Abstract
Viremia kinetics directly influence the clinical course and transmission dynamics of DENV, but many aspects of viral dynamics remain unknown. Non-human primates (NHP) have been used as a model system for DENV infection for decades. Here, we identify papers with experimentally-infected NHP and estimate the time to- and duration of viremia as well as estimate associations between these and serotype, inoculating dose, viremia assay, and species of NHP. We estimate the time to viremia in rhesus macaques to range from 2.63 to 3.32 days for DENV-2 and -1 and the duration to range from 3.13 to 5.13 days for DENV-4 and -2. We find no differences between non-human primates for time to viremia or duration, and a significant negative relationship between inoculating dose and duration of viremia. These results aide in understanding the transmission dynamics of sylvatic DENV non-human primates, an issue of growing importance as dengue vaccines become available.
Keywords: dengue virus, non-human primates, viral kinetics, within-host dynamics, sylvatic dengue
Introduction
Knowledge of the kinetics of dengue fever virus (DENV) within primate and non-primate hosts is key to understanding transmission dynamics and identifying populations at risk for infection [1]. Due to logistical and ethical obstacles, few studies have measured wildtype DENV viremia in humans over the course of an infection. Thus, non-human primates have been the major model system for comparison of viral dynamics between DENV serotypes and strains as well as evaluation of dengue therapeutics. While non-human primates differ from humans in pathological responses to DENV infection, estimates of duration of viremia that exist appear to be similar [2, 3], albeit with lower viral replication and limitation of virus to a subset of those tissues infected in humans [4].
In addition to serving as a potential model for human diseases, insight in to the replication of DENV in non-human primates is important in its own right. Four serotypes of sylvatic DENV have been shown to circulate between non-human primates and arboreal Aedes mosquitoes in Southeast Asia [5] and sylvatic DENV serotype 2 is maintained in West Africa [6]. These sylvatic viruses are ancestral to the four serotypes of DENV that are currently transmitted between humans by domestic and peridomestic Aedes [7]. Populations living in areas surrounding sylvatic hotspots of DENV transmission are at risk of infection [8, 9] from a transmission process that is poorly understood [10]. Importantly, it has recently been discovered that sylvatic DENV infection in humans can produce the most severe manifestation of dengue disease – dengue hemorrhagic fever [8, 9]. In the light of recent advances in DENV vaccines [11, 12], sylvatic reservoirs may play a key role in maintaining transmission over long time scales and may continue to expose human populations to new, genetically distinct viruses after human endemic transmission is controlled [7].
Isolations of sylvatic DENV have occurred at roughly eight year intervals in Senegal over the past 50 years [6]. The key determinants of cycle length are largely unknown. As the natural history of a pathogen has direct influence on transmission dynamics [13] knowledge of the time to detectable viremia and the length of viremia in non-human primates will be useful in ecological models of transmission [14] and may generate hypotheses for the observed serotype-specific transmission patterns [15] and clinical manifestations [16, 17] observed across DENV serotypes.
It is the goal of the present study to examine the kinetics of DENV viremia in non-human primates through systematic review and individual pooled analysis. We conducted a literature review to identify experimental DENV infections of DENV-naïve monkeys. We find associations between time from inoculation to viremia and duration of viremia and several covariates of interest using mixed effects regression models. We report robust estimates of the time to detectable viremia and the duration of viremia using recently developed methods for handling doubly-interval censored data [18].
Methods
Systematic Review
We searched PubMed, Web of Science and Google scholar for articles containing the terms “dengue primate viremia infection”, “dengue viremia primates”, “dengue viremia monkey”, “dengue vaccine primates”, and “dengue infection primates”. We narrowed our focus to primary infections where details on the infecting virus were reported. Our inclusion criteria were:
The non-human primate must be DENV naïve at the time of experimental infection (including free of exposure to experimental vaccines),
The challenge serotype (DENV-1–4) and the specific virus strain (with passage number, if applicable) must be clearly identified and the dosage of virus stated (in plaque forming units [PFU]), and
The presence of viremia must be reported on a day-by-day basis, at at least two time points, either in a graph or table, and not in a summary statistic. This does not preclude monkeys bled sporadically (e.g., every other day).
Additional (unpublished) studies were identified through expert consultation. Abstracts were doubly reviewed (BMA, DATC).
Time to Event Data
A survival analytic approach was used to determine time-to-event (viremia or clearance). If more than one method for assessing viremia was used, the method with the higher sensitivity was reported (though multiple methods were compared). Data were classified as fully observed, single- or doubly-interval censored. Observations were fully observed if the non-human primates were bled and found not to be viremic before and after being found viremic. If non-human primates were found to be viremic on the first or last sample taken, then the data point was assumed single-interval censored. If non-human primates were viremic on both the first and last sample, then the data point was assumed doubly-interval censored imposing left and right boundaries of inoculation and 16 days (estimates are insensitive to this number, see Supplementary Material). Observations missing or negative surrounded by two viremic samples were assumed to be viremic.
Methods for analyzing doubly-interval censored data have been developed previously [18]. We estimate the time to detectable viremia and the duration of viremia, both of which we assume are log-normally distributed (see Supplementary Material). We stratify by DENV serotype and compute bootstrap confidence intervals.
Associations with Time to Viremia and Duration
To explore the potential association between length of time to detectable viremia and duration of viremia, linear and random effects models were fit with time to viremia or duration as the outcome, a random effect for study and serotype, inoculating dose, viremia assay, and species of non-human primate as potential covariates of interest [19]. As these models do not directly take into account the effects of censoring, we test for differences censoring between covariates. Linear and mixed effects models are compared using the Akaike information criterion (AIC) [20].
Results
Literature
Literature searches returned 1092 unique papers (Figure 1). Of these, 117 (11%) described dengue infection in non-human primates, 226 (21%) described observational/naturally occurring dengue infection in humans and not non-human primates, 91 (8%) were about another disease, 125 (11%) had no abstracts, and 533 (49%) described experimental studies involving humans and animal models (not involving NHP).
Figure 1.
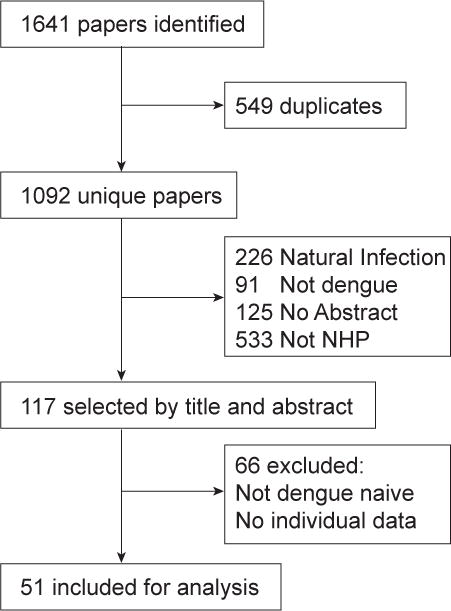
Flow Chart of Systematic Review
Fifty one published studies and three unpublished studies met the criteria for inclusion and were included in the analysis (Table 1). Thirty six included rhesus macaque (Macaca mulatta), 7 cynomolgus macaques (Macaca fascicularis), 4 each with green monkeys (Chlorocebus aethiops sabaeus) and owl monkeys (Aotus nancymaae), 3 chimpanzee (Pan troglodytes), 2 each with spider monkey (Ateles geoffroyi) and pig-tailed macaques (Macaca nemestrina), and 1 each with common marmoset (Callithrix jacchus), patas (Erythrocebus patas), squirrel monkey (Saimiri sciureus), and White Handed Gibbon (Hylobates lar). The bulk of the studies were vaccine trials/challenge studies (34/51, 67%) the rest were experimental challenge trials (18/51 35%). 59 unique DENV genotypes were represented. 72 (10%) non-human primates were infected with DENV-4 4328S, 43 (6.1%) with DENV-2 S16803, and 40 (5.6%) with DENV-1 WP74 (see Supplementary Material). Table 2 reports numbers of non-human primates by DENV serotype.
Table 1.
Summary of included studies. In table, primates studied are: rhesus macaque (Macaca mulatta), cynomolgus macaques (Macaca fascicularis), green monkeys (Chlorocebus aethiops sabaeus), owl monkeys (Aotus nancymaae), chimpanzee (Pan troglodytes), spider monkey (Ateles geoffroyi), pig-tailed macaques (Macaca nemestrina), common marmoset (Callithrix jacchus), patas (Erythrocebus patas), squirrel monkey (Saimiri sciureus), and White Handed Gibbon (Hylobates lar).
| Study | No. of primates | Species | Serotype | Ref. |
|---|---|---|---|---|
| Anez (2009) | 4 | Rhesus macaque | 4 | Anez et al. (2009) |
| Angsubhakorn (1988) | 4 | Cynomolgus macaques, rhesus macaque | 4 | Angsubhakorn et al. (1988) |
| Bernardo (2008) | 4 | Cynomolgus macaques, rhesus macaque | 1 | Bernardo et al. (2008) |
| Bray (1996) | 16 | Rhesus macaque | 1, 2, 4 | Bray et al. (1996) |
| Butrapet (2002) | 2 | Cynomolgus macaques | 1 | Butrapet et al. (2002) |
| Chen (2007) | 3 | Cynomolgus macaques | 1 | Chen et al. (2007) |
| Clements (2010) | 3 | Rhesus macaque | 2 | Clements et al. (2010) |
| Durbin (2006), Unpub.a | 6 | Rhesus macaque | 1, 2 | Unpub. |
| Durbin (2007), Unpub.a | 6 | Rhesus macaque | 1, 2 | Unpub. |
| Durbin (2008), Unpub.a | 6 | Rhesus macaque | 1, 2 | Unpub. |
| Freire (2007) | 26 | Rhesus macaque | 1, 2, 3 | Freire et al. (2007) |
| Galler (2005) | 5 | Rhesus macaque | 2 | Galler et al. (2005) |
| Goncalvez (2007) | 3 | Rhesus macaque | 4 | Goncalvez et al. (2007) |
| Guirakhoo (2000) | 8 | Rhesus macaque | 2 | Guirakhoo et al. (2000) |
| Guzman (2003) | 3 | Cynomolgus macaques | 4 | Guzman et al. (2003) |
| Halstead (1973) | 119 | Green monkey, patas, rhesus macaque | 1, 2, 3, 4 | Halstead et al. (1973) |
| Hanley (2004) | 2 | Rhesus macaque | 4 | Hanley et al. (2004) |
| Harrison (1977) | 6 | Chimpanzee, rhesus macaque | 2 | Harrison et al. (1977) |
| Hermida (2006) | 3 | Rhesus macaque | 2 | Hermida et al. (2006) |
| Houng (2000) | 3 | Rhesus macaque | 2 | Houng et al. (2000) |
| Kochel (2000) | 5 | Aotus | 1 | Kochel et al. (2000) |
| Kochel (2005) | 18 | Aotus | 1 | Kochel et al. (2005) |
| Lai (2007) | 2 | Rhesus macaque | 4 | Lai et al. (2007) |
| Marchette (1973) | 27 | Rhesus macaque | 1, 2, 3, 4 | Marchett et al. (1973) |
| Markoff (2002) | 9 | Rhesus macaque | 1 | Markoff et al. (2002) |
| Martin (2009) | 12 | Green monkey | 2 | Martin et al. (2009b) |
| Martin (2009) | 6 | Green monkey | 2 | Martin et al. (2009a) |
| Maves (2011) | 6 | Aotus | 1 | Maves et al. (2011) |
| Men (1996) | 12 | Rhesus macaque | 4 | Men et al. (1996) |
| Men (2000) | 4 | Rhesus macaque | 2 | Men et al. (2000) |
| Omatsu (2011) | 20 | Common marmoset | 1, 2, 3, 4 | Omatsu et al. (2011) |
| Onlamoon (2010) | 6 | Rhesus macaque | 2 | Onlamoon et al. (2010) |
| Pamungkas (2011) | 14 | Pig-tailed macaques (Macaca nemestrina) | 3 | Pamungkas et al. (2011) |
| Pletnev (2001) | 4 | Rhesus macaque | 4 | Pletnev et al. (2001) |
| Price (1968) | 17 | Spider monkey | 1, 3, 4 | Price et al. (1968) |
| Price (1973) | 20 | Spider monkey | 1, 2, 3, 4 | Price et al. (1973) |
| Price (1974) | 25 | Chimpanzee, cynomolgus macaques, | 1, 4 | Price et al. (1974) |
| Rhesus macaque, squirrel monkey | ||||
| Putnak (1996) | 3 | Rhesus macaque | 2 | Putnak et al. (1996) |
| Putnak (2003) | 9 | Rhesus macaque | 2 | Putnak et al. (2003) |
| Raviprakash (2000) | 5 | Rhesus macaque | 1 | Raviprakash et al. (2000) |
| Raviprakash (2006) | 5 | Rhesus macaque | 1, 2 | Raviprakash et al. (2006) |
| Raviprakash (2008) | 24 | Rhesus macaque | 1, 2, 3, 4 | Raviprakash et al. (2008) |
| Rumyantsev (2006) | 8 | Rhesus macaque | 4 | Rumyantsev et al. (2006) |
| Scherer (1978) | 10 | Chimpanzee | 1, 2, 3, 4 | Scherer et al. (1978) |
| Schiavetta (2003) | 15 | Aotus | 1, 2, 3, 4 | Schiavetta et al. (2003) |
| Simmons (2006) | 4 | Rhesus macaque | 2 | Simmons et al. (2006) |
| Simmons (2010) | 20 | Rhesus macaque | 1, 2, 3, 4 | Simmons et al. (2010) |
| Sun (2006) | 20 | Rhesus macaque | 1, 2, 3, 4 | Sun et al. (2006) |
| Tarr (1976) | 4 | Rhesus macaque | 2 | Tarr and Lubiniecki (1976) |
| Valdes (2009) | 3 | Green monkey | 2 | Valdés et al. (2009) |
| Velzing (1999) | 2 | Cynomolgus macaques | 2 | Velzing et al. (1999) |
| Whitehead, variousb | 84 | Rhesus macaque | 1, 2, 3, 4 | Blaney et al. (2006) |
| Whitehead (1970) | 33 | White handed gibbon | 1, 2, 3, 4 | Whitehead et al. (1970) |
| Widjaja (2010) | 16 | Pig-tailed macaques (Macaca nemestrina) | 1, 2, 3, 4 | Widjaja et al. (2010) |
Table 2. Summary of DENV infection by non-human primate species.
Table reports numbers of non-human primate species by infection with DENV serotypes 1–4.
| Species | DENV 1 | DENV 2 | DENV 3 | DENV 4 |
|---|---|---|---|---|
| chimpanzee (Pan troglodytes) | 2 | 6 | 2 | 12 |
| common marmoset (Callithrix jacchus) | 1 | 17 | 1 | 1 |
| cynomolgus macaques (Macaca fascicularis) | 7 | 2 | 0 | 10 |
| green monkey (Chlorocebus aethiops sabaeus) | 1 | 23 | 1 | 0 |
| owl monkeys (Aotus nancymaae) | 33 | 4 | 4 | 3 |
| patas (Erythrocebus patas) | 1 | 2 | 0 | 0 |
| pig-tailed macaques (Macaca nemestrina) | 4 | 4 | 18 | 4 |
| rhesus macaque (Macaca mulatta) | 97 | 155 | 75 | 139 |
| spider monkey (Ateles geoffroyi) | 8 | 5 | 12 | 12 |
| squirrel monkey (Saimiri sciureus) | 5 | 0 | 0 | 0 |
| white handed gibbon (Hylobates lar) | 7 | 9 | 8 | 9 |
Associations with Time to Viremia and Duration
Mixed effects models were fit with a random effect for study and were universally preferred over linear fixed effects models by AIC (see Supplementary Material). Intraclass correlation coefficients indicated strong heterogeneity by study (0.48, 95% CI: 0.37, 0.60) which could be due to differences among laboratories and assays employed. Mixed effects models assume non-human primates are exchangeable within studies, and account for heterogeneity between studies. Mixed effects models employed here do not take into account censoring, however only DENV-2 (p = 0.001) and common marmoset samples (p = 0.03) were associated with more censoring.
Tables 3 and 4 report the associations for serotype, log10 inoculating dose, assay, and species of non-human primate with length of time to detectable viremia and duration of viremia in mixed effects models. Both univariate (with only the covariate of interest included) and multivariate (with all covariates included) models were fit. The multivariate models accounting for study heterogeneity indicated the time to detectable viremia for DENV-1 was statistically significantly longer than for DENV-4 and DENV-2 and -3 were not significantly different from DENV-4. Time to detectable viremia was statistically significantly longer in patas monkeys and marginally significantly shorter in spider monkeys than rhesus macaques; and time to detectable viremia was significantly shorter in those non-human primates assayed by Immunofluorescence assays (IFA). Increasing log dose of inoculum was statistically significantly associated with shorter times to detectable viremias (Table 3). Large study heterogeneity was present, with the variance of the random intercept equal to 1 day.
Table 3. Associations with Time to Detectable Viremia.
Table reports the results of the univariate and multivariate mixed effects regression calculating associations between serotype, species, viremia assay used, and log10 inoculating dose and time to detectable viremia. Mixed effects models included random effect for study. Univariate estimates are differences in days of viremia from the reference category of each model (denoted “ref.”), and multivariate estimates are differences in days of viremia for each covariate from rhesus monkeys infected with DENV-4 assayed using plaque count. P-values calculated using likelihood ratio tests. Estimates of the fixed intercept (β0) and variance of the random intercept are presented (σ).
| Covariate | Univariate | 95% CI | p | Multivariate | 95% CI | p |
|---|---|---|---|---|---|---|
| DENV-4 | ref. | ref. | ||||
| DENV-1 | 0.53 | (0.20, 0.86) | 0.002 | 0.55 | (0.22, 0.89) | 0.001 |
| DENV-2 | −0.09 | (−0.41, 0.22) | 0.558 | −0.17 | (−0.49, 0.14) | 0.254 |
| DENV-3 | 0.20 | (−0.14, 0.54) | 0.252 | 0.24 | (−0.10, 0.57) | 0.153 |
|
| ||||||
| rhesus macaque (Macaca mulatta) | ref. | |||||
| chimpanzee (Pan troglodytes) | −0.17 | (−1.10, 0.76) | 0.730 | −0.30 | (−1.22, 0.63) | 0.483 |
| common marmoset (Callithrix jacchus) | −1.14 | (−3.15, 0.86) | 0.214 | −0.61 | (−2.75, 1.53) | 0.493 |
| cynomolgus macaques (Macaca fascicularis) | 0.66 | (−0.18, 1.50) | 0.132 | 0.74 | (−0.12, 1.60) | 0.096 |
| green monkey (Chlorocebus aethiops sabaeus) | 0.61 | (−0.32, 1.53) | 0.301 | 1.23 | (0.12, 2.35) | 0.095 |
| owl monkeys (Aotus nancymaae) | −0.21 | (−1.30, 0.89) | 0.671 | −0.20 | (−1.47, 1.08) | 0.777 |
| patas (Erythrocebus patas) | 3.64 | (2.13, 5.15) | < 0.001 | 3.89 | (2.40, 5.39) | < 0.001 |
| pig-tailed macaques (Macaca nemestrina) | −0.94 | (−2.40, 0.52) | 0.162 | −0.50 | (−2.15, 1.15) | 0.446 |
| spider monkey (Ateles geoffroyi) | −1.29 | (−2.74, 0.15) | 0.056 | −2.49 | (−5.20, 0.22) | 0.040 |
| squirrel monkey (Saimiri sciureus) | −0.35 | (−1.73, 1.03) | 0.620 | −0.96 | (−2.36, 0.43) | 0.162 |
| white handed gibbon (Hylobates lar) | 0.62 | (−1.35, 2.58) | 0.508 | −0.27 | (−2.34, 1.80) | 0.755 |
|
| ||||||
| plaque count | ref. | |||||
| ELISA | −0.75 | (−2.31, 0.82) | 0.329 | −1.53 | (−3.51, 0.45) | 0.161 |
| FFA | 0.02 | (−1.49, 1.52) | 0.974 | 0.22 | (−1.41, 1.86) | 0.746 |
| IFA | −0.75 | (−1.43, −0.07) | 0.025 | −0.89 | (−1.74, −0.04) | 0.016 |
| RTPCR | −0.67 | (−1.37, 0.03) | 0.050 | −0.51 | (−1.39, 0.37) | 0.170 |
| suckling mice | −1.16 | (−2.34, 0.02) | 0.043 | 0.64 | (−1.71, 2.99) | 0.561 |
|
| ||||||
| log10 dose | −0.15 | (−0.32, 0.01) | 0.069 | −0.21 | (−0.39, −0.04) | 0.021 |
|
| ||||||
| Intercept (β0) | 2.68 | (1.63, 3.74) | < 0.001 | |||
| Random Effect (σ) | 0.99 | (0, 2.93) | ||||
Table 4. Associations with Duration of Viremia.
Table reports the results of the univariate and multivariate mixed effects regression calculating associations between serotype, species, viremia assay used, and log10 inoculating dose and duration of viremia. Mixed effects models included random effect for study. Univariate estimates are differences in days of viremia from the reference category of each model (denoted “ref.”), and multivariate estimates are differences in days of viremia for each covariate from rhesus monkeys infected with DENV-4 assayed using plaque count. P-values calculated using likelihood ratio tests. Durations of DENV-1 and -2 viremia are significantly longer than DENV-4 after adjusting for study, species, assay and log10 dose. Estimates of the fixed intercept (β0) and variance of the random intercept are presented (σ).
| Covariate | Univariate | 95% CI | p | Multivariate | 95% CI | p |
|---|---|---|---|---|---|---|
| DENV-4 | ref. | ref. | ||||
| DENV-1 | 0.63 | (0.17, 1.09) | 0.008 | 0.74 | (0.26, 1.21) | 0.002 |
| DENV-2 | 1.02 | (0.58, 1.46) | < 0.001 | 1.21 | (0.77, 1.65) | < 0.001 |
| DENV-3 | −0.34 | (−0.81, 0.13) | 0.151 | −0.30 | (−0.77, 0.17) | 0.225 |
|
| ||||||
| rhesus macaque (Macaca mulatta) | ref. | |||||
| chimpanzee (Pan troglodytes) | 0.16 | (−1.26, 1.57) | 0.836 | 0.26 | (−1.06, 1.59) | 0.624 |
| common marmoset (Callithrix jacchus) | −0.15 | (−4.05, 3.76) | 0.936 | −1.80 | (−5.09, 1.49) | 0.185 |
| cynomolgus macaques (Macaca fascicularis) | 0.23 | (−1.09, 1.55) | 0.732 | 0.00 | (−1.24, 1.24) | 0.941 |
| green monkey (Chlorocebus aethiops sabaeus) | −1.36 | (−2.84, 0.12) | 0.062 | −1.48 | (−3.08, 0.12) | 0.050 |
| owl monkeys (Aotus nancymaae) | −0.46 | (−2.54, 1.62) | 0.637 | −1.29 | (−3.23, 0.66) | 0.125 |
| patas (Erythrocebus patas) | −2.07 | (−4.21, 0.06) | 0.057 | −2.35 | (−4.45, −0.25) | 0.026 |
| pig-tailed macaques (Macaca nemestrina) | −0.40 | (−3.21, 2.42) | 0.764 | −0.64 | (−3.16, 1.88) | 0.514 |
| spider monkey (Ateles geoffroyi) | −1.11 | (−3.91, 1.69) | 0.400 | 0.34 | (−3.77, 4.46) | 0.834 |
| squirrel monkey (Saimiri sciureus) | −0.72 | (−2.72, 1.29) | 0.470 | −1.39 | (−3.36, 0.59) | 0.170 |
| white handed gibbon (Hylobates lar) | 0.00 | (−3.87, 3.86) | 0.998 | 0.09 | (−3.09, 3.28) | 0.932 |
|
| ||||||
| plaque count | ref. | |||||
| ELISA | 0.02 | (−2.81, 2.85) | 0.987 | 1.34 | (−1.61, 4.30) | 0.304 |
| FFA | 0.17 | (−2.59, 2.93) | 0.889 | 0.27 | (−2.22, 2.75) | 0.788 |
| IFA | 0.98 | (−0.29, 2.24) | 0.110 | 1.34 | (0.05, 2.64) | 0.018 |
| RTPCR | 1.89 | (0.59, 3.18) | 0.004 | 2.52 | (1.19, 3.85) | < 0.001 |
| suckling mice | −0.16 | (−2.39, 2.06) | 0.880 | −0.37 | (−3.92, 3.18) | 0.807 |
|
| ||||||
| log10 dose | −0.40 | (−0.65, −0.14) | 0.002 | −0.44 | (−0.69, −0.18) | < 0.001 |
|
| ||||||
| Intercept (β0) | 5.00 | (3.47, 6.53) | < 0.001 | |||
| Random Effect (σ) | 2.32 | (0, 5.30) | ||||
Duration of viremia was statistically significantly longer for DENV-1 and -2 as compared to DENV-4 after accounting for study heterogeneity. Duration for DENV-3 was not significantly different from DENV-4 (Table 4). Adjusting for study, species, assay, and dose increased the difference in durations between DENV-1 and -2 and DENV-4. Changing the reference serotype to DENV-2 shows DENV-1, -3, and -4 to have statistically significantly shorter durations of viremia than DENV-2 (see Supplementary Material). Significantly longer durations of viremia were observed when assayed by RT-PCR and IFA compared to plaque-forming assays, adjusting for study, species, assay, and dose. No significant differences in viremia duration were observed across species, besides a significant shortening in patas monkeys (however, only 3 patas monkeys were tested) and a marginally significant shortening in green monkeys from rhesus monkeys. Duration of viremia was negatively associated with dose of inoculum, with durations decreasing by 0.44 days (95% CI: 0.18, 0.7) per log10 increase in dose. Again, the variance of the random intercept was quite large (2.32 days).
Estimates of Time to Detectable Viremia and Duration of Viremia
In rhesus macaques the median time to detectable viremia of DENV was 3.32 days (95% CI: 3.01, 3.65), 2.63 days (95% CI: 2.40, 2.89), 3.02 days (95% CI: 2.71, 3.34), and 3.23 days (95% CI: 2.99, 3.47) for DENV-1, -2, -3, and -4, respectively (Table 5 and Figure 2). The median duration of viremia was 4.67 days (95% CI: 4.27, 5.12), 5.13 days (95% CI: 4.82, 5.48), 3.22 days (95% CI: 2.83, 3.72), and 3.13 days (95% CI: 2.86, 3.46) for DENV-1, -2, -3, and -4, respectively. As no significant differences were observed in duration of viremia between species (see above), estimates of duration were pooled across all species. The median time to detectable viremia of DENV was 3.23 days (95% CI: 3.00, 3.45), 2.44 days (95% CI: 2.22, 2.65), 2.89 days (95% CI: 2.67, 3.11), and 3.17 days (95% CI: 2.98, 3.37) for DENV-1, -2, -3, and -4, respectively (see Supplementary Material). The median duration of viremia was 4.33 days (95% CI: 4.03, 4.67), 4.84 days (95% CI: 4.52, 5.15), 3.34 days (95% CI: 3.01, 3.68), and 3.24 days (95% CI: 3.01, 3.51) for DENV-1, -2, -3, and -4, respectively.
Table 5. Summary of DENV Virus Kinetics in Rhesus Macaques.
Table reports median days to viremia and duration of viremia with 95% bootstrap confidence intervals for 5th, 25th, 50th, 75th, and 95th percentile.
| Serotype | Percentile | Time to Viremia Days (95% CI) |
Duration Days (95% CI) |
|---|---|---|---|
| 1 | 5th | 1.43 (1.20, 1.68) | 2.02 (1.73, 2.44) |
| n = 97 | 25th | 2.35 (2.08, 2.64) | 3.31 (2.97, 3.76) |
| 50th | 3.32 (3.01, 3.65) | 4.67 (4.27, 5.12) | |
| 75th | 4.69 (4.28, 5.11) | 6.59 (6.01, 7.18) | |
| 95th | 7.73 (6.89, 8.57) | 10.82 (9.51, 12.12) | |
|
| |||
| 2 | 5th | 0.98 (0.82, 1.18) | 2.24 (1.98, 2.59) |
| n = 155 | 25th | 1.76 (1.55, 2.00) | 3.65 (3.35, 4.00) |
| 50th | 2.63 (2.40, 2.89) | 5.13 (4.82, 5.48) | |
| 75th | 3.95 (3.67, 4.22) | 7.21 (6.73, 7.70) | |
| 95th | 7.06 (6.48, 7.64) | 11.76 (10.48, 13.01) | |
|
| |||
| 3 | 5th | 1.30 (1.07, 1.57) | 1.03 (0.84, 1.33) |
| n = 75 | 25th | 2.14 (1.85, 2.41) | 2.02 (1.73, 2.41) |
| 50th | 3.02 (2.71, 3.34) | 3.22 (2.83, 3.72) | |
| 75th | 4.26 (3.78, 4.74) | 5.14 (4.56, 5.84) | |
| 95th | 6.99 (5.91, 8.13) | 10.05 (8.70, 11.58) | |
|
| |||
| 4 | 5th | 1.44 (1.32, 1.58) | 1.10 (0.95, 1.31) |
| n = 139 | 25th | 2.32 (2.16, 2.51) | 2.04 (1.83, 2.32) |
| 50th | 3.23 (2.99, 3.47) | 3.13 (2.86, 3.46) | |
| 75th | 4.49 (4.12, 4.84) | 4.82 (4.38, 5.28) | |
| 95th | 7.22 (6.48, 7.89) | 8.96 (7.96, 10.00) | |
Figure 2. Days of Viremia for Primates.
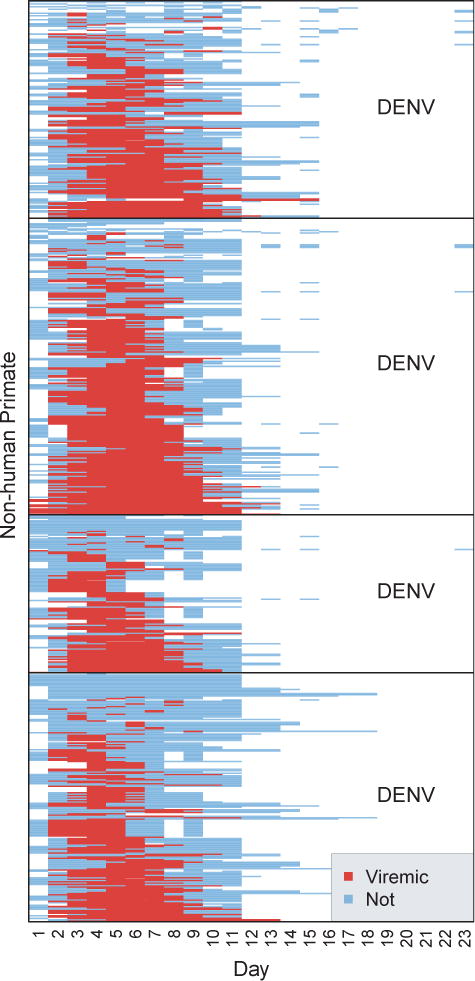
Figure shows the days of viremia for each non-human primate stratified by DENV serotype. Blue bars indicate DENV negative blood samples and red indicates positive samples. White indicates no samples. Data were sorted by DENV serotype, then by days of viremia.
Discussion
The results of our meta-analysis indicate that the median time to detectable viremia and duration of viremia of DENV was not statistically significantly different between non-human primate species. In rhesus macaques (Macaca mulatta), median times to detectable viremia ranged from 2.63 (95% CI: 2.40, 2.89) days for DENV-2 to 3.32 (95% CI: 3.01, 3.65) days for DENV-1 and median duration of viremia from 3.13 (95% CI: 2.86, 3.46) days for DENV-4 to 5.13 (95% CI: 4.82, 5.48) days for DENV-2. These estimates are shorter than those previously reported in humans. Tricou et al. reported a median duration of viremia of 6.2 days (IQR 5.8 to 7.2) for all serotypes and 6.8 days (IQR 6 to 7.3) for DENV-1 [21]. Vaughn et al. reported a mean duration of viremia in humans of 5.5 days for primary DENV-1 infection and 4.6 days for primary DENV-3 infection [22]. Murgue et al. found a mean duration of 4.4 days for primary DENV infection in a cohort of French Polynesian children [23]. However, all three of these studies estimate the duration of viremia in individuals hospitalized with dengue, and thus likely not on the first day of viremia. This would tend to underestimate the true duration of viremia. Additionally, due to selection of dengue cases based on severity (i.e. hospitalized patients) the cases included in these studies may not be representative of all dengue infections. The non-human primate studies identified here skirt these two problems directly.
We found no statistically significant differences in time to- or duration of viremia between the 11 species of non-human primates studied here save for patas monkeys. Patas monkeys were found to have significantly longer times to detectable viremia and shorter duration of viremia, however, only 3 patas were infected in one study [2]. More measurements in patas monkeys would be an important contribution as it is one of the few species from which sylvatic DENV has been isolated [6]. Similarly, spider monkeys had a marginally significantly shorter time to detectable viremia (p = 0.04) than rhesus monkeys. Interestingly, the monkeys examined here included several species of old and new world non-human primates, otherwise expected to exhibit differing physiologic and immune responses [24].
Interestingly, the duration of DENV-4 viremia was significantly shorter than DENV-1 and -2 after adjusting for study, species, assay and dose of inoculum. There has been clear demonstration of differences in transmission patterns [15] and in clinical manifestations [16, 17] across the four serotypes of DENV. Shorter duration of DENV-4 viremia may account for the reduced severity observed in this serotype. Fried et al. found cases of dengue hemorrhagic fever (DHF) to be twice as likely in secondary DENV-2 and -3 infections than in secondary DENV-4 [25]. Conversely, we found DENV-2 to be statistically significantly longer than DENV-1, and -3, and -4. Blamaseda et al. found nearly double the odds of shock and internal hemorrhage with DENV-2 infection in outbreaks of DENV in Nicaragua [16]. Nisalak et al. found DENV-3 to be associated with severe outbreaks of dengue in hospitalized cases in Bangkok, Thailand [15]. Fox et al. found time to undetectable DENV-2 NS1 protein to be significantly longer than DENV-1 [26]. Extended durations of viremia for DENV-2 and -3 may be the cause of the increased severity of these infections and may be the reason sylvatic DENV-2 is the only serotype to have emerged in Africa from southeast Asia.
We found significantly longer durations of viremia when assayed using RT-PCR or immunofluorescence as compared to plaque-forming assays. This is most likely due to a higher sensitivity of RT-PCR as compared to other, older methods for determining viremia such as plaque counting and inoculation of suckling mice. Though some of this may be due to detection of viral RNA, and not actively replicating virus. More modern methods such as ELISA and focus-forming assays were not found to be significantly different from plaque-forming assays, but this could be due to small sample sizes. The effect estimates for ELISA and FFA were 1.34 and 0.27 days longer, respectively, adjusting for study heterogeneity, serotype, species, and inoculating dose. Importantly, these differences in detection of viremia were robust to adjustment for study heterogeneity, which was considerable. Intraclass correlation coefficients indicated nearly half of the observed variance was due to differences between studies. This underlines the importance of using random effects models to account for differences between studies, and using care when interpreting results of viremia assays.
Surprisingly, increasing doses were associated with both shorter times to detectable viremia and shorter durations of viremia. This phenomenon was observed by Martin et al. in green monkeys [27], in yellow fever virus (YFV) infections in rhesus monkeys [28], chimeric YF-DENV vaccine in cynomolgus macaques [29] and in humans receiving a live, attenuated Japanese Encephalitis vaccine (ChimeriVax-JE) [30, 31] and a live, attenuated West Nile virus vaccine [32]. It could be that a large inoculating dose causes a rapid initial rise in viremia inducing a stronger innate immune response leading to quicker clearance. Studies in humans have found that higher peak viremia titers were positively associated with more severe disease [21, 22, 33]; however the evidence for an association between the magnitude and duration of viremia remains inconclusive. Vaughn et al. found the time from peak viremia to clearance was more rapid in DHF cases than in DF cases [22], while Fox et al. found no difference in rate of clearance between DHF and DF [26].
Not all studies included in this meta-analysis reported daily levels of viremia. Additional studies examining directly the relationship between inoculating dose, peak viremia and duration of viremia are necessary, as well as studies investigating the effects of preexisting immunity on time to viremia and duration.
Our methods separately accounted for the two largest potential sources of bias: random effects models accounted for study heterogeneity and doubly-interval censored survival analysis accounted for the large amount of censoring (right-, left- and both) present in reported days of non-human primate viremia. While the random effects model did not take into account the effects of censoring, the amount of censoring only differed in DENV-2 and common marmoset samples, and inferences drawn from them are useful for examining associations between covariates of interest and the time to detectable viremia and duration of viremia. Even though the random effects model accounts for most of the heterogeneity between studies, some caution must still be used when interpreting the results of the associations as some residual confounding may exist from remaining heterogeneity between studies. Finally, while all efforts were made to find all studies reporting non-human primate viremia, it is possible that some studies were missed, or that some data were not published [34].
Our study provides estimates of the times to detectable viremia and durations of DENV-1–4 viremia in multiple non-human primate species, both Old World and New World, and identifies how these differ across serotype, viremia assay, non-human primate species and inoculating dose. Few if any studies have directly compared DENV infection in multiple non-human primates. Our results further understanding of within host DENV replication kinetics which are especially important in how they influence transmission dynamics. In the light of new dengue vaccine trials [35, 36], sylvatic DENV infection in non-human primates could provide a source of infectious introductions. An accurate and thorough understanding of the sylvatic cycle of dengue, including the roles of the various non-human primate species in transmission, may allow prediction of epidemics within non-human primates and thereby lessen the impact of spillover on humans living in areas of overlap with non-human primate hosts. Our results also are important in parameterizing dynamic models of dengue [14], and further understanding of DENV transmission dynamics in general, including differences in serotype-specific cycles.
Supplementary Material
Figure 3. Time to Detectable Viremia for DENV in Rhesus Macaque.
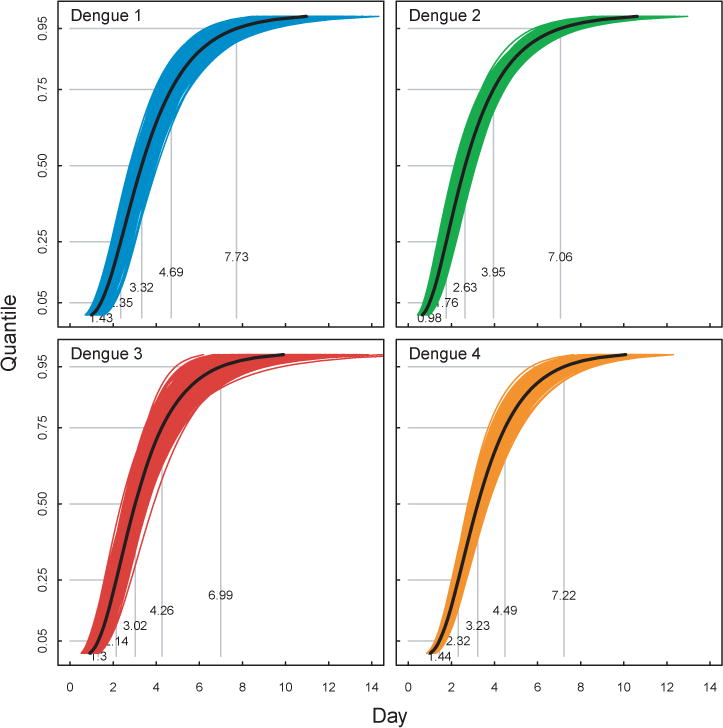
Figure shows estimates of the time to detectable viremia for DENV-1–4 in Rhesus primates. Black lines indicate estimates from full dataset and light colored lines indicate bootstrap replicates. Grey lines indicate the 5th, 25th, 50th, 75th and 95th quantiles.
Figure 4. Duration of DENV Infection in Rhesus Macaque.
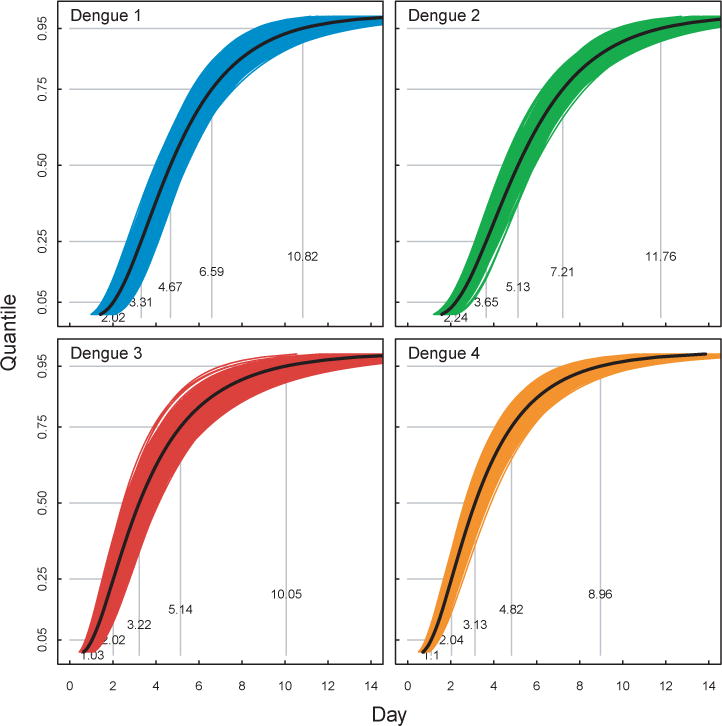
Figure shows estimates of the duration of infection for DENV-1–4 in Rhesus primates. Black lines indicate estimates from full dataset and light colored lines indicate bootstrap replicates. Grey lines indicate the 5th, 25th, 50th, 75th and 95th quantiles.
Acknowledgments
The authors wish to thank Drs. A. Pletnev, A. Goncalvez, R. Purcell and S. Whitehead for kindly providing data, and Josh Pascual for reviewing abstracts. This work was funded by NIH grant AI069145 (PI: SCW, KH, DATC) as well as US NIH National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 1U54GM088491-0109 (DATC). BMA acknowledges an NSF Graduate Research Fellowship (grant no. DGE-0707427), and the Omidyar Postdoctoral Fellowship program. DATC holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simmons CP, Farrar JJ, Nguyen vVC, Wills B. Dengue. N Engl J Med. 2012 Apr;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 2.Halstead S, Shotwell H, Casals J. Studies on pathogenesis of dengue infection in monkeys 1. clinical laboratory responses to primary infection. Journal of Infectious Diseases. 1973;128(1):7–14. doi: 10.1093/infdis/128.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Koraka P, Benton S, van Amerongen G, Stittelaar KJ, Osterhaus ADME. Characterization of humoral and cellular immune responses in cynomolgus macaques upon primary and subsequent heterologous infections with dengue viruses. Microbes Infect. 2007 Jul;9:940–6. doi: 10.1016/j.micinf.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Zompi S, Harris E. Animal models of dengue virus infection. Viruses. 2012 Jan;4:62–82. doi: 10.3390/v4010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudnick A, Lim T, Ireland J. Dengue fever studies in Malaysia. Institute for Medical Research; 1986. [Google Scholar]
- 6.Diallo M, Ba Y, Sall AA, Diop OM, Ndione JA, Mondo M, Girault L, Mathiot C. Amplification of the sylvatic cycle of dengue virus type 2, senegal, 1999–2000: entomologic findings and epidemiologic considerations. Emerg Infect Dis. 2003 Mar;9:362–7. doi: 10.3201/eid0903.020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011 Jul;9:532–41. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardosa J, Ooi MH, Tio PH, Perera D, Holmes EC, Bibi K, Abdul Manap Z. Dengue virus serotype 2 from a sylvatic lineage isolated from a patient with dengue hemorrhagic fever. PLoS Negl Trop Dis. 2009;3(4):e423. doi: 10.1371/journal.pntd.0000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco L, Palacios G, Martinez JA, Vázquez A, Savji N, De Ory F, Sanchez-Seco MP, Martín D, Lipkin WI, Tenorio A. First report of sylvatic denv-2-associated dengue hemorrhagic fever in west africa. PLoS Negl Trop Dis. 2011 Aug;5:e1251. doi: 10.1371/journal.pntd.0001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasilakis N, Weaver SC. The history and evolution of human dengue emergence. Adv Virus Res. 2008;72:1–76. doi: 10.1016/S0065-3527(08)00401-6. [DOI] [PubMed] [Google Scholar]
- 11.Guy B, Saville M, Lang J. Development of sanofi pasteur tetravalent dengue vaccine. Hum Vaccin. 2010;6 doi: 10.4161/hv.6.9.12739. [DOI] [PubMed] [Google Scholar]
- 12.Durbin AP, Whitehead SS. Dengue vaccine candidates in development. Curr Top Microbiol Immunol. 2010;338:129–43. doi: 10.1007/978-3-642-02215-9_10. [DOI] [PubMed] [Google Scholar]
- 13.Keeling MJ, Rohani P. Modeling infectious diseases in humans and animals. Princeton: Princeton University Press; 2008. [Google Scholar]
- 14.Althouse BM, Lessler J, Sall AA, Diallo M, Hanley KA, Watts DM, Weaver SC, Cummings DA. Synchrony of sylvatic dengue isolations: A multi-host, multi-vector sir model of dengue virus transmission in senegal. PLoS neglected tropical diseases. 2012;6(11):e1928. doi: 10.1371/journal.pntd.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, Burke DS, Hoke CH, Innis BL, Vaughn DW. Serotype-specific dengue virus circulation and dengue disease in bangkok, thailand from 1973 to 1999. Am J Trop Med Hyg. 2003 Feb;68:191–202. [PubMed] [Google Scholar]
- 16.Balmaseda A, Hammond SN, Pérez L, Tellez Y, Saborío SI, Mercado JC, Cuadra R, Rocha J, Pérez MA, Silva S, Rocha C, Harris E. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006 Mar;74:449–56. [PubMed] [Google Scholar]
- 17.Halsey ES, Marks MA, Gotuzzo E, Fiestas V, Suarez L, Vargas J, Aguayo N, Madrid C, Vimos C, Kochel TJ, Laguna-Torres VA. Correlation of serotype-specific dengue virus infection with clinical manifestations. PLoS Negl Trop Dis. 2012;6(5):e1638. doi: 10.1371/journal.pntd.0001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reich N, Lessler J, Cummings D, Brookmeyer R. Estimating incubation period distributions with coarse data. Statistics in medicine. 2009;28(22):2769–2784. doi: 10.1002/sim.3659. [DOI] [PubMed] [Google Scholar]
- 19.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. 2. Hoboken, N.J: Wiley; 2011. (Wiley series in probability and statistics). [Google Scholar]
- 20.Burnham KP, Anderson DR, Burnham KP. Model selection and multimodel inference: a practical information-theoretic approach. 2. New York: Springer; 2002. [Google Scholar]
- 21.Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP. Kinetics of viremia and ns1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl Trop Dis. 2011 Sep;5:e1309. doi: 10.1371/journal.pntd.0001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000 Jan;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 23.Murgue B, Roche C, Chungue E, Deparis X. Prospective study of the duration and magnitude of viraemia in children hospitalised during the 1996–1997 dengue-2 outbreak in french polynesia. J Med Virol. 2000 Apr;60:432–8. doi: 10.1002/(sici)1096-9071(200004)60:4<432::aid-jmv11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Nunn CL, Gittleman JL, Antonovics J. Promiscuity and the primate immune system. Science. 2000 Nov;290:1168–70. doi: 10.1126/science.290.5494.1168. [DOI] [PubMed] [Google Scholar]
- 25.Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon IK, Jarman RG, Green S, Rothman AL, Cummings DAT. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in bangkok, thailand from 1994 to 2006. PLoS Negl Trop Dis. 2010;4(3):e617. doi: 10.1371/journal.pntd.0000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox A, Le NMH, Simmons CP, Wolbers M, Wertheim HFL, Pham TK, Tran THN, Trinh TML, Nguyen TL, Nguyen VT, Nguyen DH, Farrar J, Horby P, Taylor WR, Nguyen VK. Immunological and viral determinants of dengue severity in hospitalized adults in ha noi, viet nam. PLoS Negl Trop Dis. 2011;5(3):e967. doi: 10.1371/journal.pntd.0000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín J, Hermida L, Castro J, Lazo L, Martínez R, Gil L, Romero Y, Puente P, Zaragoza S, Cosme K, Guzmán MG, Cardosa J, Guillén G. Viremia and antibody response in green monkeys (chlorocebus aethiops sabaeus) infected with dengue virus type 2: a potential model for vaccine testing. Microbiol Immunol. 2009 Apr;53:216–23. doi: 10.1111/j.1348-0421.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- 28.FOX J, PENNA H. Behavior of 17d yellow fever virus in rhesus monkeys relation to substrain, dose, and neural or extraneural inoculation. American Journal of Epidemiology. 1943;38(2):152–172. [Google Scholar]
- 29.Guirakhoo F, Pugachev K, Zhang Z, Myers G, Levenbook I, Draper K, Lang J, Ocran S, Mitchell F, Parsons M, Brown N, Brandler S, Fournier C, Barrere B, Rizvi F, Travassos A, Nichols R, Trent D, Monath T. Safety and efficacy of chimeric yellow fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J Virol. 2004 May;78:4761–75. doi: 10.1128/JVI.78.9.4761-4775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monath TP, Guirakhoo F, Nichols R, Yoksan S, Schrader R, Murphy C, Blum P, Woodward S, McCarthy K, Mathis D, Johnson C, Bedford P. Chimeric live, attenuated vaccine against japanese encephalitis (chimerivax-je): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated japanese encephalitis antigen. J Infect Dis. 2003 Oct;188:1213–30. doi: 10.1086/378356. [DOI] [PubMed] [Google Scholar]
- 31.Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, Marchesani R, Knauber M, Wells KH, Arroyo J, Guirakhoo F. Clinical proof of principle for chimerivax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002 Jan;20:1004–18. doi: 10.1016/s0264-410x(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 32.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, McCarthy K, Johnson C, Ermak T, Shin S, Arroyo J, Guirakhoo F, Kennedy JS, Ennis FA, Green S, Bedford P. A live, attenuated recombinant west nile virus vaccine. Proc Natl Acad Sci U S A. 2006 Apr;103:6694–9. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang WK, Chao DY, Kao CL, Wu HC, Liu YC, Li CM, Lin SC, Ho ST, Huang JH, King CC. High levels of plasma dengue viral load during defervescence in patients with dengue hemorrhagic fever: implications for pathogenesis. Virology. 2003 Jan;305:330–8. doi: 10.1006/viro.2002.1704. [DOI] [PubMed] [Google Scholar]
- 34.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990 Mar;263:1385–9. [PubMed] [Google Scholar]
- 35.Halstead SB. Dengue vaccine development: a 75% solution? Lancet. 2012 Sep; doi: 10.1016/S0140-6736(12)61510-4. [DOI] [PubMed] [Google Scholar]
- 36.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, cyd tetravalent dengue vaccine in thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012 Sep; doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 37.Blaney J, Durbin A, Murphy B, Whitehead S. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral immunology. 2006;19(1):10–32. doi: 10.1089/vim.2006.19.10. [DOI] [PubMed] [Google Scholar]
- 38.Añez G, Men R, Eckels KH, Lai CJ. Passage of dengue virus type 4 vaccine candidates in fetal rhesus lung cells selects heparin-sensitive variants that result in loss of infectivity and immunogenicity in rhesus macaques. J Virol. 2009 Oct;83:10384–94. doi: 10.1128/JVI.01083-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angsubhakorn S, Yoksan S, Bhamarapravati N, Moe JB, Marchette NJ, Pradermwong A, Sahaphong S. Dengue-4 vaccine: neurovirulence, viraemia and immune responses in rhesus and cynomolgus monkeys. Trans R Soc Trop Med Hyg. 1988;82(5):746–9. doi: 10.1016/0035-9203(88)90224-6. [DOI] [PubMed] [Google Scholar]
- 40.Bernardo L, Izquierdo A, Alvarez M, Rosario D, Prado I, López C, Martínez R, Castro J, Santana E, Hermida L, Guillen G, Guzmán MG. Immunogenicity and protective efficacy of a recombinant fusion protein containing the domain iii of the dengue 1 envelope protein in non-human primates. Antiviral Res. 2008 Nov;80:194–9. doi: 10.1016/j.antiviral.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Bray M, Men R, Lai CJ. Monkeys immunized with intertypic chimeric dengue viruses are protected against wild-type virus challenge. J Virol. 1996 Jun;70:4162–6. doi: 10.1128/jvi.70.6.4162-4166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butrapet S, Rabablert J, Angsubhakorn S, Wiriyarat W, Huang C, Kinney R, Punyim S, Bhamarapravati N. Chimeric dengue type 2/type 1 viruses induce immune responses in cynomolgus monkeys. Southeast Asian J Trop Med Public Health. 2002 Sep;33:589–99. [PubMed] [Google Scholar]
- 43.Chen L, Ewing D, Subramanian H, Block K, Rayner J, Alterson KD, Sedegah M, Hayes C, Porter K, Raviprakash K. A heterologous dna prime-venezuelan equine encephalitis virus replicon particle boost dengue vaccine regimen affords complete protection from virus challenge in cynomolgus macaques. J Virol. 2007 Nov;81:11634–9. doi: 10.1128/JVI.00996-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clements DE, Coller BAG, Lieberman MM, Ogata S, Wang G, Harada KE, Putnak JR, Ivy JM, McDonell M, Bignami GS, Peters ID, Leung J, Weeks-Levy C, Nakano ET, Humphreys T. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010 Mar;28:2705–15. doi: 10.1016/j.vaccine.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freire MS, Marchevsky RS, Almeida LFC, Yamamura AMY, Caride EC, Brindeiro PA, Motta MCA, Nogueira RMR, Kubelka CF, Bonaldo MC, Galler R. Wild dengue virus types 1, 2 and 3 viremia in rhesus monkeys. Mem Inst Oswaldo Cruz. 2007 May;102:203–8. doi: 10.1590/s0074-02762007005000011. [DOI] [PubMed] [Google Scholar]
- 46.Galler R, Marchevsky RS, Caride E, Almeida LFC, Yamamura AMY, Jabor AV, Motta MCA, Bonaldo MC, Coutinho ESF, Freire MS. Attenuation and immunogenicity of recombinant yellow fever 17d-dengue type 2 virus for rhesus monkeys. Braz J Med Biol Res. 2005 Dec;38:1835–46. doi: 10.1590/s0100-879x2005001200012. [DOI] [PubMed] [Google Scholar]
- 47.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A. 2007 May;104:9422–7. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guirakhoo F, Weltzin R, Chambers TJ, Zhang ZX, Soike K, Ratterree M, Arroyo J, Georgakopoulos K, Catalan J, Monath TP. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol. 2000 Jun;74:5477–85. doi: 10.1128/jvi.74.12.5477-5485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guzmán MG, Rodríguez R, Rodríguez R, Hermida L, Alvarez M, Lazo L, Muné M, Rosario D, Valdés K, Vázquez S, Martinez R, Serrano T, Paez J, Espinosa R, Pumariega T, Guillén G. Induction of neutralizing antibodies and partial protection from viral challenge in macaca fascicularis immunized with recombinant dengue 4 virus envelope glycoprotein expressed in pichia pastoris. Am J Trop Med Hyg. 2003 Aug;69:129–34. [PubMed] [Google Scholar]
- 50.Hanley KA, Manlucu LR, Manipon GG, Hanson CT, Whitehead SS, Murphy BR, Blaney JE., Jr Introduction of mutations into the non-structural genes or 3′ untranslated region of an attenuated dengue virus type 4 vaccine candidate further decreases replication in rhesus monkeys while retaining protective immunity. Vaccine. 2004 Sep;22:3440–8. doi: 10.1016/j.vaccine.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 51.Harrison VR, Eckels KH, Sagartz JW, Russell PK. Virulence and immunogenicity of a temperature-sensitive dengue-2 virus in lower primates. Infect Immun. 1977 Oct;18:151–6. doi: 10.1128/iai.18.1.151-156.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermida L, Bernardo L, Martín J, Alvarez M, Prado I, López C, Sierra BdlC, Martínez R, Rodríguez R, Zulueta A, Pérez AB, Lazo L, Rosario D, Guillén G, Guzmán MG. A recombinant fusion protein containing the domain iii of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine. 2006 Apr;24:3165–71. doi: 10.1016/j.vaccine.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 53.Houng HH, Hritz D, Kanesa-thasan N. Quantitative detection of dengue 2 virus using fluorogenic rt-pcr based on 3′-noncoding sequence. J Virol Methods. 2000 Apr;86:1–11. doi: 10.1016/s0166-0934(99)00166-4. [DOI] [PubMed] [Google Scholar]
- 54.Kochel TJ, Raviprakash K, Hayes CG, Watts DM, Russell KL, Gozalo AS, Phillips IA, Ewing DF, Murphy GS, Porter KR. A dengue virus serotype-1 dna vaccine induces virus neutralizing antibodies and provides protection from viral challenge in aotus monkeys. Vaccine. 2000 Jul;18:3166–73. doi: 10.1016/s0264-410x(00)00105-5. [DOI] [PubMed] [Google Scholar]
- 55.Kochel TJ, Watts DM, Gozalo AS, Ewing DF, Porter KR, Russell KL. Cross-serotype neutralization of dengue virus in aotus nancymae monkeys. J Infect Dis. 2005 Mar;191:1000–4. doi: 10.1086/427511. [DOI] [PubMed] [Google Scholar]
- 56.Lai CJ, Goncalvez AP, Men R, Wernly C, Donau O, Engle RE, Purcell RH. Epitope determinants of a chimpanzee dengue virus type 4 (denv-4)-neutralizing antibody and protection against denv-4 challenge in mice and rhesus monkeys by passively transferred humanized antibody. J Virol. 2007 Dec;81:12766–74. doi: 10.1128/JVI.01420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marchett N, Halstead S, Falkler W, Stenhous A, Nash D. Studies on pathogenesis of dengue infection in monkeys 3. sequential distribution of virus in primary and heterologous infections. Journal of Infectious Diseases. 1973;128(1):23–30. doi: 10.1093/infdis/128.1.23. [DOI] [PubMed] [Google Scholar]
- 58.Markoff L, Pang X, Houng Hs H-s, Falgout B, Olsen R, Jones E, Polo S. Derivation and characterization of a dengue type 1 host range-restricted mutant virus that is attenuated and highly immunogenic in monkeys. J Virol. 2002 Apr;76:3318–28. doi: 10.1128/JVI.76.7.3318-3328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martín J, Hermida L, Castro J, Romero Y, Cardosa J, Guillén G. Viremia and the magnitude of the immune response upon infection of green monkeys with dengue virus type 2 are strain-dependent. Curr Microbiol. 2009 Dec;59:579–83. doi: 10.1007/s00284-009-9488-6. [DOI] [PubMed] [Google Scholar]
- 60.Maves RC, Oré RMC, Porter KR, Kochel TJ. Immunogenicity and protective efficacy of a psoralen-inactivated dengue-1 virus vaccine candidate in aotus nancymaae monkeys. Vaccine. 2011 Mar;29:2691–6. doi: 10.1016/j.vaccine.2011.01.077. [DOI] [PubMed] [Google Scholar]
- 61.Men R, Bray M, Clark D, Chanock RM, Lai CJ. Dengue type 4 virus mutants containing deletions in the 3′ noncoding region of the rna genome: analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J Virol. 1996 Jun;70:3930–7. doi: 10.1128/jvi.70.6.3930-3937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Men R, Wyatt L, Tokimatsu I, Arakaki S, Shameem G, Elkins R, Chanock R, Moss B, Lai CJ. Immunization of rhesus monkeys with a recombinant of modified vaccinia virus ankara expressing a truncated envelope glycoprotein of dengue type 2 virus induced resistance to dengue type 2 virus challenge. Vaccine. 2000 Jul;18:3113–22. doi: 10.1016/s0264-410x(00)00121-3. [DOI] [PubMed] [Google Scholar]
- 63.Omatsu T, Moi ML, Hirayama T, Takasaki T, Nakamura S, Tajima S, Ito M, Yoshida T, Saito A, Katakai Y, Akari H, Kurane I. Common marmoset (callithrix jacchus) as a primate model of dengue virus infection: development of high levels of viraemia and demonstration of protective immunity. J Gen Virol. 2011 Oct;92:2272–80. doi: 10.1099/vir.0.031229-0. [DOI] [PubMed] [Google Scholar]
- 64.Onlamoon N, Noisakran S, Hsiao HM, Duncan A, Villinger F, Ansari AA, Perng GC. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood. 2010 Mar;115:1823–34. doi: 10.1182/blood-2009-09-242990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pamungkas J, Iskandriati D, Saepuloh U, Affandi M, Arifin E, Paramastri Y, Dewi F, Sajuthi D. Dissemination in pigtailed macaques after primary infection of dengue-3 virus. Microbiology Indonesia. 2011;5(2):7. [Google Scholar]
- 66.Pletnev AG, Bray M, Hanley KA, Speicher J, Elkins R. Tick-borne langat/mosquito-borne dengue flavivirus chimera, a candidate live attenuated vaccine for protection against disease caused by members of the tick-borne encephalitis virus complex: evaluation in rhesus monkeys and in mosquitoes. J Virol. 2001 Sep;75:8259–67. doi: 10.1128/JVI.75.17.8259-8267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Price WH. Sequential immunization as a vaccination procedure against dengue viruses. Am J Epidemiol. 1968 Nov;88:392–7. doi: 10.1093/oxfordjournals.aje.a120899. [DOI] [PubMed] [Google Scholar]
- 68.Price WH, Casals J, Thind I, O’Leary W. Sequential immunization procedure against group b arboviruses using living attenuated 17d yellow fever virus, living attenuated langat e5 virus, and living attenuated dengue 2 virus (new guinea c isolate) Am J Trop Med Hyg. 1973 Jul;22:509–23. doi: 10.4269/ajtmh.1973.22.509. [DOI] [PubMed] [Google Scholar]
- 69.Price WH, Casals J, O’Leary W. Studies on the sequential immunization against group b arboviruses in squirrel monkeys, cynomolgus monkeys, rhesus monkeys, and chimpanzees. Am J Trop Med Hyg. 1974 Jan;23:118–30. doi: 10.4269/ajtmh.1974.23.118. [DOI] [PubMed] [Google Scholar]
- 70.Putnak R, Barvir DA, Burrous JM, Dubois DR, D’Andrea VM, Hoke CH, Sadoff JC, Eckels KH. Development of a purified, inactivated, dengue-2 virus vaccine prototype in vero cells: immunogenicity and protection in mice and rhesus monkeys. J Infect Dis. 1996 Dec;174:1176–84. doi: 10.1093/infdis/174.6.1176. [DOI] [PubMed] [Google Scholar]
- 71.Putnak R, Fuller J, VanderZanden L, Innis BL, Vaughn DW. Vaccination of rhesus macaques against dengue-2 virus with a plasmid dna vaccine encoding the viral pre-membrane and envelope genes. Am J Trop Med Hyg. 2003 Apr;68:469–76. [PubMed] [Google Scholar]
- 72.Raviprakash K, Porter KR, Kochel TJ, Ewing D, Simmons M, Phillips I, Murphy GS, Weiss WR, Hayes CG. Dengue virus type 1 dna vaccine induces protective immune responses in rhesus macaques. J Gen Virol. 2000 Jul;81:1659–67. doi: 10.1099/0022-1317-81-7-1659. [DOI] [PubMed] [Google Scholar]
- 73.Raviprakash K, Apt D, Brinkman A, Skinner C, Yang S, Dawes G, Ewing D, Wu SJ, Bass S, Punnonen J, Porter K. A chimeric tetravalent dengue dna vaccine elicits neutralizing antibody to all four virus serotypes in rhesus macaques. Virology. 2006 Sep;353:166–73. doi: 10.1016/j.virol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Raviprakash K, Wang D, Ewing D, Holman DH, Block K, Woraratanadharm J, Chen L, Hayes C, Dong JY, Porter K. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J Virol. 2008 Jul;82:6927–34. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rumyantsev AA, Chanock RM, Murphy BR, Pletnev AG. Comparison of live and inactivated tick-borne encephalitis virus vaccines for safety, immunogenicity and efficacy in rhesus monkeys. Vaccine. 2006 Jan;24:133–43. doi: 10.1016/j.vaccine.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 76.Scherer WF, Russell PK, Rosen L, Casals J, Dickerman RW. Experimental infection of chimpanzees with dengue viruses. Am J Trop Med Hyg. 1978 May;27:590–9. doi: 10.4269/ajtmh.1978.27.590. [DOI] [PubMed] [Google Scholar]
- 77.Schiavetta AM, Harre JG, Wagner E, Simmons M, Raviprakash K. Variable susceptibility of the owl monkey (aotus nancymae) to four serotypes of dengue virus. Contemp Top Lab Anim Sci. 2003 Sep;42:12–20. [PubMed] [Google Scholar]
- 78.Simmons M, Porter KR, Hayes CG, Vaughn DW, Putnak R. Characterization of antibody responses to combinations of a dengue virus type 2 dna vaccine and two dengue virus type 2 protein vaccines in rhesus macaques. J Virol. 2006 Oct;80:9577–85. doi: 10.1128/JVI.00284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simmons M, Burgess T, Lynch J, Putnak R. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology. 2010 Jan;396:280–8. doi: 10.1016/j.virol.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 80.Sun W, Nisalak A, Gettayacamin M, Eckels KH, Putnak JR, Vaughn DW, Innis BL, Thomas SJ, Endy TP. Protection of rhesus monkeys against dengue virus challenge after tetravalent live attenuated dengue virus vaccination. J Infect Dis. 2006 Jun;193:1658–65. doi: 10.1086/503372. [DOI] [PubMed] [Google Scholar]
- 81.Tarr GC, Lubiniecki AS. Chemically induced temperature-sensitive mutants of dengue virus type 2: comparison of temperature sensitivity in vitro with infectivity suckling mice, hamsters, and rhesus monkeys. Infect Immun. 1976 Mar;13:688–95. doi: 10.1128/iai.13.3.688-695.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valdés I, Hermida L, Martín J, Menéndez T, Gil L, Lazo L, Castro J, Niebla O, López C, Bernardo L, Sánchez J, Romero Y, Martínez R, Guzmán MG, Guillén G. Immunological evaluation in nonhuman primates of formulations based on the chimeric protein p64k-domain iii of dengue 2 and two components of neisseria meningitidis. Vaccine. 2009 Feb;27:995–1001. doi: 10.1016/j.vaccine.2008.11.106. [DOI] [PubMed] [Google Scholar]
- 83.Velzing J, Groen J, Drouet MT, van Amerongen G, Copra C, Osterhaus AD, Deubel V. Induction of protective immunity against dengue virus type 2: comparison of candidate live attenuated and recombinant vaccines. Vaccine. 1999 Mar;17:1312–20. doi: 10.1016/s0264-410x(98)00393-4. [DOI] [PubMed] [Google Scholar]
- 84.Whitehead RH, Chaicumpa V, Olson LC, Russell PK. Sequential dengue virus infections in the white-handed gibbon (hylobates lar) Am J Trop Med Hyg. 1970 Jan;19:94–102. doi: 10.4269/ajtmh.1970.19.94. [DOI] [PubMed] [Google Scholar]
- 85.Widjaja S, Winoto I, Sturgis J, Maroef C, Listyaningsih E, Tan R, Pamungkas J, Iskandriati D, Blair P, Sadjuti D, et al. Macaca nemestrina and dengue virus infectivy: a potential model for evaluating dengue vaccine candidates. Microbiology Indonesia. 2010;4(2) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


