Abstract
Objective
To compare energy intake, total daily energy expenditure (TDEE), non-exercise energy expenditure (NEEx), resting metabolic rate (RMR), non-exercise physical activity (NEPA), and sedentary time between participants with weight loss <5% (non-responders) vs. ≥5% (responders) in response to exercise.
Methods
Overweight/obese (BMI 25–40 kg/m2), adults (18–30 yrs.) were randomized to exercise: 5 day/week, 400 or 600 kcal/session, 10 months.
Results
Forty participants responded and 34 did not respond to the exercise protocol. Non-responder energy intake was higher vs. responders, significant only in men (p=0.034). TDEE increased only in responders (p=0.001). NEEx increased in responders and decreased in non-responders, significant only in men (p=0.045). There were no within or between-group differences for change in RMR. NEPA increased in responders and decreased in non-responders (group-by-time interactions: total sample, p=0.049; men, p=0.016). Sedentary time decreased in both groups, significant only in men.
Conclusion
Men who did not lose weight in response to exercise (<5%) had higher energy intake and lower NEEx compared to men losing ≥5%. No significant differences in any parameters assessed were observed between women who lost <5% vs. those losing ≥5. Factors associated with the weight loss response to exercise in women warrant additional investigation.
Keywords: compensation, inter-individual variability, exercise training, non-exercise energy expenditure
Introduction
Exercise is an integral component in weight management (1–4). Our group and others have demonstrated clinically significant weight loss (range=−4 to −8.4%) resulting from aerobic exercise without energy restriction when sufficient levels of exercise energy expenditure (EEEx ~2,000 kcal/wk.) are completed (5–10). This level of weight loss meets or exceeds weight loss observed in several intensive behavioral interventions (range: −1.3% to −6.5%) which included both energy restriction and increased physical activity (11–14). However, several reports have indicated that even when exercise of sufficient energy expenditure is supervised and closely monitored, there is considerable variability in the magnitude and direction of weight change (5–7, 15–18). Behavioral adaptations including non-compliance with the exercise protocol, changes in energy intake and/or non-exercise energy expenditure (NEEx), and metabolic adaptations such as decreased resting metabolic rate (RMR), may be associated with the high level of individual variability in weight change in response to exercise (19–21). As highlighted in two recent systematic reviews, the literature regarding changes in both energy intake and NEEx in response to exercise is inconclusive and conflicting (22, 23). Reductions in RMR may play a larger role when weight loss is induced by energy restriction (24) as weight loss by exercise may prevent significant reductions in RMR (25, 26). However, the contribution of changes in RMR to individual variability in exercise induced weight loss is unclear.
To date, the few trials that have assessed the association of change in these factors with the weight loss response to exercise have been conducted over short durations (8–12 weeks) (7, 27, 28) and utilized sub-optimal assessments of energy intake and energy expenditure. Only 2 trials, both conducted in women, have included simultaneous assessments of changes in energy intake and NEEx on exercise induced weight loss (28, 29). In addition, trials have classified compensators and non-compensators based on the assumption that an energy deficit of 3500 kcal will induce a weight loss of 1 pound (30), an assumption shown to overestimate theoretical weight loss (31).
Data from the Midwest Exercise Trial-2 (MET-2) afforded an opportunity to compare changes in energy intake, RMR, NEEx, non-exercise physical activity (NEPA) and sedentary time in men and women who lost ≥5% of baseline body weight (responders) with those who lost <5% (non-responders) in response to a 10 month supervised exercise training program with verified levels of EEEx. The 5% cut point was selected as it represents a clinically important level of weight loss associated with improved chronic disease risk (2). Briefly, MET-2 was a 10 month randomized efficacy trial, 5 day/week supervised exercise intervention at 2 levels of EEEx (400 or 600 kcal/session) or non-exercise control. A detailed description of the design and methods for MET-2 (32), results for the primary outcome (weight change) (6), and changes in NEEx and NEPA have been published (33).
METHODS
Participants
Participants were healthy, overweight/obese, sedentary men and women (age 18–30 years, BMI 25–40 kg/m2) who were able to exercise. Participants reported being weight stable (±4.5 kg) for the 3 months prior to enrolling in the study. Participants provided written informed consent and were compensated for participation. Study approval was obtained from the Human Subjects Committee at the University of Kansas-Lawrence. Data from participants assigned to one of the two exercise groups are included in this report.
Randomization and blinding
One-hundred forty one individuals who met eligibility criteria were stratified by sex and randomized by the study statistician (~80% exercise; ~20% control). Participants were instructed to continue their typical ad-libitum diet and NEPA over the duration of the intervention. Investigators/research staff were blinded at the level of outcome assessments, data entry and analysis.
Exercise intervention
A description of the exercise intervention has been reported elsewhere (32). Exercise was primarily treadmill walking/jogging in a dedicated exercise facility. Alternate activities (stationary biking, walking/jogging outside) were permitted for 20% of exercise sessions to provide variety and decrease overuse injuries. Exercise progressed from 150 kcal/session to the target EEEx of 400 or 600 kcal/session at the end of month 4, and remained at target for the final 6 months.
Exercise energy expenditure
Exercise duration to elicit either 400 or 600 kcal/session was determined from EEEx measured by indirect calorimetry (ParvoMedics TrueOne2400, Sandy UT) during treadmill exercise at 70% and 80% of maximal heart rate. This procedure was repeated monthly and exercise duration was adjusted to achieve the target EEEx.
Exercise supervision and compliance
All exercise sessions were supervised and exercise intensity and duration was verified by heart rate monitor (RS400; Polar Electro, Woodbury, NY). Compliance was defined as completing >90% of scheduled exercise sessions. Participants who were non-compliant during any 3 month interval (months 0–3, 3–6, 6–9) or during the final month were dismissed.
OUTCOME MEASURES
Anthropometrics, energy intake, NEPA and sedentary time were assessed at baseline, 3.5, 7, and 10 months. TDEE, RMR and NEEx were assessed at baseline and 10 months.
Anthropometrics: Weight/Height/waist circumference/body composition
Body weight was measured between 7 a.m. and 10 a.m. following a 12 hour, fast while wearing a standard hospital gown using a digital scale accurate to ±0.1 kg (PS6600, Befour Inc., Saukville, WI). Height was measured using a stadiometer (Model PE-WM-60–84, Perspective Enterprises, Portage, MI) and BMI was calculated as weight (kg)/height (m2). The average of 3 measures of waist circumference (WC) was recorded. Dual energy x-ray absorptiometry (DXA) was used to determine fat-free mass (FFM), fat mass (FM) and percent body fat (Lunar DPX-IQ). Women completed pregnancy testing before each DXA test.
Dietary Intake
Energy and macronutrient intake was assessed over 7-day periods (minimum of 2 meal/days on weekdays and 1 meal/day on weekends) of ad-libitum eating in a University of Kansas cafeteria. Two digital photographs (90° and 45° angle) were obtained before and after consumption of each meal with the cafeteria trays placed in docking station to standardize the camera angle. Notes were placed on the tray to identify beverages (e.g., diet vs. regular soft-drink; skim vs. whole milk, etc.) and other food items that would be difficult to identify from the photo. Foods consumed outside the cafeteria (e.g., snacks, non-cafeteria meals) were assessed using multiple-pass recalls. Types and amounts of food and beverages consumed at the cafeteria and results from the recalls were entered into the Nutrition Data System for Research (NDS-R Versions 2005, 2006, University of Minnesota, Minneapolis, MN) for the quantification of energy intake. Baseline data from the current study indicated that digital photography provided estimates of energy intake over 7 days within ~6% of TDEE assessed by doubly labelled water (DLW).
RMR
RMR was assessed by indirect calorimetry (ParvoMedics TrueOne 2400 System, Sandy, UT) between 6 and 10 a.m. after a 12 hour fast and 48 hour abstention from exercise (34). Participants rested for 15 minutes in a temperature controlled (21–24º C) room and were placed in a ventilated hood for assessment of VO2 and VCO2 for a minimum of 35 minutes. The criterion for a valid RMR was a minimum of 30 minutes of measured values with <10% average standard deviation. RMR (kcal d−1) was calculated using the Weir equation (35).
Total Daily Energy Expenditure (TDEE)/NEEx
TDEE was assessed using DLW over a 14-day period. The end study assessment was obtained during the final 2 weeks exercise training. Participants reported to our laboratory between 8 and 9 a.m. after an overnight fast. Baseline urine specimens were collected prior to oral dosing with a mixed solution of 2H218O. The isotope provided was based on body weight (0.10g·kg−1 of 2H2O and 0.15g·kg−1 H218O) and was followed with a rinse solution of 100ml of tap water. A weighed 1:400 dilution of each participant’s dose was prepared and a sample of the tap water was stored at −70ºC for later analysis. Two additional urine samples (3 hrs. apart) were collected on days 1 and 14. Samples were analyzed in duplicate for 2H2O and H218O by isotope ratio mass spectrometry as previously described by Herd et al (36). TDEE was estimated using the equation of Elia (37): TDEE (MJ/d)=(15.48/RQ+5.55) X rCO2 (L/d). NEEx, i.e., energy expenditure not associated with exercise training, was calculated as [(TDEE*0.9)–RMR]–net EEEx (i.e., EEEx–RMR). This approach assumes the thermic effect of food represents 10% of TDEE (38). Note: Net EEEx at baseline equals zero.
NEPA/Sedentary time
NEPA was assessed by an accelerometer (Actigraph GT1M, Pensacola, FL) worn at the waist, over the non-dominant hip, for 7 consecutive days, using 1-minute epochs with a minimum of 10 hours constituting a valid day. Three valid days were required to be included in the analysis. Non-wear time was identified as ≥60 consecutive minutes with 0 cts·min−1, with allowance for 1–2 minutes of accelerometer counts between 0–100 (39). Data were processed using a custom SAS program. NEPA (≥100 cts min−1) was calculated by removing accelerometer data over the duration of exercise sessions from the daily accelerometer data. Sedentary time was defined as time with accelerometer readings <100 cts·min−1 (39). On average, approximately 6 valid days of accelerometer data were available. The number of valid days did not differ between responders and non-responders at any assessment points. There were no significant differences in wear time between study groups or over time.
Statistical Analysis
Participants randomized to exercise groups who were adherent to the study protocol (attended ≥90% of supervised exercise sessions and complied with the energy intake assessment protocol) were included in the analysis. Baseline characteristics were summarized by descriptive statistics; responders/non-responder differences were examined using t-test and chi-square test, as appropriate. Linear mixed modeling was used to estimate the responder/non-responder differences (group effect), changes over time (time effect), and differences for change (group-by-time interaction) in energy intake, energy expenditure, and physical activity, accounting for age and sex. All analyses were conducted using SAS Software v9.3 (SAS Institute, 2002–2012).
Results
Participants
Seventy-four of 115 participants randomized to the exercise groups (64%) completed the intervention and complied with the study protocol. Approximately 44% of participants failed to comply with the exercise training protocol. There were no significant differences in baseline characteristic between participants who did or did not complete the intervention. Weight loss was ≥5% in 40 (54%) (responders, n=20 men, 20 women) and <5% in 34 (46%) of participants (non-responders, n=17 men, 17 women). Mean weight loss was −8.4 ±3.8% in responders and −0.04±2.5% in non-responders (p<0.001), with a similar response in both men and women (Figure 1). Due to technical problems, or failure to comply with the assessment protocols, this report includes DLW data from 36 responders and 32 non-responders at baseline and 31 responders and 31 non-responders at 10 months. Accelerometer data were available on all participants at baseline and on 38 responders and 33 non-responders at 10 months.
Figure 1.
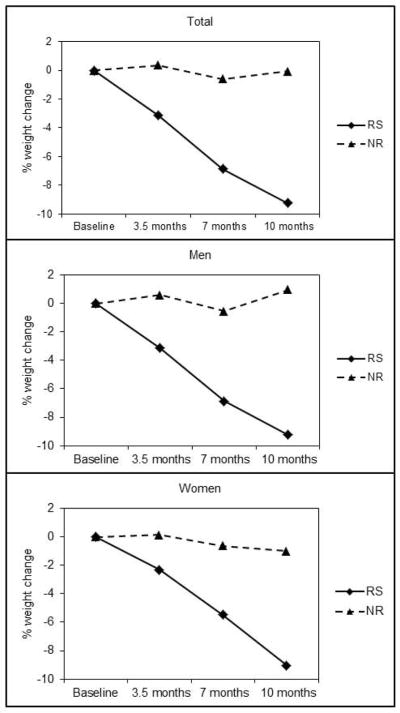
Percent weight changes across 10 months in responders and non-responders to an aerobic exercise intervention.
Baseline characteristics (Table 1)
Table 1.
Baseline characteristics in responders and non-responders to an aerobic exercise intervention.
| Variable | Responder
|
Non-Responder
|
p | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Age (years) | 23.0 | 3.4 | 22.1 | 4.9 | 0.363 |
| Height (cm) | 170.8 | 9.4 | 171.5 | 8.7 | 0.760 |
| Weight (kg) | 91.6 | 18.1 | 91.8 | 19.0 | 0.975 |
| BMI (kg·m−2) | 31.2 | 4.4 | 31.2 | 5.2 | 0.979 |
| Body Fat (%) | 40.3 | 6.9 | 38.2 | 10.2 | 0.307 |
| Waist Circumference (cm) | 92.5 | 11.4 | 92.5 | 12.4 | 0.993 |
| Fat-free Mass (kg) | 51.8 | 10.6 | 53.5 | 12.5 | 0.531 |
| Fat Mass (kg) | 35.3 | 9.8 | 34.7 | 10.0 | 0.799 |
Note.; M = mean, SD = standard deviation; BMI = body mass index; Responders = weight loss ≥5%; Non-responders = weight loss <5%
There were no significant responders/non-responders differences in baseline demographics. The proportion of responders/non-responders did not differ by exercise group. Therefore, for the purpose of the responder/non-responder comparison, results for the 400 and 600 kcal/session groups were combined.
Body composition
There were no significant between or within group differences for change in FFM in the total sample, or men or women. In the total sample FFM was stable from baseline to 10 months in both responders (0.2±1.8 kg; p=0.469) and non-responders (−0.1±4.0 kg; p=0.849). In men, FFM was unchanged in both responders (0.1±1.9 kg; p=0.816) and non-responders (0.3±1.8 kg; p=0.446). However, FFM at baseline and 10 months was significantly higher in men who did not responded (baseline=67.9±8.9 kg, 10 months=68.2±8.1 kg) compared to men who did respond to exercise (62.3±7.7 kg, p=0.049; 10 months: 62.4±7.5 kg, p=0.031). There were no significant changes in FFM in women: responders (0.3±1.6 kg; p=0.401), non-responders (−0.6±5.3 kg; p=0.655). FM decreased in responders; total sample (−7.8±3.9 kg), men (−8.6±4.4 kg), women (−7.0±3.2 kg) (all p <0.001) but not in non-responders; total sample (−0.5±3.8 kg; p=0.449), men (0.3±2.3 kg; p=0.644), women (−1.2±4.8 kg; p=0.304). WC decreased in responders; total sample (−6.6±5.0 cm), men (−8.0±4.8 cm), and women (−5.0±4.9 cm) (all p <0.001). The magnitude of the reductions in WC observed in non-responders was smaller than those observed in responders; total sample (−1.3±3.1 cm, p=0.020), men (−0.41±2.7 cm; p=0.547), and women (−2.6±3.3 cm, p=0.015).
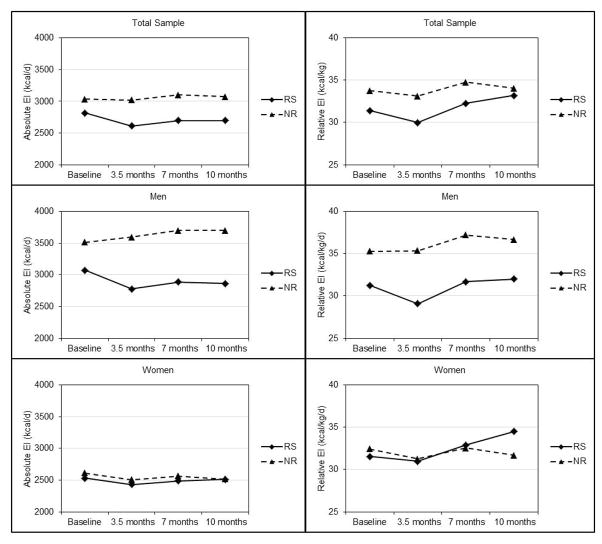
Energy Intake (Table 2, Figure 2)
Table 2.
Absolute (kcal/day) and relative energy intake (kcal/kg/day) across 10 months in responders and non-responders to an aerobic exercise intervention
| n | Responder
|
n | Non-Responder
|
p-between | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Energy Intake (kcal/day) | |||||||
| Total Sample | |||||||
| Baseline | 40 | 2817 | 650 | 34 | 3036 | 694 | 0.166 |
| 3.5 months | 40 | 2613 | 532 | 34 | 3020 | 881 | 0.022 |
| 7 months | 40 | 2693 | 661 | 34 | 3100 | 784 | 0.019 |
| 10 months | 40 | 2696 | 603 | 34 | 3074 | 967 | 0.053 |
| p-within | 0.181 | 0.649 | |||||
| Men | |||||||
| Baseline | 21 | 3075 | 739 | 18 | 3514 | 578 | 0.058 |
| 3.5 months | 21 | 2775 | 588 | 18 | 3596 | 911 | 0.002 |
| 7 months | 21 | 2885 | 727 | 18 | 3702 | 525 | 0.001 |
| 10 months | 21 | 2864 | 616 | 18 | 3700 | 646 | 0.000 |
| p-within | 0.104 | 0.038 | |||||
| Women | |||||||
| Baseline | 19 | 2533 | 382 | 16 | 2611 | 484 | 0.586 |
| 3.5 months | 19 | 2431 | 404 | 16 | 2509 | 432 | 0.573 |
| 7 months | 19 | 2488 | 523 | 16 | 2566 | 554 | 0.665 |
| 10 months | 19 | 2509 | 545 | 16 | 2518 | 866 | 0.971 |
| p-within | 0.861 | 0.499 | |||||
| Energy intake (kcal/kg/day) | |||||||
| Total Sample | |||||||
| Baseline | 40 | 31.4 | 6.5 | 34 | 33.8 | 7.9 | 0.160 |
| 3.5 months | 40 | 30.0 | 6.2 | 33 | 33.1 | 8.1 | 0.066 |
| 7 months | 39 | 32.3 | 8.0 | 34 | 34.7 | 9.0 | 0.221 |
| 10 months | 40 | 33.2 | 7.6 | 34 | 34.0 | 10.9 | 0.705 |
| p-within | 0.085 | 0.797 | |||||
| Men | |||||||
| Baseline | 21 | 31.3 | 7.4 | 16 | 35.3 | 8.7 | 0.139 |
| 3.5 months | 21 | 29.1 | 6.1 | 16 | 35.4 | 9.0 | 0.018 |
| 7 months | 20 | 31.7 | 8.0 | 16 | 37.2 | 7.4 | 0.042 |
| 10 months | 21 | 32.0 | 6.4 | 16 | 36.7 | 8.4 | 0.062 |
| p-within | 0.571 | 0.113 | |||||
| Women | |||||||
| Baseline | 19 | 31.5 | 5.5 | 18 | 32.4 | 7.2 | 0.670 |
| 3.5 months | 19 | 31.0 | 6.3 | 18 | 31.3 | 7.2 | 0.670 |
| 7 months | 19 | 32.9 | 8.2 | 18 | 32.6 | 9.9 | 0.911 |
| 10 months | 19 | 34.5 | 8.9 | 18 | 31.7 | 12.5 | 0.434 |
| p-within | 0.081 | 0.684 | |||||
Note. M = Mean; SD = Standard deviation; Responders = weight loss ≥5%; Non-responders = weight loss <5%
Figure 2.
Absolute (kcal/day) and relative energy intake (kcal/kg/day) across 10 months in responders and non-responders to an aerobic exercise intervention.
In the total sample, there was no effect of time or group-by-time interaction; however, there was a significant effect for group (p=0.034) with higher absolute energy intake (kcal/day) in non-responders compared with responders across 10 months. Absolute energy intake was significantly higher in non-responders compared with responders at 3.5 and 7 months and was nearly significantly higher at 10 months (p=0.053). There were no significant effects of group, time, or group-by-time interaction for relative energy intake (kcal/kg/day).
There was a group-by-time interaction (p=0.044) for absolute energy intake in men. Absolute energy intake was significantly higher in non-responders compared with responders at 3.5, 7, and 10 months. Over the 10 month intervention there was a non-significant decrease in energy intake in responders and a significant increase in non-responders (p=0.038). There were no significant effects of group, time, or group-by-time interaction for relative energy intake in men. Nevertheless, relative energy intake was significantly higher in non-responders compared with responders at 3.5, and 7 months and nearly significantly higher at 10 months (p=0.062). There were no significant effects for group, time or group-by-time interaction for either absolute or relative energy intake in women.
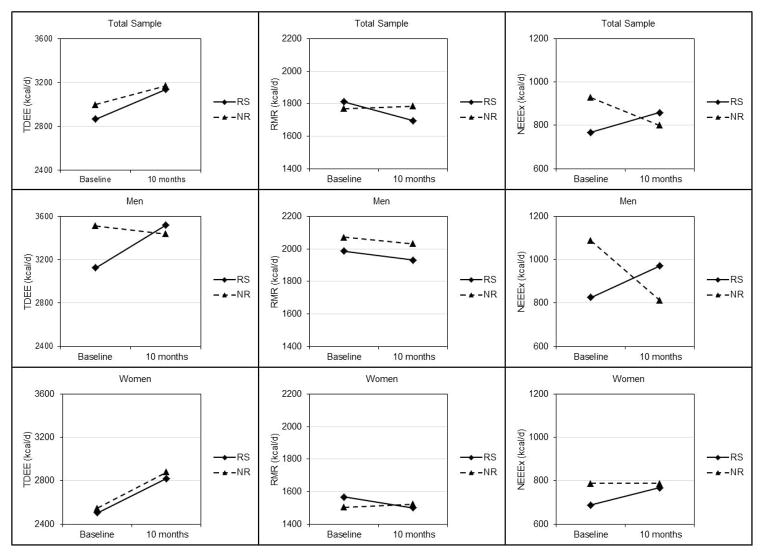
EEEx (Table 3, Figure 3)
Table 3.
Total daily energy expenditure (TDEE), non-exercise energy expenditure (NEEx) and resting metabolic rate (RMR) at baseline and 10 months in responders and non-responders of an aerobic exercise intervention.
| Variable | n | Responder
|
n | Non-Responder
|
p-between | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| TDEE (kcal·d−1) | |||||||
| Total Sample | |||||||
| Baseline | 36 | 2866 | 598 | 32 | 3000 | 737 | 0.418 |
| 10 months | 31 | 3137 | 658 | 31 | 3169 | 675 | 0.847 |
| p-within | 0.001 | 0.141 | |||||
| Men | |||||||
| Baseline | 21 | 3125 | 466 | 15 | 3514 | 615 | 0.038 |
| 10 months | 14 | 3519 | 703 | 16 | 3440 | 600 | 0.741 |
| p-within | 0.056 | 0.906 | |||||
| Women | |||||||
| Baseline | 15 | 2504 | 587 | 17 | 2546 | 505 | 0.829 |
| 10 months | 17 | 2822 | 421 | 15 | 2881 | 647 | 0.758 |
| p-within | 0.006 | 0.037 | |||||
| RMR (kcal·d−1) | |||||||
| Total Sample | |||||||
| Baseline | 36 | 1811 | 355 | 32 | 1771 | 380 | 0.652 |
| 10 months | 31 | 1695 | 299 | 31 | 1786 | 385 | 0.305 |
| p-within | 0.070 | 0.897 | |||||
| Men | |||||||
| Baseline | 21 | 1986 | 297 | 15 | 2073 | 207 | 0.336 |
| 10 months | 14 | 1933 | 198 | 16 | 2032 | 295 | 0.297 |
| p-within | 0.058 | 0.808 | |||||
| Women | |||||||
| Baseline | 15 | 1566 | 282 | 17 | 1504 | 284 | 0.537 |
| 10 months | 17 | 1500 | 214 | 15 | 1524 | 285 | 0.790 |
| p-within | 0.539 | 0.796 | |||||
| NEEx (kcal·d−1) | |||||||
| Total Sample | |||||||
| Baseline | 36 | 768 | 316 | 32 | 929 | 437 | 0.091 |
| 10 months | 31 | 860 | 444 | 31 | 801 | 416 | 0.594 |
| p-within | 0.190 | 0.172 | |||||
| Men | |||||||
| Baseline | 21 | 826 | 276 | 15 | 1089 | 454 | 0.059 |
| 10 months | 14 | 971 | 539 | 16 | 813 | 344 | 0.339 |
| p-within | 0.334 | 0.063 | |||||
| Women | |||||||
| Baseline | 15 | 687 | 359 | 17 | 788 | 381 | 0.450 |
| 10 months | 17 | 767 | 338 | 15 | 788 | 493 | 0.890 |
| p-within | 0.401 | 0.978 | |||||
Note. M = mean; SD = standard deviation; kcal = kilocalories; Responders = ≥5% weight loss; Non-Responders = < 5% weight loss
Figure 3.
Total daily energy expenditure (TDEE), non-exercise energy expenditure (NEEx) and resting metabolic rate (RMR) at baseline and 10 months in responders and non-responders to an aerobic exercise intervention.
The study design, required compliance with the exercise protocol, thus EEEx over the 14-day DLW assessment was nearly identical in responders and non-responders in the total sample (268±69 vs. 266±89 kcal/day), and in men (263±66 vs. 251±65 kcal/day), and women (272±73 vs. 281±93 kcal/day). TDEE: In the total sample, TDEE increased in responders (327±470 kcal/day, p=0.001) but not in non-responders (159±575 kcal/day, p=0.141); however, neither the effect of group nor group-by-time interaction was significant. In men, there was a significant group-by-time interaction (p=0.002). TDEE increased in responder men (310±555 kcal/day, p=0.056) and essentially unchanged in non-responders (−17±551 kcal/day, p=0.906). In women, only the time effect was significant (p=0.001). TDEE increased significantly in both responders (344±387 kcal/day, p=0.006) and non-responders (335±562 kcal/day, p=0.037).
RMR
In the total sample, men, and women, RMR decreased in responders (total sample=−83±234 kcal/day, p=0.070, men=−126±226 kcal/day, p=0.058, women =−41±242 kcal/day, p=0.539) and was essentially unchanged in non-responders (total sample=5±190 kcal/day, p=0.897; men=−8±119 kcal/day, p =0.808; women=17±246 kcal/day, p=0.796). However, the effect of group, time, or group-by-time interaction was not significant in the total sample, men, and women.
NEEx
There was a group-by-time interaction for NEEx in the total sample (p=0.049) and in men (p=0.016). NEEx increased over 10 months in responders (total sample=116±456 kcal/day, p=0.190; men=142±531 kcal/day, p=0.334) and decreased in non-responders (total sample=−128±502 kcal/day, p=0.172; men=−260±499 kcal/day, p=0.063). There was no significant effect of group, time or group-by-time interaction for NEEx in women.
We also evaluated changes in TDEE, NEEx and RMR relative to body weight (i.e. kcal/kg/day). Results in the total sample, men, and women paralleled those for absolute energy intake described above.
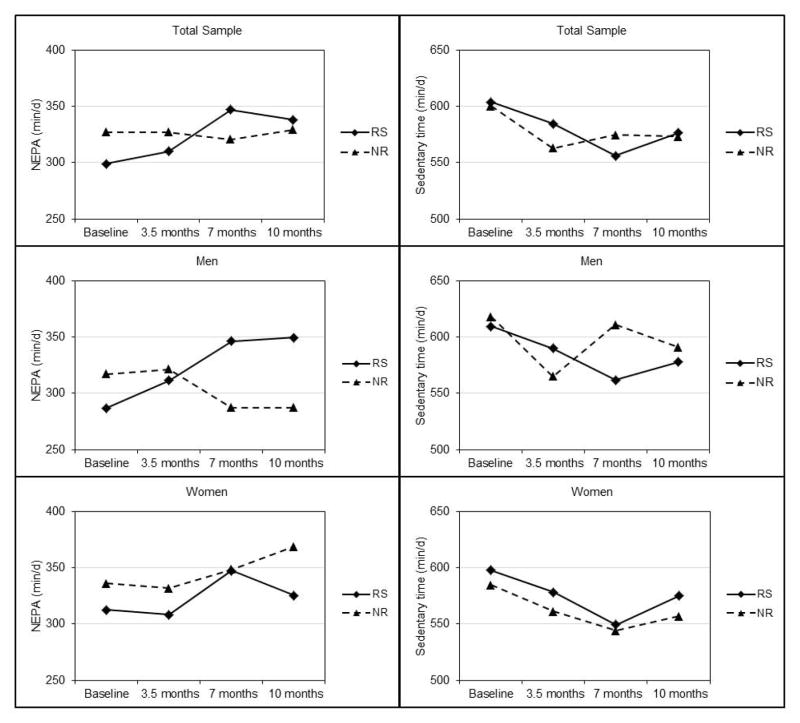
NEPA & Sedentary Time (Table 4, Figure 4)
Table 4.
Non-exercise physical activity (NEPA) and sedentary time in responders and non-responders to an aerobic exercise intervention
| n | Responder
|
n | Non-Responder
|
p-between | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| NEPA (min·d−1) | |||||||
| Total Sample | |||||||
| Baseline | 40 | 299 | 76 | 34 | 327 | 138 | 0.296 |
| 3.5 months | 37 | 310 | 73 | 33 | 327 | 89 | 0.383 |
| 7 months | 39 | 347 | 81 | 33 | 321 | 97 | 0.214 |
| 10 months | 38 | 338 | 95 | 33 | 329 | 99 | 0.699 |
| p-within | 0.012 | 0.924 | |||||
| Men | |||||||
| Baseline | 21 | 287 | 70 | 16 | 317 | 157 | 0.484 |
| 3.5 months | 20 | 312 | 69 | 15 | 322 | 101 | 0.736 |
| 7 months | 21 | 347 | 72 | 15 | 287 | 91 | 0.036 |
| 10 months | 20 | 350 | 82 | 16 | 287 | 73 | 0.023 |
| p-within | 0.003 | 0.375 | |||||
| Women | |||||||
| Baseline | 19 | 313 | 81 | 18 | 336 | 122 | 0.492 |
| 3.5 months | 17 | 308 | 81 | 18 | 332 | 80 | 0.390 |
| 7 months | 18 | 348 | 92 | 18 | 349 | 95 | 0.970 |
| 10 months | 18 | 326 | 109 | 17 | 369 | 105 | 0.239 |
| p-within | 0.699 | 0.453 | |||||
| Sedentary Time (min·d−1) | |||||||
| Total Sample | |||||||
| Baseline | 40 | 604 | 81 | 34 | 600 | 101 | 0.866 |
| 3.5 months | 37 | 585 | 83 | 33 | 563 | 109 | 0.352 |
| 7 months | 39 | 556 | 83 | 33 | 575 | 127 | 0.480 |
| 10 months | 38 | 577 | 75 | 33 | 573 | 97 | 0.877 |
| p-within | 0.054 | 0.159 | |||||
| Men | |||||||
| Baseline | 21 | 610 | 96 | 16 | 618 | 89 | 0.782 |
| 3.5 months | 20 | 590 | 84 | 15 | 565 | 90 | 0.411 |
| 7 months | 21 | 562 | 75 | 15 | 611 | 98 | 0.096 |
| 10 months | 20 | 578 | 71 | 16 | 591 | 64 | 0.566 |
| p-within | 0.081 | 0.149 | |||||
| Women | |||||||
| Baseline | 19 | 598 | 63 | 18 | 585 | 111 | 0.662 |
| 3.5 months | 17 | 579 | 84 | 18 | 561 | 125 | 0.639 |
| 7 months | 18 | 550 | 93 | 18 | 544 | 143 | 0.894 |
| 10 months | 18 | 575 | 81 | 17 | 557 | 120 | 0.598 |
| p-within | 0.381 | 0.439 | |||||
Note. M = mean; SD = standard deviation; Responder = ≥5% weight loss; Non-responder = < 5% weight loss. .
Figure 4.
Non-exercise physical activity (NEPA) and sedentary time across 10 months in responders and non-responders to an aerobic exercise intervention.
NEPA
There were group-by-time interactions for NEPA over 10 months in the total sample (p=0.023) and in men (p=0.003). NEPA increased from baseline to 10 months in responders (total sample=38±90 min/day, p=0.012; men=66±86 min/day, p=0.003) and decreased in non-responders (total sample=−2±131 min/day, p=0.925, men=−30±131 min/day, p=0.375). There was no significant effect of group, time or group-by-time interaction for NEPA in women.
Sedentary time
There was no significant group, time, or group-by-time interaction for sedentary time (min/day) over 10 months in the total sample or in women. In men, effects of group and the group-by-time interaction were non-significant; however, there was a significant effect of time. Sedentary time decreased from baseline to 10 months in both responders (−27±71 min/day, p=0.149) and non-responders (−39±93 min/day, p=0.081). We also analyzed NEPA and sedentary time using the percentage of time participants engaged in these activities after removing the time spent in exercise training. The results for these approaches were the same; thus, we have presented the results for NEPA and sedentary time as min/day as these units are more easily interpreted.
Discussion
Approximately 46% of overweight and obese young adults who completed a 10 month moderate-to-vigorous intensity aerobic exercise program, with ad-libitum eating, failed to achieve >5% weight loss. EEEx, measured by indirect calorimetry, was nearly identical in responders and non-responders, thus eliminating the possibility that the variability in weight loss was due to differential compliance with the exercise prescription.
Non-responders had higher levels of energy intake across 10 months and a smaller increase in TDEE from baseline to 10 months, as a result of decreased NEEx compared with responders. During the intervention, energy intake in non-responders was significantly higher (~200–400 kcal/day) than responders which induced a smaller energy deficit at 10 months in non-responders (~95 kcal/day) compared with responders (~441 kcal/day). NEEx, was reduced in non-responders (−128 kcal/day) and increased in responders (116 kcal/day) which contributed to an increase in TDEE among non-responders that was ~168 kcal/day less than observed in responders. Results for NEPA and sedentary time parallel the DLW results. That is, NEPA increased significantly and sedentary time decreased significantly in responders while NEPA was essential unchanged and sedentary time decreased in non-responders. The overall differences between responders and non-responders were primarily due to differences in energy intake and energy expenditure parameters observed in men, but not in women.
Our results are in general agreement with those from the limited number of trials that have compared energy intake, TDEE, NEEx or NEPA between participants who failed to achieve significant weight loss, or failed to achieve the magnitude of weight loss expected based on the level of EEEx. For example, King et al (7) compared changes in energy intake, assessed using a 24-hr. test meal protocol, in middle-age men and women whose actual weight loss in response to supervised exercise protocol (12 wks,5 day/wk., 500 kcal/session) was greater than or equal to (responders, n=17) or less than predicted weight loss (non-responder, n=18). Energy intake decreased in responders and increased in non-responders (p-between <0.05). In a subsequent paper, King et al., (27) reported on an additional 23 middle-age men and women (total n=58), who completed an identical 12 week supervised exercise trial as previously described (7). Responders (n=32) and non-responders (n=26) were defined as having changes in body composition (fat mass/fat-free mass) greater or less than expected based on EEEx. In agreement with their earlier report (7), there was a significant difference (p=0.04) for change in energy intake between responders and non-responders. NEPA, assessed by accelerometer every 4 weeks during a free-living probe day, did not differ between responders and non-responders. Manthou et al.,(40) compared change in energy intake (7-day weighed food diary), and change in TDEE and NEEx (7-day activity diary combined with individual heart rate/VO2 calibration) between overweight young adult women who achieved less than (n=23) vs. greater than or equal to predicted fat loss (n=11) in response to a supervised exercise protocol (8 wks., 150 min/wk.). Although not statistically significant, the increase in energy intake in non-responders was ~20% greater than responders. TDEE increased in both responders and non-responder, however, the between group difference was not significant. NEEx decreased in non-responders and increased in responders (p=0.046).
Factors associated with failure to achieve weight loss of at least 5% in response to aerobic exercise showed potentially important sex differences, that if replicated, may be worthy of further evaluation. In men, energy intake at baseline and across 10 months was higher in non-responders compared with responders. Higher energy intake at baseline may indicate these men were in a positive energy balance despite reporting being weight stable. NEEx decreased in non-responders and increased in responders from baseline to 10 months. The decrease in NEEx in non-responders was of sufficient magnitude to compensate for the increase energy expenditure associated with the exercise intervention and resulted in no change in TDEE. However, in women, factors differentiating responders from non-responders were unclear. Energy intake was nearly identical in both responders and non-responders at baseline and across the intervention, while NEEx was essentially unchanged in both groups.
Results from the available literature, and the current trial, indicate that individuals who fail to lose weight by aerobic exercise show higher levels of energy intake and reduced NEEx. These observations suggest that behavioral counseling, in conjunction with an exercise intervention, to minimize or eliminate these compensatory changes, may improve exercise induced weight loss. The ultimate goal would be to identify baseline characteristics of participants who are likely to be non-responsive to exercise for weight loss. This would allow the development of targeted exercise interventions in terms of EEEx, rate of progression to the goal EEEx, frequency and intensity of behavioral counselling etc., to maximize the effect on weight loss.
Strengths of the current investigation include the use of a randomized efficacy design with an intervention over 10 months, inclusion of both men and women, the use of supervised exercise at verified levels of EEEx, multiple assessments of energy intake using digital photography and assessments of NEEx, NEPA and sedentary time. However this study was not designed to detect differences in energy intake or energy expenditure between participants who did or did not achieve at least 5% weight loss in response to exercise. Additionally, we did not include assessments of eating behaviors, such as cognitive restraint, uncontrolled eating or emotional eating, or menstrual cycle stage or contraceptive use in women, all of which may have provided additional insights into differences between responders and non-responders.
In summary, our results indicated that young adult men who failed to lose 5% or more of their baseline body weight in response to aerobic exercise training had higher levels of energy intake and a smaller increase in TDEE across the intervention, as a result of decreased NEEx, compared with those whose weight loss was ≥ 5%. However, the results among women are less clear. Additional randomized trials, designed to evaluate the effect of participant characteristics including age, sex, race/ethnicity, body weight/composition, aerobic capacity, eating behaviors etc. and exercise factors including mode, frequency, intensity, time of day, and level of EEEx on compensatory responses in energy intake and expenditure to aerobic exercise training are warranted. Results from these trials would inform the design and targeting of weight management interventions using exercise alone, or exercise in combination with energy restriction.
What is already known about this subject?
Clinically significant weight loss (range=−4 to −8.4%) can be achieved with aerobic exercise without energy restriction when sufficient levels of exercise energy expenditure (EEEx ~2,000 kcal/wk.) are completed.
Even when exercise of sufficient energy expenditure is supervised and closely monitored, there is considerable variability in the magnitude and direction of weight change.
Previous trials that have assessed the association between of compensatory changes in factors such as resting metabolic rate, energy intake and non-exercise energy expenditure, and the variability in weight loss weight loss response to aerobic exercise have been conducted over short durations (8–12 weeks) and utilized sub-optimal assessments of both energy intake and non-exercise energy expenditure.
What does this study add?
Compares changes in energy intake, resting metabolic rate, non-exercise energy expenditure, non-exercise physical activity and sedentary time in men and women who lost ≥5% of baseline body weight with those who lost <5% in response to a 10 month supervised exercise training program with controlled and documented levels of EEEx.
Acknowledgments
Disclosure of funding: This study was supported by the National Institutes of Health grant R01-DK049181. Trial Registration: clinicaltrials.gov Identifier: NCT01186523
Footnotes
Conflict of Interest
The authors report no conflict of interest
Author Contribution
RW, JH, JH, EW and JD conceived the project design of this secondary analysis; JL, SD, JH and EW were involved in the analysis of the data. JH carried out experiments from the primary RCT. All authors were involved in writing the paper and had final approval of the submitted and published versions. Competing interests: the authors have no competing interests
References
- 1.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and Science in Sports Exercise. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 2.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports Exercise. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 3.Shaw K, Gennat H, O'Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database of Systematic Reviews (Online) 2006:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services, Physical Activity Advisory Committee. Physical Activity Advisory Committee Report, 2008. U.S. Department of Health and Human Services; Washington D.C: 2008. [Google Scholar]
- 5.Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Archives of Internal Medicine. 2003;163:1343–1350. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly JE, Honas JJ, Smith BK, Mayo MS, Gibson CA, Sullivan DK, et al. Aerobic exercise alone results in clinically significant weight loss for men and women: midwest exercise trial 2. Obesity. 2013;21:E219–228. doi: 10.1002/oby.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. International Journal of Obesity (2005) 2008;32:177–184. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 8.Nordby P, Auerbach PL, Rosenkilde M, Kristiansen L, Thomasen JR, Rygaard L, et al. Endurance training per se increases metabolic health in young, moderately overweight men. Obesity. 2012;20:2202–2212. doi: 10.1038/oby.2012.70. [DOI] [PubMed] [Google Scholar]
- 9.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. The Journals of Gerontology. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obesity Research. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 11.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. New England Journal of Medicine. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Archives of Internal Medicine. 2012;172:565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross R, Lam M, Blair SN, Church TS, Godwin M, Hotz SB, et al. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Archives of Internal Medicine. 2012;172:414–424. doi: 10.1001/archinternmed.2011.1972. [DOI] [PubMed] [Google Scholar]
- 14.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. New England Journal of Medicine. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchard C, Tremblay A, Despres JP, Theriault G, Nadeau A, Lupien PJ, et al. The response to exercise with constant energy intake in identical twins. Obesity Research. 1994;2:400–410. doi: 10.1002/j.1550-8528.1994.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 16.Caudwell P, Hopkins M, King NA, Stubbs RJ, Blundell JE. Exercise alone is not enough: weight loss also needs a healthy (Mediterranean) diet? Public Health Nutrition. 2009;12:1663–1666. doi: 10.1017/S1368980009990528. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins M, Gibbons C, Caudwell P, Hellström P, Näslund E, King N, et al. The adaptive metabolic response to exercise-induced weight loss influences both energy expenditure and energy intake. European Journal of Clinical Nutrition. 2014;68:581–586. doi: 10.1038/ejcn.2013.277. [DOI] [PubMed] [Google Scholar]
- 18.King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP, et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity. 2007;15:1373–1383. doi: 10.1038/oby.2007.164. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exercise and Sport Sciences Reviews. 2005;33:169–174. doi: 10.1097/00003677-200510000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Major G, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. International Journal of Obesity. 2007;31:204–212. doi: 10.1038/sj.ijo.0803523. [DOI] [PubMed] [Google Scholar]
- 21.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Medicine and Science in Sports Exercise. 2013;45:1600–1609. doi: 10.1249/MSS.0b013e31828ba942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnelly JE, Herrmann SD, Lambourne K, Szabo AN, Honas JJ, Washburn RA. Does Increased Exercise or Physical Activity Alter Ad-Libitum Daily Energy Intake or Macronutrient Composition in Healthy Adults? A Systematic Review. PloS One. 2014;9:e83498. doi: 10.1371/journal.pone.0083498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Washburn R, Lambourne K, Szabo A, Herrmann S, Honas J, Donnelly J. Does increased prescribed exercise alter non–exercise physical activity/energy expenditure in healthy adults? A systematic review. Clinical Obesity. 2014;4:1–20. doi: 10.1111/cob.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tremblay A, Royer M, Chaput J, Doucet E. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. International Journal of Obesity. 2013;37:759–764. doi: 10.1038/ijo.2012.124. [DOI] [PubMed] [Google Scholar]
- 25.Potteiger JA, Kirk EP, Jacobsen DJ, Donnelly JE. Changes in resting metabolic rate and substrate oxidation after 16 months of exercise training in overweight adults. International Journal of Sport Nutrition and Exercsie Metabolism. 2008;18:79–95. doi: 10.1123/ijsnem.18.1.79. [DOI] [PubMed] [Google Scholar]
- 26.Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Medicine. 2006;36:239–262. doi: 10.2165/00007256-200636030-00005. [DOI] [PubMed] [Google Scholar]
- 27.King NA, Caudwell PP, Hopkins M, Stubbs JR, Naslund E, Blundell JE. Dual-process action of exercise on appetite control: increase in orexigenic drive but improvement in meal-induced satiety. The American Journal of Clinical Nutrition. 2009;90:921–927. doi: 10.3945/ajcn.2009.27706. [DOI] [PubMed] [Google Scholar]
- 28.Manthou E, Gill J, Wright A, Malkova D. Mechanisms opposing exercise-induced perturbations in energy balance in overweight women. Proceedings of the Nutrition Society. 2008;67:E225. [Google Scholar]
- 29.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4:e4515. doi: 10.1371/journal.pone.0004515. Epub 2009/02/19. doi: 10.1371/journal.pone. 0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wishnofsky M. CALORIC EQUIVALENTS OF GAINED OR LOST WEIGHT. Journal of the American Medical Association. 1960;173:85–85. [Google Scholar]
- 31.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. The Lancet. 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnelly JE, Washburn RA, Smith BK, Sullivan DK, Gibson C, Honas JJ, et al. A randomized, controlled, supervised, exercise trial in young overweight men and women: The Midwest Exercise Trial II (MET2) Contemporary Clinical Trials. 2012;33:804–810. doi: 10.1016/j.cct.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis EA, Herrmann SD, Honas JJ, Lee J, Donnelly JE, Washburn RA. Nonexercise Energy Expenditure and Physical Activity in the Midwest Exercise Trial 2. Medicine and Science in Sports Exercise. 2014;46:2286–2294. doi: 10.1249/MSS.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haugen HA, Melanson EL, Tran ZV, Kearney JT, Hill JO. Variability of measured resting metabolic rate. The American Journal of Clinical Nutrition. 2003;78:1141–1145. doi: 10.1093/ajcn/78.6.1141. [DOI] [PubMed] [Google Scholar]
- 35.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of Physiology. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herd S, Vaughn W, Goran M. Comparison of zinc reduction with platinum reduction for analysis of deuterium-enriched water samples for the doubly-labeled water technique. Obesity Research. 1999;8(4):302–8. doi: 10.1038/oby.2000.36. [DOI] [PubMed] [Google Scholar]
- 37.Elia M, Wood S, Khan K, Pullicino E. Ketone body metabolism in lean male adults during short-term starvation, with particular reference to forearm muscle metabolism. Clinical Science. 1990;78:579–584. doi: 10.1042/cs0780579. [DOI] [PubMed] [Google Scholar]
- 38.Weststrate JA. Resting metabolic rate and diet-induced thermogenesis: a methodological reappraisal. The American Journal of Clinical Nutrition. 1993;58:592–601. doi: 10.1093/ajcn/58.5.592. [DOI] [PubMed] [Google Scholar]
- 39.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and Science in Sports Exercise. 2008;40:181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 40.Manthou E, Gill JMR, Wright A, Malkova D. Behavioral compensatory adjustments to exercise training in overweight women. Medicine and Science in Sports Exercise. 2010;42:1221–1228. doi: 10.1249/MSS.0b013e3181c524b7. [DOI] [PubMed] [Google Scholar]