortality risk.
Conclusions
Severe obesity is associated with increased post-stroke mortality in middle-aged and older adults. Stroke patients with class 2 obesity had the lowest mortality risk. More research is needed to determine weight management goals among stroke survivors.
Keywords: Stroke, obesity, mortality
Over 35% of US adults are obese.1 Obesity accounts for almost 10% of all medical spending reaching as high as $147 billion annually in the US.2 In 2008, 3.5% of Americans were severely obese (Body Mass Index (BMI) ≥40 kg/m2) which roughly corresponds to 100 pounds (45 kg) or more overweight.3 Over 50% of severely obese Americans have hypertension, the most important stroke risk factor.4 Increasing BMI is associated with an increased risk of incident stroke5 and an increased risk of all-cause mortality in the general population.6
In contrast to the general population, research has shown that higher BMI may be neutral or have a protective association with mortality in patients with clinical cardiovascular disease (CVD), including stroke patients, a phenomenon described as the “obesity paradox”.7–12 However, less is known about the relationship between severe obesity and mortality in patients with stroke. Moreover, little is known about the relationship between obesity and post-stroke mortality among Latinos, particularly Mexican Americans (MAs), a subgroup with high rates of severe obesity and incident stroke.1, 13
For these reasons, we sought to explore the association of BMI and all-cause mortality among acute ischemic stroke (AIS) patients in a bi-ethnic population-based stroke surveillance study. We hypothesized that all-cause mortality would be higher in severely obese AIS patients compared with normal weight AIS patients.
Methods
The Brain Attack Surveillance in Corpus Christi (BASIC) study is a population-based complete case capture stroke surveillance study in the bi-ethnic community of Nueces County, Texas. Nueces County is a geographically isolated, urban area. Ninety percent of the population of Nueces County (population 340,223) resides in Corpus Christi.14 Sixty one percent are MAs, and 33% are non-Hispanic whites (NHWs).14 Of the Hispanic population in the county over age 45, <3% declare ancestry from countries other than Mexico.15
The BASIC methods of stroke case capture have been detailed previously.13, 16, 17 Briefly, stroke cases ages 45 and older were identified by active surveillance of hospital admission and emergency department logs of the 7 hospitals in the community. Passive surveillance of stroke hospital and emergency department discharges using International Classification of Disease, Ninth Revision, code searches was also used. Using source documentation, all screened cases were validated by a study neurologist who was blinded to the patient’s ethnicity and age. The study population consisted of cases of the first ischemic stroke (incident or recurrent) identified by the BASIC study between June 1, 2005 and December 31, 2010. Mexican American ethnicity was determined from the medical record for which we have found excellent agreement (97%) with self-report in this community.13 Stroke cases of a race/ethnicity other than MAs or NHWs were excluded due to the limited sample sizes.
Covariates
Our primary predictor of interest was BMI. Patients’ height and weight were abstracted from the medical record, and index BMI was calculated. For descriptive analyses, underweight was defined as BMI<18.5 kg/m2, normal weight as BMI 18.5–24.9 kg/m2, overweight as 25–29.9 kg/m2, class 1 obesity as 30–34.9 kg/m2, class 2 obesity as 35–39.9 and severe obesity ≥40 kg/m2.18 BMI was modeled as a continuous variable when evaluating the association with all-cause mortality in multivariable models.
A review of the literature and our previous work was used to identify potential confounders of the association between BMI and all-cause mortality in AIS patients.19–22 Based on this assessment, the following covariates were abstracted from the stroke hospitalization medical record and included in the multivariable model: age, race/ethnicity, gender, stroke severity, hypertension, atrial fibrillation, coronary artery disease, diabetes, heart failure, high cholesterol, chronic obstructive pulmonary disease, dementia, end stage renal disease (ESRD), cancer, history of stroke or transient ischemic attack (prior to the BASIC study), excessive alcohol use and smoking.17 Second stroke during the study period was identified through BASIC stroke surveillance. Stroke severity was defined as the National Institutes of Health Stroke Scale (NIHSS) at the time of the index stroke and was taken from the medical record or abstracted from the medical record based on the validated Williams method.23
Outcome
All-cause mortality was ascertained from the time of the first AIS through December 31, 2010. In-hospital deaths were identified using the hospital medical record. Out of hospital deaths were identified using outcome assessments at 90 days, the Social Security Death Index and the Texas Department of Health death certificate database. The Texas Department of Health death certificate database was not available for 2010 at the time of analysis. However, based on previous years of data less than 3% of deaths in our population are based on this data source alone and thus few mortality cases for this year should have been missed. Mortality is ascertained from multiple sources and the date of death for stroke patients who died outside of Nueces county is still obtained.
Statistical Analysis
Patients’ demographics and stroke risk factors were summarized using frequencies/percents and medians/interquartile ranges (IQR) and compared across BMI groups using non-parametric Kruskal Wallis tests for continuous variables and chi-square tests for categorical variables. Kaplan-Meier curves and a log rank test were used to assess the crude association between categorical BMI (underweight, normal weight, overweight, class 1 obesity, class 2 obesity and severe obesity) and all-cause mortality. Cox proportional hazards models were then used to analyze the association of BMI modeled as a continuous covariate and mortality accounting for demographics and risk factors. All covariates were added simultaneously to the multivariable Cox regression model. Second stroke identified during BASIC was modeled as a time-varying covariate.
Scatter plots of martingale residuals were used to determine the appropriate form of the continuous variables and to investigate outliers. In the fully adjusted model, the functional forms (i.e., linear versus non-linear) of BMI, age, and NIHSS were assessed. Visual inspection of a smoothed martingale residuals plot showed that BMI was non-linearly associated with all-cause mortality, and that 11 observations with a BMI over 50 could potentially drive the non-linear association. Thus, a penalized cubic spline model with degrees of freedom selected via Akaike’s information criteria (AIC) was used to model the BMI-mortality association, with the 11 patients with BMI>50 removed from our analyses. 24 Using similar methods, it was determined that NIHSS was best fit using natural log NIHSS plus one and age was best modeled linearly. Using Schoenfield residuals, we determined that the natural log of NIHSS violated the assumption of proportional hazards and thus we modeled this variable as having a time dependent coefficient. No other violations of model assumptions were identified.
The fully adjusted model was used to determine predicted mortality risk at 1, 2 and 3 years for individuals with otherwise average characteristics but various baseline BMI values. For a range of baseline BMI values, we then computed hazard ratios (HR) associated with a 1-kg/m2 difference in BMI to determine at what BMI individuals with higher weight have higher mortality (e.g., mortality HR comparing those with BMI of 25 vs. 24, 26 vs. 25, etc.). In the fully adjusted model, we also tested whether age modified the association of BMI with all-cause mortality by estimating a model where the BMI spline term differed by age, and comparing it with the model without the interaction using a likelihood ratio test. Based on the significant association, we stratified by age to visualize the nature of the effect modification by age. The same methods were used to assess whether smoking modified the association of BMI with all-cause mortality.
We then performed a series of sensitivity analyses to determine the robustness of our main findings. First, because modeling BMI as a continuous predictor can sometimes exaggerate its relationship with mortality,25 we repeated the fully adjusted analysis with BMI coded in 1 unit categories compared to a reference group of normal weight (BMI=24) stroke patients. To ensure all categories had a sufficient number of individuals, some BMI categories were collapsed. Second, to explore the possibility that the results are influenced by including patients with prior history of stroke/TIA we ran the fully adjusted model excluding stroke patients who reported a previous history of stroke or TIA. Because recurrent stroke may be on the causal pathway from BMI to mortality, we ran the fully adjusted model excluding recurrent stroke as a covariate. Finally, to ensure that truncating the distribution of the primary predictor by excluding the 11 patients with BMI over 50 kg/m2 did not bias the model estimates, the fully adjusted model was rerun including these observations. This project was approved by the Institutional Review Boards at the University of Michigan and both of the Nueces County hospital systems.
Results
From June 1, 2005 to December 31, 2010, a total of 1,870 patients were identified with an AIS during BASIC surveillance. Sixty-five (4%) patients were excluded due to missing height and/or weight data, 11(0.6%) patients were excluded because their BMI was over 50 kg/m2,1 patient was excluded for inaccurate recording of weight (weight was recorded as 20 pounds) and 2 patients were excluded due to missing multiple data fields. There was no difference in age or stroke severity among those with and without BMI data (data not shown). The median BMI of the 1,791 patients in our study population was 27.1 (IQR 23.7–31.2). Of these, 54 (3%) were underweight, 543 (30%) were normal weight, 629 (35%) were overweight, 311 (17%) were class 1 obesity, 157 (9%) were class 2 obesity and 97 (5%) had severe obesity.
The median age was 72 (IQR 60–81) years and 56% of the AIS patients were Mexican American. Baseline characteristics of the cohort are reported in Table 1. As obesity increased, the age of AIS patients decreased (p<0.01). MAs comprised 73% of the severely obese AIS patients and 65% of class 2 obesity patients. Women were more likely than men to be underweight (5% vs. 1%, p<0.01), normal weight (34% vs. 26%, p<0.01) and class 2 obesity (11% vs. 7%, p<0.01). Men were more likely than women to be overweight (42% vs. 28%, p<0.01). There was no difference in class 1 obesity (p=0.15) or severe obesity (p=0.10). Underweight patients with AIS had a higher NIHSS compared to the other groups. Those with higher BMI were more likely to have diabetes. In contrast, under-weight patients were more likely to have atrial fibrillation.
Table 1.
Sociodemographic Characteristics and Stroke Risk Factors among Patients with Acute Ischemic Stroke (n=1,791): The Brain Attack Surveillance in Corpus Christi (BASIC) Project, June 1, 2005- December 31, 2010.
| Underweight N=54 3% n(%) |
Normal weight N=543 30% n(%) |
Overweight N=629 35% n(%) |
Class 1 obesity N=311 17% n(%) |
Class 2 obesity N=157 9% n(%) |
Severe obesity N=97 5% n(%) |
P value | |
|---|---|---|---|---|---|---|---|
| Age median (IQR) | 85 (76, 90) | 79 (67, 85) | 72 (60, 81) | 67 (57, 77) | 63 (54- 73) | 57 (52- 65) | <0.01 |
| Mexican Americans | 19 (35) | 252 (46) | 363 (58) | 195 (63) | 108 (65) | 71 (73) | <0.01 |
| Females | 43 (80) | 309 (57) | 257 (41) | 95 (61) | 152 (60) | 57 (59) | <0.01 |
| NIH score median (IQR) | 7 (2, 14) | 5 (2, 9) | 4 (2, 8) | 4 (2, 8) | 3 (2, 7) | 3.5 (1, 6) | <0.01 |
| Hypertension | 43 (80) | 407 (75)* | 495 (79) | 267 (86) | 141 (90) | 90 (93) | <0.01 |
| Atrial fibrillation | 16 (30) | 133 (25)* | 96 (15) | 35 (11) | 19(12) | 6(6) | <0.01 |
| CAD | 22 (41) | 177 (33)* | 222 (35) | 106 (34) | 54 (34) | 35 (36) | 0.84 |
| Diabetes | 14 (26) | 161 (30)* | 245 (39) | 166 (53) | 105 (67) | 76 (78) | <0.01 |
| High cholesterol | 17 (31) | 191 (35)+ | 272 (43) | 159 (51) | 73 (47) | 43 (44) | <0.01 |
| Congestive Heart Failure | 20 (37) | 92 (17)* | 87(14) | 41 (13) | 23 (15) | 22 (23) | <0.01 |
| Cancer | 8 (15) | 75 (14)* | 80 (13) | 32 (10) | 9 (6) | 8 (8) | 0.06 |
| ESRD | 1 (2) | 33 (6)* | 35 (6) | 15 (5) | 9 (6) | 7 (7) | 0.77 |
| COPD | 9 (17) | 70 (13)* | 56 (9) | 28 (9) | 18 (11) | 9 (9) | 0.15 |
| Dementia | 14 (26) | 79 (15)* | 53 (8) | 14 (5) | 5 (3) | 1 (1) | <0.01 |
| Hx stroke or TIA | 21 (39) | 157 (29)* | 182 (29) | 80 (26) | 41 (26) | 21 (22) | 0.24 |
| Recurrent stroke | 4 (7) | 63 (12) | 67 (11) | 35 (11) | 23 (15) | 12 (12) | 0.71 |
| Excessive alcohol use | 3 (6) | 38 (7)* | 38 (6) | 20 (6)* | 7(4) | 3 (3) | 0.68 |
| Smoker | 11 (20) | 117 (22)+ | 125 (20)‡ | 57 (18)* | 30 (19) | 17 (18)* | 0.79 |
1 missing
2 missing
6 missing
IQR = interquartile range, CAD = coronary artery disease, ESRD = end stage renal disease, COPD = chronic obstructive pulmonary disease, TIA = transient ischemic attack
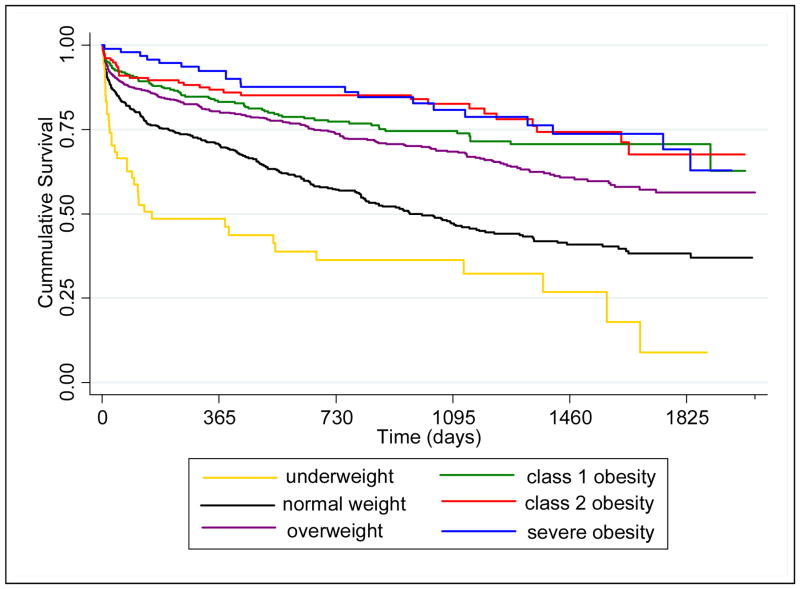
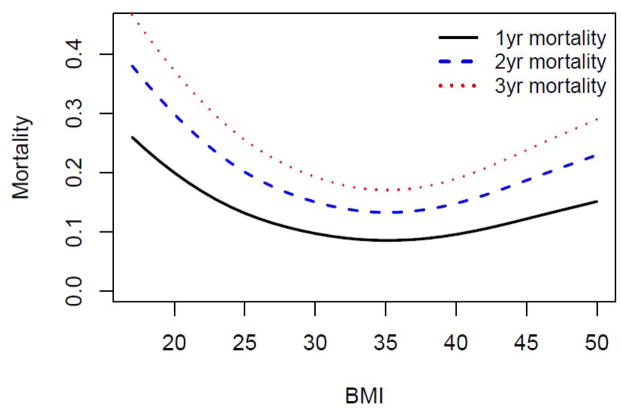
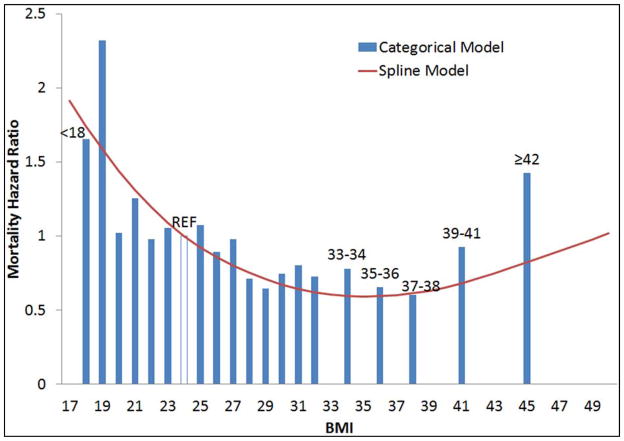
The median follow-up was 660 days (range 0 – 2038 days). A total of 625 (35%) patients died during the study period. Survival was different among the six BMI groups (Figure 1, log rank P-value ≤ 0.001), with higher survival among those with higher BMI. After adjusting for demographics, stroke severity, stroke and mortality risk factors, the relationship between BMI and mortality was U-shaped (Figure 2). For BMI levels ranging from 17 to approximately 35, mortality risk was lower among those with higher BMI. The lowest mortality risk was observed at an approximate BMI of 35 kg/m2. At BMI levels above 35 kg/m2, mortality risk was higher among those with higher BMI. Results of the fully adjusted with BMI modeled categorically did not differ from the spline model (Figure 3).
Figure 1.
Kaplan-Meier Survival Curves among Patients with Acute Ischemic Stroke (n=1,791) Stratified by Weight: The Brain Attack Surveillance in Corpus Christi (BASIC) Project, June 1, 2005- December 31, 2010.
(p<0.01, log rank test).
Figure 2. Predicted Mortality by Baseline BMI among Patients with Acute Ischemic Stroke: The Brain Attack Surveillance in Corpus Christi (BASIC) Project, June 1, 2005-December 31, 2010 (n=1,775).
Adjusted for age, race/ethnicity, gender, stroke severity, hypertension, atrial fibrillation, coronary artery disease, diabetes, high cholesterol, heart failure, chronic obstructive pulmonary disease, dementia, end stage renal disease, cancer, history of stroke or transient ischemic attack (prior to the BASIC study), recurrent stroke during the study period, excessive alcohol use and smoking.
Figure 3.
Mortality hazard ratio comparing BMI categories to reference BMI of 24 with superimposed spline term for continuous BMI. The Brain Attack Surveillance in Corpus Christi (BASIC) Project, June 1, 2005-December 31, 2010 (n=1,775).
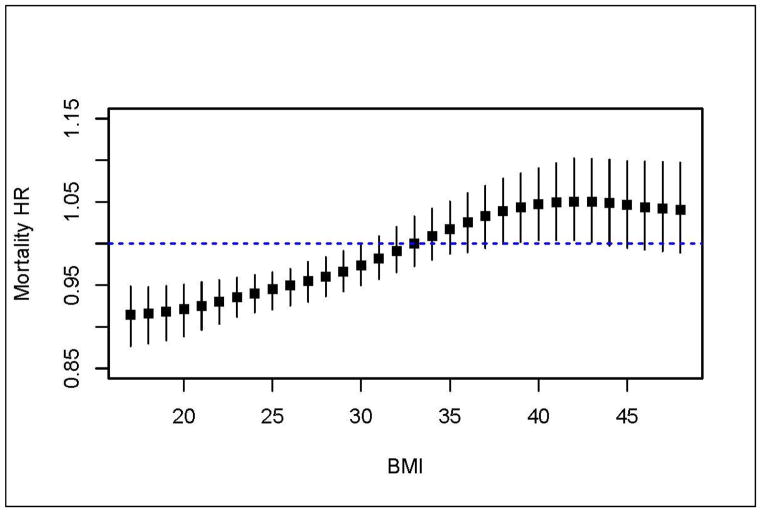
To further explore the U-shaped phenomenon, we sought to determine the threshold where a one unit higher BMI was no longer associated with lower mortality and where it became associated with higher mortality. Using the fully adjusted Cox model we compared the relative difference in mortality risk associated with 1-kg/m2 higher baseline BMI using hazard ratios for a range of baseline BMIs (Figure 4). The loss of a protective association between BMI and mortality occurred when comparing a baseline BMI of 31 to a baseline BMI of 32 kg/m2 (HR 0.98, 95%CI 0.96–1.01). Conversely, the transition to a harmful association between BMI and mortality occurred at the point of comparing baseline BMI of 38 to 39 kg/m2 (HR 1.04, 95%CI 1.00–1.08).
Figure 4. Hazard Ratio for Mortality for a 1-kg/m2 increase in BMI across the range of baseline BMI among Patients with Acute Ischemic Stroke (n=1,775): The Brain Attack Surveillance in Corpus Christi (BASIC) Project, June 1, 2005- December 31, 2010.
Adjusted for age, race/ethnicity, gender, stroke severity, hypertension, atrial fibrillation, coronary artery disease, diabetes, high cholesterol, heart failure, chronic obstructive pulmonary disease, dementia, end stage renal disease, cancer, history of stroke or transient ischemic attack (prior to the BASIC study), recurrent stroke during the study period, excessive alcohol use and smoking.
Using the fully adjusted model, we found evidence that age modified the association of BMI with mortality (P=0.001). The above described BMI mortality association was strongest among younger compared to older patients. That is, higher age at stroke onset attenuated the BMI-mortality association. There was no suggestion that smoking status modified the association of BMI with mortality (P=0.15). Limiting the population to patients with first stroke, removing the recurrent stroke covariate, or including those with a BMI ≥ 50 kg/m2 did not meaningfully alter the main results (data not shown).
Discussion
In this population-based stroke surveillance study, we found a U-shaped relationship between BMI at stroke onset and all-cause mortality in patients with AIS. After accounting for demographics, stroke severity, stroke and mortality risk factors, a 1 unit higher BMI had a protective association with mortality when BMI was lower than 31 kg/m2. Higher BMI beyond 31 kg/m2 did not render significant protective associations, and further, higher values of BMI above 38 kg/m2 were associated with higher mortality in the fully adjusted model. The large number of severely obese AIS patients in our study population allowed us to differentiate the detrimental association of severe obesity from the beneficial association of class 1 on the risk of post-stroke mortality.
Some previous studies suggest that the association of obesity and post-stroke mortality is age-dependent, although the direction of the interaction has not been consistent across studies.8, 9, 12 Our results are similar to work in the US and Korea showing that stroke patients with younger ages have a stronger BMI mortality association than older stroke patients.8, 12 Additionally, our findings are in general agreement with the previous studies showing a protective association of obesity on post-stroke mortality,8–12 but we were able to address some of the limitations of previous work in this area.8–12, 26 First, we conducted a population-based study limited to AIS patients and performed a comprehensive adjustment for possible confounders including the non-linear association of NIHSS with mortality and the time-dependent effect of recurrent stroke. Second, we avoided potential misclassification bias by treating BMI as a continuous predictor and by not combining obese and severely obese patients and normal-weight and underweight patients for analyses which could introduce bias. For example, lumping underweight individuals who have higher risk with normal weight individuals to define the “normal” weight comparison group would overestimate the protective association between obese and normal weight groups. Additionally, we were able to adjust for potential factors related to mortality in underweight patients with AIS including cancer, smoking status, dementia and ESRD.27 Reasons for the reduced mortality among obese stroke patients are unknown. Researchers have speculated that this may be due to the more frequent use of anti-hypertensive medications among obese stroke patients but this requires additional research.9
Severe obesity is associated with increased all-cause mortality in the general population.6, 28 Data suggest that the prevalence of vascular risk factors including diabetes, hypertension and hyperlipidemia among severely obese people mediate the increased mortality risk.28 However in our study of AIS patients, severely obese AIS patients continued to have increased mortality after accounting for these conditions. Another possibility to explain our results is that similar to the general population,29 severely obese AIS patients may have more depressive symptoms which in turn is associated with increased post-stroke mortality.30 We did not explore depressive symptoms in our study population. Alternate explanations for the increased mortality among severely obese AIS patients may include decreased diagnostic testing due to size limitations of diagnostic equipment (MRI scanners), decreased utilization or intensity of post-stroke rehabilitation due to the difficulty of therapists working with larger patients or weight limits of rehabilitation equipment, or missed follow-up appointments due to difficulty using standard transportation for larger patients. More research is needed to explore ways in which severe obesity is associated with increased post-stroke all-cause mortality.
Our findings have important clinical implications. The current secondary stroke prevention guidelines recommend weight loss as part of comprehensive therapy for hypertension and metabolic syndrome.31 To date, however, there is no randomized controlled trial (RCT) evidence that weight management or reduction prevents recurrent stroke or death after AIS. Additionally, weight management by calorie restriction and/or physical activity poses potential risks in adults with AIS, particularly those with older age, diabetes or neurologic disability. Thus in the absence of hypertension and metabolic syndrome, providers, health systems and payers may focus their efforts on improving currently sub-optimal adherence to secondary stroke preventive strategies with proven efficacy such as statins, and anti-thrombotic drugs.31
Limitations of this study warrant discussion. BMI may not accurately reflect obesity among the elderly and does not account for body fat distribution.32 We studied BMI (a measure of overall obesity) and did not examine the role of body composition or abdominal adiposity. BMI was abstracted from the medical record and is dependent on the accuracy of medical documentation. We cannot exclude that weight may be difficult to obtain in obese stroke patients particularly with severe disabilities and therefore measurement error is possible. BMI measures over time in individual patients were not available. Thus, we cannot determine the influence of changes in BMI on mortality. In addition to BMI, other factors that have been shown to influence post-stroke mortality and that may be associated with BMI, such as admission to a stroke unit, depression and social support were not available. Our model may be over adjusted given that recurrent stroke may be on the causal pathway between BMI and post-stroke mortality. However, a recent study showed no association between obesity and recurrent stroke33 and our sensitivity analysis excluding recurrent stroke did not meaningfully change the study results. Our study population is limited to MA and NHW AIS patients who were older than age 45. Thus, our results are not generalizable to the entire US AIS population. Like most observational studies we cannot exclude the possibility that an unrecognized or unmeasured confounder accounts for the observed association. Finally, we cannot exclude the possibility that obese patients died prior to their stroke and thus the obese people that have survived until the time of their stroke and thus entry into BASIC are a healthier subset.
Conclusion
In conclusion, in this population-based stroke surveillance study, we demonstrated a heterogeneous association of BMI on all-cause mortality in middle-aged and older adults with AIS with a protective association for obesity and a detrimental association of severe obesity. Further study is needed to confirm these findings and to determine the safety and effectiveness of weight loss interventions among the obese and severely obese AIS patients.
Acknowledgments
Study Funding: NIH/NINDS R01NS038916, NINDS K23 NS073685 (Skolarus), K23AG040278 (Levine)
Footnotes
No disclosures.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. JAMA: The Journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Affairs. 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. American journal of preventive medicine. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA: The Journal of the American Medical Association. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: Meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418–426. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 6.Prospective Studies C. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. The Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 8.Towfighi A, Ovbiagele B. The impact of body mass index on mortality after stroke. Stroke. 2009;40:2704–2708. doi: 10.1161/STROKEAHA.109.550228. [DOI] [PubMed] [Google Scholar]
- 9.Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, Manios E, Georgiopoulos G, Alevizaki M. Association between obesity and mortality after acute first-ever stroke: The obesity-stroke paradox. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- 10.Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body mass index and poststroke mortality. Neuroepidemiology. 2008;30:93–100. doi: 10.1159/000118945. [DOI] [PubMed] [Google Scholar]
- 11.Kiyohara Y, Kubo M, Kato I, Tanizaki Y, Tanaka K, Okubo K, Nakamura H, Iida M. Ten-year prognosis of stroke and risk factors for death in a japanese community: The hisayama study. Stroke. 2003;34:2343–2347. doi: 10.1161/01.STR.0000091845.14833.43. [DOI] [PubMed] [Google Scholar]
- 12.Kim BJ, Lee S-H, Jung K-H, Yu K-H, Lee B-C, Roh J-K. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology. 2012;79:856–863. doi: 10.1212/WNL.0b013e318266fad1. [DOI] [PubMed] [Google Scholar]
- 13.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, Longwell PJ, McFarling DA, Akuwumi O, Al-Wabil A, Al-Senani F, Brown DL, Moye LA. Excess stroke in mexican americans compared with non-hispanic whites: The brain attack surveillance in corpus christi project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Accessed Janaury 12, 2012];United States Census 2010. http://quickfacts.census.gov/qfd/states/48/48355.html.
- 15.United States Census Bureau. [Accessed July 18, 2013]; http://factfinder2.census.gov/faces/nav/jsf/pages/community_facts.xhtml.
- 16.Piriyawat P, Smajsova M, Smith MA, Pallegar S, Al-Wabil A, Garcia NM, Risser JM, Moye LA, Morgenstern LB. Comparison of active and passive surveillance for cerebrovascular disease: The brain attack surveillance in corpus christi (basic) project. Am J Epidemiol. 2002;156:1062–1069. doi: 10.1093/aje/kwf152. [DOI] [PubMed] [Google Scholar]
- 17.Smith MA, Risser JM, Moye LA, Garcia N, Akiwumi O, Uchino K, Morgenstern LB. Designing multi-ethnic stroke studies: The brain attack surveillance in corpus christi (basic) project. Ethn Dis. 2004;14:520–526. [PubMed] [Google Scholar]
- 18.Obesity: Preventing and managing the global epidemic. Report of a who consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 19.Vernino S, Brown RD, Jr, Sejvar JJ, Sicks JD, Petty GW, O’Fallon WM. Cause-specific mortality after first cerebral infarction: A population-based study. Stroke. 2003;34:1828–1832. doi: 10.1161/01.STR.0000080534.98416.A0. [DOI] [PubMed] [Google Scholar]
- 20.Lisabeth LD, Risser JM, Brown DL, Al-Senani F, Uchino K, Smith MA, Garcia N, Longwell PJ, McFarling DA, Al-Wabil A, Akuwumi O, Moye LA, Morgenstern LB. Stroke burden in mexican americans: The impact of mortality following stroke. Ann Epidemiol. 2006;16:33–40. doi: 10.1016/j.annepidem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Smith EE, Shobha N, Dai D, Olson DM, Reeves MJ, Saver JL, Hernandez AF, Peterson ED, Fonarow GC, Schwamm LH. Risk score for in-hospital ischemic stroke mortality derived and validated within the get with the guidelines-stroke program. Circulation. 2010;122:1496–1504. doi: 10.1161/CIRCULATIONAHA.109.932822. [DOI] [PubMed] [Google Scholar]
- 22.Saposnik G, Kapral MK, Liu Y, Hall R, O’Donnell M, Raptis S, Tu JV, Mamdani M, Austin PC Network obotIotRotCS, the Stroke Outcomes Research Canada Working Group. . Iscore: A risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011;123:739–749. doi: 10.1161/CIRCULATIONAHA.110.983353. [DOI] [PubMed] [Google Scholar]
- 23.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the nih stroke scale. Stroke. 2000;31:858–862. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 24.Eilers PH, Marx BD. Flexible smoothing with B-splines and penalties. Statistical science. 1996:89–102. [Google Scholar]
- 25.Welch HG, Schwartz LM, Woloshin S. The exaggerated relations between diet, body weight and mortality: The case for a categorical data approach. Canadian Medical Association Journal. 2005;172:891–895. doi: 10.1503/cmaj.1041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oki I, Nakamura Y, Okamura T, Okayama A, Hayakawa T, Kita Y, Ueshima H. Body mass index and risk of stroke mortality among a random sample of japanese adults: 19-year follow-up of nippon data80. Cerebrovascular Diseases. 2006;22:409–415. doi: 10.1159/000094860. [DOI] [PubMed] [Google Scholar]
- 27.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of u.S. Adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 28.McTigue K, Larson JC, Valoski A, Burke G, Kotchen J, Lewis CE, Stefanick ML, Van Horn L, Kuller L. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA: The Journal of the American Medical Association. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 29.Dixon JB, Dixon ME, O’Brien PE. Depression in association with severe obesitychanges with weight loss. Archives of Internal Medicine. 2003;163:2058–2065. doi: 10.1001/archinte.163.17.2058. [DOI] [PubMed] [Google Scholar]
- 30.House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32:696–701. doi: 10.1161/01.str.32.3.696. [DOI] [PubMed] [Google Scholar]
- 31.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 32.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ovbiagele B, Bath PM, Cotton D, Vinisko R, Diener H-C. Obesity and recurrent vascular risk after a recent ischemic stroke. Stroke. 2011;42:3397–402. doi: 10.1161/STROKEAHA.111.624957. [DOI] [PubMed] [Google Scholar]