Abstract
Importance
Osteoporosis and cardiovascular disease may share common biological pathways, with inflammation playing a role in the development of both. Although observational studies have suggested that statins are associated with a lower risk of fractures, randomized trial data addressing this issue are scant.
Objective
We sought to determine whether statin therapy reduces the risk of fracture and in a secondary analysis, whether baseline levels of the inflammatory biomarker high-sensitivity C-reactive protein (hs-CRP) are associated with the risk of fracture.
Design, Setting, Participants
The JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial was an international, randomized, double-blind, placebo controlled study enrolling 17,802 men over the age of 50 and women over the age of 60 with hs-CRP ≥ 2 mg/L. Participants were screened from 2003 to 2006 and followed prospectively for up to 5 years (median follow-up 1.9 years).
Intervention
Rosuvastatin, 20 mg daily or placebo
Main Outcomes and Measures
Incident fracture was a pre-specified secondary endpoint of the JUPITER trial. Fractures were confirmed by radiographs, computed tomography, bone scan or other methods. Cox proportional hazards models were used to calculate hazard ratios (HR) and associated 95% confidence intervals (CI) for the risk of fracture according to randomized treatment assignment as well as increasing tertiles of hs-CRP, controlling for potential confounders.
Results
During the study, 431 incident fractures were reported and confirmed. Among participants allocated to rosuvastatin, 221 fractures were confirmed, as compared with 210 among those allocated to placebo such that the incidence rates of fracture in the rosuvastatin and placebo groups were 1.20 and 1.14 per 100 person-years, respectively (adjusted HR 1.06, 95% CI 0.88–1.28, p=0.53). Overall, increasing baseline hs-CRP was not associated with an increased risk of fractures, (adjusted HR for each unit increase in hs-CRP tertile 1.06, 95% CI 0.94–1.20, ptrend=0.34)
Conclusions and Relevance
Among men and women with elevated hs-CRP enrolled in a large trial of rosuvastatin therapy for cardiovascular disease, statin therapy did not reduce the risk of fracture. Higher baseline hs-CRP was not associated with an increased risk of incident fracture.
Background
Osteoporotic fractures contribute significantly to the burden of disease facing an aging population. Cardiovascular disease (CVD) and osteoporosis are both age-related systemic diseases that may share common biological pathways,1,2 and several epidemiologic studies have linked them together. Inflammation is key to the pathogenesis of atherosclerosis, and may also play an important role in the development of osteoporosis. Chronic inflammation promotes bone loss, and extensive reciprocal relationships exist between bone metabolism and the immune system.3–5
There are several mechanisms by which statins may exert positive biologic effects on bone. In an early rodent study, statin injection was shown to stimulate bone formation.6 Statins and nitrogen-containing bisphosphonate drugs both act in the mevalonate pathway of cholesterol synthesis.7 These observations have fueled interest in the role of statins in bone metabolism and the hypothesis that statins may have clinical benefits beyond CVD prevention.
Several observational studies found a reduced risk of fractures in users of statins8–11, but others found no association12,13. Several studies have also shown an association between statin use and greater bone mineral density.14–16 Post-hoc analyses of randomized clinical trials of statin therapy have not demonstrated a reduced risk of fracture.17,18 Such analyses have been limited by their post-hoc consideration of fractures, use of statins that may be less effective on bone in-vitro, and insufficient power.19
In the JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial we sought on an a priori and pre-specified basis to determine (a) whether treatment with rosuvastatin is associated with a lower risk of fractures and; (b) in an exploratory analysis, whether higher baseline hs-CRP is associated with an increased risk of fracture.
Methods
Trial design
Incident fracture was a pre-specified secondary endpoint of the JUPITER trial. The JUPITER trial was a randomized, double-blind, placebo-controlled, multinational trial performed at 1315 centers in 26 countries. Details of the study design and main results of the trial have been described in detail previously.20 Men and women over the age of 50 and 60, respectively, were eligible for participation if they had no prior history of CVD or diabetes mellitus and if at the screening visit, hs-CRP was ≥2 mg/L and LDL <130 mg/dL. The baseline hs-CRP level was obtained by averaging the screening and baseline visit levels, and therefore baseline hs-CRP levels less than 2 mg/L were not protocol violations. Blood was drawn locally and shipped to a central laboratory where it was analyzed unbatched using a validated high sensitivity assay with the Behring nephelometer and reagent.21
Exclusion criteria relevant to the development of fractures were recent history of alcohol abuse, cancer within the 5 years prior to enrollment (except basal or squamous cell skin cancers), diabetes mellitus, chronic inflammatory conditions such as lupus, severe arthritis or inflammatory bowel disease and use of post-menopausal hormone replacement therapy or chronic oral glucocorticoids. 17,802 participants were randomized to receive either rosuvastatin 20 mg daily or placebo and were followed for up to 5 years (median 1.9 years). The primary endpoint of the study was the first occurrence of a major cardiovascular event.
At baseline, participants had a detailed medical history taken and underwent a screening physical exam prior to randomization. Participants were then followed at 3-month intervals and queried for the occurrence of trial endpoints including fractures and adverse events. A trial flow diagram is shown in eFigure 1 (supplement). The trial protocol was approved by the local institutional review board of each participating study site.
Endpoints
Each case of fracture was reported on a designated secondary endpoint case report form. The type of fracture and imaging test used for confirmation was recorded. Fractures were confirmed by the site principal investigator using radiographs, computed tomography, bone scan or other methods. Only the first confirmed fracture was included in the primary analysis. In the 13 participants who had multiple confirmed fractures occurring on the same day, only one fracture was included in the primary analysis. However, all confirmed fractures were included in the analysis of the effect of rosuvastatin on each individual fracture type. In a sensitivity analysis, we excluded these participants, hypothesizing these fractures may have been due to extreme trauma. Comment fields on case report forms were searched manually for pathologic fractures and severe trauma, leading to the exclusion of 7 cases of fracture. We also performed an analysis of all first reported fractures including those identified from adverse event reports, and fractures occurring during safety monitoring after the trial ended. On March 30, 2008, the JUPITER trial was terminated after the data and safety monitoring committee determined that the gathered evidence on efficacy and safety demonstrated that prolonged use of rosuvastatin was clearly indicated for specific patients. Although follow-up for the trial’s primary endpoint terminated on that day, participants remained on study drug and were followed in a blinded manner for the occurrence of adverse events and safety endpoints, including fracture, until their close-out study visit. The final study visit occurred on August 30, 2008.
Statistical Methods
To compare the rates of fracture in the placebo and rosuvastatin groups, we fitted Cox proportional hazards models to estimate hazard ratios (HR) and associated 95% confidence intervals (CI). The multivariate model chosen based on apriori knowledge included: age (continuous), sex, blood pressure ≥140/90 mmHg or use of anti-hypertensive medications (yes/no), randomized treatment assignment, current tobacco use (yes/no), body mass index (categories: <25, 25–30, ≥30 kg/m2) exercise (categories: rarely or less than once per week, once/week, 2–6 times/week, daily), race, alcohol use (categories: ≤1–3 times/month, 1–6 times/week, 1–3 times/day, ≥4 times/day), baseline hemoglobin A1c in quartiles (<5.5, 5.5–5.7, 5.7–5.9, >5.9%), and history of previous fracture. To evaluate potential effect modification, multiplicative interaction terms between drug assignment and various baseline characteristics were inserted into the unadjusted Cox model. Rates of incident fracture in the two groups were compared using the Kaplan-Meier method, with differences between the two groups tested using the log-rank test. The proportional hazards assumption was tested using a multiplicative interaction term between each covariate and the mean centered logarithm of study time. We did not detect a violation of the proportional hazards assumption. All analyses were performed according to the intent-to-treat principle and a two-sided p-value <0.05 was considered significant.
We also performed an exploratory analysis to determine whether baseline hs-CRP in tertiles as well as continuous log transformed hs-CRP predicts incident fracture within the JUPITER study using the same multivariate model as above. Since women on average have higher levels of hs-CRP and different fracture risks than men, we also performed additional analyses using gender specific tertiles of hs-CRP.22 Baseline use of thiazide diuretics, bisphosphonates, calcium, vitamin D, and inhaled or oral steroids did not result in a change in effect estimates and thus were omitted from the multivariate model.
Results
Study participants
Of the 17,802 participants in the JUPITER trial, 38.2 % were women and the median age of participants was 66 years. History of a previous fracture was reported by 36.6% of participants, with 57.7% of those participants reporting 1–3 fractures in the 20 years prior to randomization. The median hs-CRP level was 4.3 mg/L in participants in the placebo group and 4.2 mg/L in the rosuvastatin group (Table 1).
Table 1.
Baseline Characteristics
| Characteristics | Placebo, n=8901, (%) | Rosuvastatin, n=8901, (%) |
|---|---|---|
| Age, y | 66.0 (60–71) | 66.0 (60–71) |
| Female | 3375 (37.9) | 3426 (38.5) |
| Race | ||
| White | 6325 (71.1) | 6358 (71.5) |
| Black | 1124 (12.6) | 1100 (12.4) |
| Asian | 136 (1.5) | 147 (1.7) |
| Hispanic | 1140 (12.8) | 1121 (12.6) |
| Other | 176 (2.0) | 173 (1.9) |
| Current smoking | 1420 (16.0) | 1400 (15.7) |
| Hypertension | 4921 (57.5) | 4864 (56.9) |
| Previous fracture | ||
| Total | 3291 (37.0) | 3228 (36.3) |
| Men | 2234 (40.4) | 2160 (39.5) |
| Female | 1057 (31.3) | 1068 (31.2) |
| Number of fractures in past 20 yrs | ||
| None | 1285 (39.9) | 1274 (40.2) |
| 1 | 1520 (47.2) | 1494 (47.1) |
| 2–3 | 382 (11.9) | 366 (11.5) |
| >3 | 37 (1.2) | 37 (1.2) |
| Body mass index, kg/m2 | ||
| <25 | 2038 (22.9) | 2046 (23.1) |
| ≥25–30 | 3509 (39.5) | 3489 (39.3) |
| ≥30 | 3336 (37.6) | 3338 (37.6) |
| hs-CRP†, mg/liter | 4.3 (2.9–7.2) | 4.2 (2.8–7.1) |
| Metabolic syndrome | 3584 (42.1) | 3519 (41.5) |
| Alcohol intake | ||
| 1–3/month | 5164 (58.0) | 5237 (58.9) |
| 1–6 times/week | 2045 (23.0) | 2035 (22.9) |
| 1–3 times/day | 1376 (15.5) | 1349 (15.2) |
| ≥4 times/day | 313 (3.5) | 276 (3.1) |
| Exercise | ||
| Rarely | 4588 (51.6) | 4490 (50.5) |
| <1/week | 461 (5.2) | 462 (5.2) |
| 1/week | 590 (6.6) | 568 (6.4) |
| 2–3 times/week | 1460 (16.4) | 1574 (17.7) |
| 4–6 times/week | 713 (8.0) | 697 (7.9) |
| Daily | 1086 (12.2) | 1105 (12.4) |
| Bisphosphonate use | 372 (4.2) | 393 (4.4) |
| Calcium | 626 (7.0) | 629 (7.6) |
| Vitamin D | 257 (3.0) | 236 (2.8) |
| Steroids | ||
| Systemic | 43 (0.5) | 32 (0.4) |
| Inhaled | 485 (5.5) | 495 (5.6) |
| Thiazide Diuretics | 1114 (12.9) | 1119 (12.6) |
Values are median (interquartile range) or n (%).
Race is self-reported.
Hypertension was defined as blood pressure ≥140/90 mmHg or taking anti-hypertensive agents.
Some participants who reported a previous fracture did not report their fracture history in the previous 20 years.
The metabolic syndrome was defined according to consensus criteria of the National Heart, Lung, Blood Institute and American Heart Association.
Values of hs-CRP are the average of the values obtained at the first two study visits.
hs-CRP=high-sensitivity C-reactive protein
Rosuvastatin and Risk of Fracture
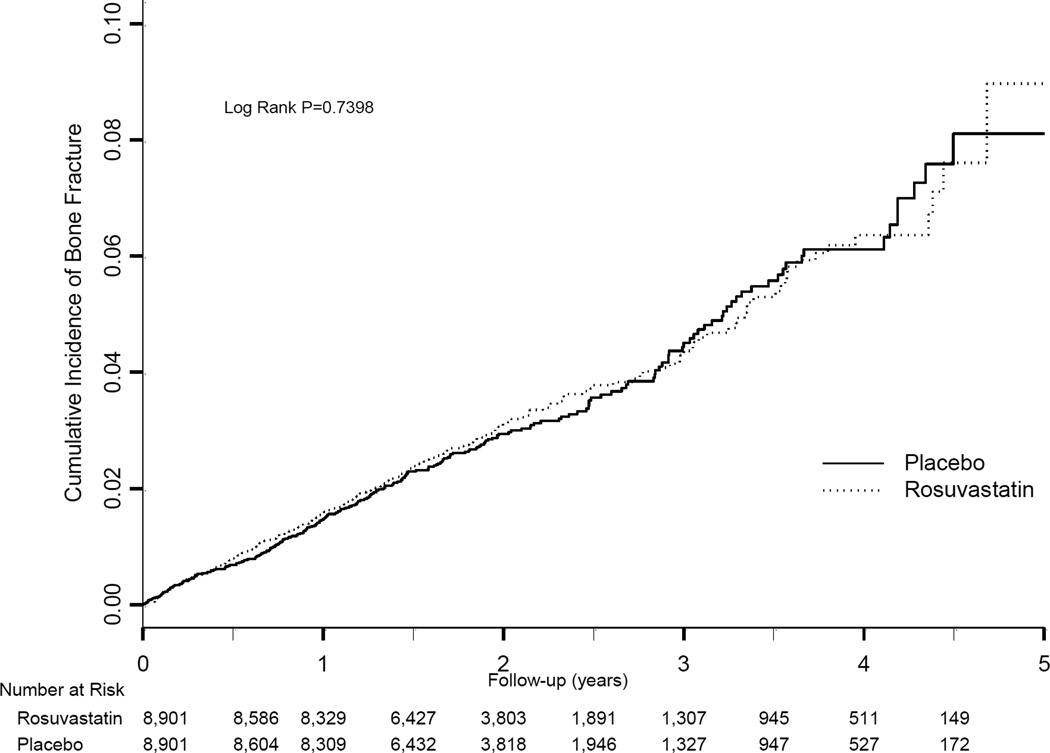
During the study period there were 431 confirmed fractures; 221 participants in the rosuvastatin group and 210 participants assigned to placebo (Table 2). Overall, the incidence rates of fracture in the rosuvastatin and placebo groups were 1.20 and 1.14 per 100 person-years, respectively (adjusted HR 1.06, 95% CI 0.88–1.28). The risk of fractures during follow-up was increased in women as compared with men: adjusted HR 2.06, 95% CI 1.66–2.56 (see eTable 1 in supplement). Among women, the incidence rate of fracture among those treated with rosuvastatin was 1.80 per 100 person-years (adjusted HR 1.16, 95% CI 0.89–1.50) and among men, 0.85 per 100 person-years (adjusted HR 0.97 95% CI 0.74–1.28) (Table 2). The HR for each of the covariates in the adjusted model are shown in eTable 1 (supplement). The cumulative incidence curves for bone fracture in the placebo and rosuvastatin groups are shown in Figure 1. There were 137 upper extremity fractures (including 71 wrist fractures), and 135 lower extremity fractures (including 55 ankle fractures)(Table 2). There were no significant differences in the rate of specific fractures between the two study groups (Table 2).
Table 2.
Occurrence of fracture according to study group.
| Rosuvastatin (n=8901 | Placebo (n=8901) | Hazard ratio | p* | |||
|---|---|---|---|---|---|---|
| No. of patients |
Incidence rate (per 100 person- years) |
No. of patients |
Incidence rate (per 100 person-years) |
|||
| Incident fracture† | 221 | 1.20 | 210 | 1.14 | 1.06(0.88–1.28) | 0.53 |
| Men | 99 | 0.85 | 105 | 0.89 | 0.97(0.74–1.28) | 0.83 |
| Women | 122 | 1.80 | 105 | 1.58 | 1.16(0.89–1.50) | 0.28 |
| Hip | 23 | 0.12 | 14 | 0.07 | 1.67(0.85–3.23) | 0.14 |
| Vertebral | 22 | 0.12 | 18 | 0.10 | 1.23(0.66–2.30) | 0.52 |
| Upper Extremity | 72 | 0.39 | 65 | 0.35 | 1.12(0.80–1.56) | 0.53 |
| Lower Extremity | 71 | 0.38 | 64 | 0.34 | 1.13(0.80–1.58) | 0.50 |
| Skull, face, finger, toe | 29 | 0.16 | 25 | 0.13 | 1.17(0.69–2.00) | 0.57 |
| Other | 25 | 0.13 | 35 | 0.19 | 0.73(0.43–1.21) | 0.22 |
Adjusted for age (continuous), sex, blood pressure ≥140/90 mmHg or use of anti-hypertensive medications (yes/no), current tobacco use (yes/no), body mass index (categories: <25, 25–30, ≥30 kg/m2) exercise (categories: rarely or less than once per week, once/week, 2–6 times/week, daily), race, alcohol use (categories: ≤1–3 times/month, 1–6 times/week, 1–3 times/day, ≥4 times/day), baseline HbA1c in quartiles (<5.5, 5.5–5.7, 5.7–5.9, >5.9%), hs-CRP in tertiles (<3.2, 3.2–5.8, ≥5.8) and previous fracture history.
Only the first fracture is included in incident fracture. Some participants had multiple fractures. All confirmed fractures are included in hip, vertebral, upper extremity, lower extremity, skull, face, finger, toe and other categories.
Figure 1. Cumulative Incidence of Fracture according to treatment assignment.
Shown is the incidence of bone fracture in the rosuvastatin and placebo groups. The P value was calculated using a log-rank test of the effect of rosuvastatin, using a proportional hazards model.
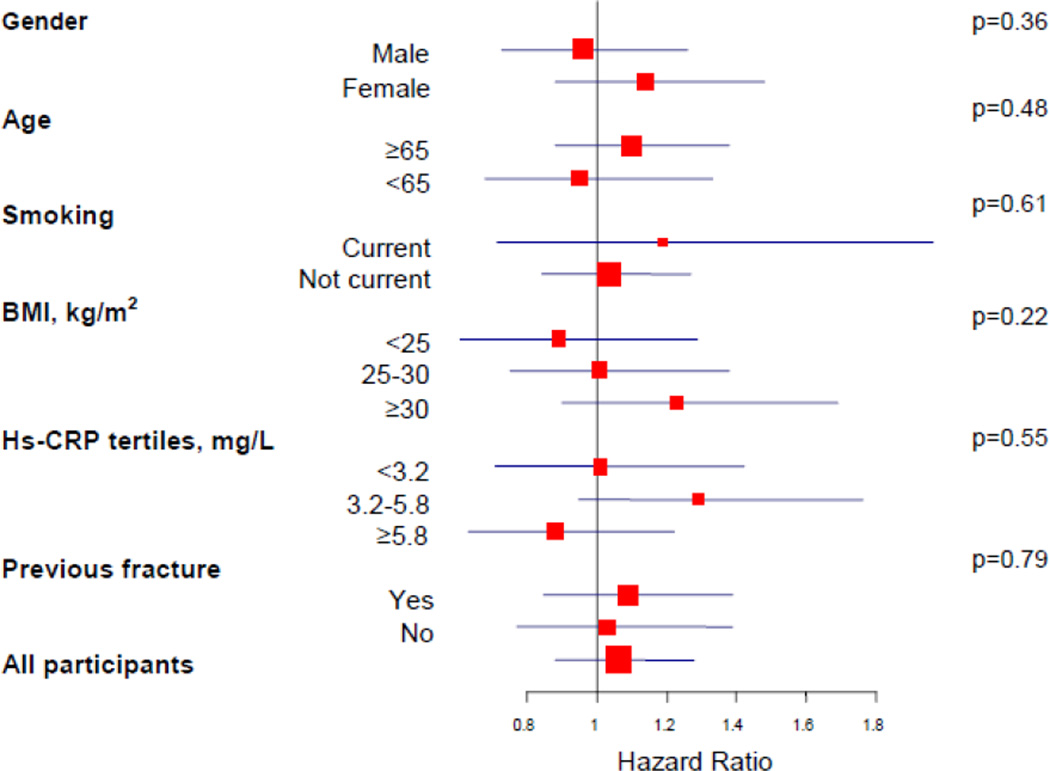
The effect of rosuvastatin on risk of fracture appeared consistent across major subgroups without evidence of treatment benefit in men or women, or among those with and without history of previous fracture (Figure 2). There was no evidence of effect modification by number of previous fractures (p=0.73).
Figure 2. Effect of Rosuvastatin on risk of fracture, according to baseline characteristics of study participants.
Shown are the hazard ratios (red square boxes) for rosuvastatin as compared with placebo. The size of the square box is inversely proportional to the confidence interval (horizontal blue lines). Interaction p values are shown to the right.
Hs-CRP= high sensitivity C-reactive protein
Sensitivity Analyses
The inclusion of 10 additional confirmed fractures occurring after trial termination during safety monitoring did not alter our overall results (adjusted HR 1.06, 95% CI 0.88–1.28) nor did the inclusion of all 571 cases of reported (not confirmed) fracture (adjusted HR1.04 95% CI 0.88–1.22). The exclusion of fractures less likely to be associated with osteoporosis such as skull, face, finger, and toe fractures led to consistent findings (hazard ratio 1.05, 95% CI 0.86–1.28). The exclusion of vertebral fractures, which can be challenging to diagnose without baseline radiographs yielded similar results (adjusted HR 1.05, 95% CI 0.86–1.29). Since participants with multiple fractures occurring on the same day may have had extreme trauma we also performed an analysis excluding these participants (adjusted HR 1.03 95% CI 0.85–1.25).
Per-protocol analysis
At the time of trial termination, 75% of participants remained on study drug. Compliance was assessed at each clinic visit by pill count. In an analysis of the 14,594 participants who remained on study drug or did not initiate off-label statin therapy at one year, rosuvastatin was not associated with a reduced risk of fracture (adjusted HR 1.06, 95% CI 0.86–1.29).
Hs-CRP and Risk of Bone Fracture
Increasing tertiles of baseline hs-CRP were not associated with the risk of fracture, (adjusted HR for each unit increase in hs-CRP tertile 1.06, 95% CI 0.94–1.20, ptrend=0.34) (Table 2). Results were similar in the continuous hs-CRP analysis (adjusted HR for 1 unit increase in log transformed hs-CRP 1.09, 95% CI 0.95–1.24, p=0.23) as well as in gender specific analyses (Table 2).
Discussion
In this randomized, double-blind placebo controlled trial of rosuvastatin therapy for primary CVD prevention, allocation to rosuvastatin did not reduce the risk of fracture. Furthermore, higher baseline hs-CRP was not associated with an increased risk of fracture. Our results were consistent in sensitivity analyses and various subgroups.
Our findings with regard to statin therapy are similar to prior analyses of cardiovascular trials17,18,26 and some observational and case-control studies12,13. In exploratory analyses of the Scandinavian Simvastatin Survival Study (4S) and LIPID trials, statin therapy did not reduce the rate of adverse event reports of fracture.17,18 In a pre-specified analysis of the Heart Protection Study, which enrolled subjects with vascular disease or diabetes, simvastatin 40 mg daily was not associated with a decreased risk of hospitalization for fracture.26 Among postmenopausal women participating in the Women’s Health Initiative, statin use was not associated with a lower fracture risk or improved bone density.13
However, multiple observational studies have observed a salutary effect of statins on fracture risk. In a meta-analysis of four large observational studies, statin use was associated with a lower risk of hip fracture (OR 0.43 95% CI 0.25–0.75).19 Furthermore, case control studies among varied populations in the United Kingdom11, elderly Medicare and Medicaid beneficiaries10, and American veterans9 have suggested a reduced risk of fracture in those exposed to statins with odds ratios ranging from 0.29 to 0.64. While the effect estimates in these observational reports are compelling, the limitations of observational data are noteworthy. These studies may suffer from selection bias, confounding by indication, or a “healthy user” phenomenon, as well as residual confounding by other cardiovascular medications, frailty, and obesity.28–30 The discordance between observational studies and randomized trials may also be explained by other factors. For example, it is possible that chronic statin therapy, intermittent use of statins over a long time period, or statin therapy in selected populations reduces the risk of fracture.
Elevation of the inflammatory biomarker hs-CRP, has been associated with an increased risk of fractures in several observational cohort studies.23–25 In one cohort of elderly women, increasing hs-CRP was associated with a higher risk of fractures (adjusted HR for highest hs-CRP tertile 2.40, 95% CI 1.10–5.24).23 Since JUPITER enrolled participants with elevated hs-CRP, our analysis of baseline hs-CRP levels and fracture risk is limited to those at the upper end of the range of hs-CRP values. The truncated range of hs-CRP may have obscured our ability to detect a relationship between hs-CRP and fracture risk. In addition, the relatively short follow-up and younger age of participants in our study in contrast to observational studies may have contributed to the observed lack of association.
To our knowledge, our study is the first randomized controlled trial examining the relationship between statin therapy and incident fracture in a pre-specified manner. This design is a major strength of our analysis as it minimizes the risk of bias and residual confounding inherent in observational investigations. Furthermore, the use of rosuvastatin, a potent statin, extends prior investigations in the LIPID, 4S, and HPS trials using pravastatin and simvastatin, respectively.
Our study design has important limitations. First, the relatively short treatment duration may not have been sufficient to achieve a beneficial effect of statins on bone. We note however, that our Kaplan-Meier curves offered no suggestion of an emerging benefit in those participants treated for 3–5 years. Second, we administered a fixed dose of statin and are unable to exclude the possibility that higher doses of statin may prevent fracture. Orally administered statins have low bioavailability and the dose employed in animal studies of statins and bone metabolism is approximately 10-fold higher than that used in human studies.28 However, the 20 mg dose of rosuvastatin was sufficient to achieve cardiovascular benefits in this trial and higher doses may convey other risks. Additionally, as our study endpoint was fracture, we are unable to evaluate subclinical effects on bone or elucidate further on the mechanism by which statins may mediate effects on bone.
Whether the study was designed with adequate statistical power is a concern in light of the null finding. However, with the accrual of 431 confirmed, incident fractures in JUPITER, the log-rank test with 2-sided alpha set at 0.05 has an estimated 96% power to detect a 30% reduction in the hazard of first fracture associated with statin assignment (hazard ratio 0.7), 84% power to detect a 25% reduction (hazard ratio 0.75), and 64% power to detect a 20% reduction (hazard ratio 0.8).
In conclusion, in this large randomized trial of rosuvastatin therapy among men and women with evidence of inflammation, randomization to rosuvastatin did not reduce the risk of fracture and higher baseline hs-CRP was not associated with an increased risk of fracture. Our study does not support the use of statins in doses used for cardiovascular disease prevention to reduce the risk of fracture.
Supplementary Material
Table 3.
Baseline hs-CRP and risk of fracture
| Tertile | hs-CRP (mg/L) |
Patients (n) |
No. Fractures |
Incidence Rate (per 100 person- years) |
HR | 95% CI | ptrend* |
|---|---|---|---|---|---|---|---|
| Total Cohort | |||||||
| Highest | ≥5.8 | 6102 | 142 | 1.15 | 1.13 | 0.88–1.44 | |
| Middle | 3.2–5.8 | 5939 | 161 | 1.30 | 1.20 | 0.95–1.52 | |
| Lowest | < 3.2 | 5761 | 128 | 1.06 | Ref. | Ref. | 0.34 |
| Men | |||||||
| Highest | ≥5.4 | 3790 | 71 | 0.90 | 1.34 | 0.94–1.90 | |
| Middle | 3.1–5.4 | 3502 | 80 | 1.07 | 1.42 | 1.01–1.99 | |
| Lowest | < 3.1 | 3709 | 53 | 0.66 | Ref. | Ref. | 0.10 |
| Women | |||||||
| Highest | ≥6.3 | 2317 | 71 | 1.58 | 0.97 | 0.69–1.36 | |
| Middle | 3.6–6.3 | 2099 | 71 | 1.67 | 1.03 | 0.74–1.42 | |
| Lowest | < 3.6 | 2385 | 85 | 1.82 | Ref. | Ref. | 0.86 |
Adjusted for age (continuous), sex, blood pressure ≥140/90 mmHg or use of anti-hypertensive medications (yes/no), randomized treatment assignment, current tobacco use (yes/no), body mass index (categories: <25, 25–30, ≥30 kg/m2) exercise (categories: rarely or less than once per week, once/week, 2–6 times/week, daily), race, alcohol use (categories: ≤1–3 times/month, 1–6 times/week, 1–3 times/day, ≥4 times/day), baseline HbA1c in quartiles (<5.5, 5.5–5.7, 5.7–5.9, >5.9%) and history of previous fracture.
Acknowledgment
Jessica Peña had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Funding/Support, The JUPITER trial was supported by AstraZeneca. Dr. Peña was supported by T32 HL07575 from the National Heart, Lung, and Blood Institute. Dr. Ridker has received investigator initiated grant support from the NHLBI, Pfizer, AstraZeneca, Novartis, and Amgen; is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to inflammatory biomarkers in cardiovascular disease that have been licensed to AstraZeneca and Seimens; and has served as a consultant to Genzyme, Vascular Biogenics, Boston Heart, Amgen, and ISIS, Inc. Dr. Glynn has received grant support from AstraZeneca through research grants to Brigham and Women’s Hospital for analyses of JUPITER data, as well as investigator-initiated grant support from Novartis.
Dr. Solomon receives salary support through research grants to Brigham and Women’s Hospital from Amgen, Lilly, Pfizer and the CORRONA Foundation. He also serves in unpaid roles on trials sponsored by Bristol Myers Squibb and Pfizer. He receives royalties from UpToDate on chapters regarding NSAIDs and coxibs. He receives an honorarium from the American Orthopedic Association for serving on a Multispecialty Board related to their Own the Bone program and is on the Governance Committee of the National Bone Health Alliance.
Role of the Sponsor: AstraZeneca collected the trial data and monitored study sites but did not design the study protocol, conduct analyses, interpret the data or have any role in the preparation, review or approval of this manuscript.
Footnotes
Trial registration: ClinicalTrials.gov number NCT00239681
Authors Contribution: Dr. Peña had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and design: All authors.
Acquisition of data: MacFadyen, Peña, Glynn, Ridker.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Peña, Aspberg.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: MacFadyen, Peña, Aspberg.
Obtained funding: Ridker.
Administrative, technical, or material support: Ridker, MacFadyen, Peña, Aspberg.
Conflicts of Interest and Financial Disclosures:
References
- 1.McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23(1):1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 2.Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 3.Greenblatt MB, Shim J-H. Osteoimmunology: A Brief Introduction. Immune Netw. 2013;13(4):111. doi: 10.4110/in.2013.13.4.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amelio P, Isaia G, Isaia GC. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. J Endocrinol Invest. 2009;32(4 Suppl):6–9. [PubMed] [Google Scholar]
- 5.Baud’huin M, Lamoureux F, Duplomb L, Rédini F, Heymann D. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell Mol Life Sci CMLS. 2007;64(18):2334–2350. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 7.Cummings SR, Bauer DC. Do statins prevent both cardiovascular disease and fracture? JAMA. 2000;283(24):3255–3257. doi: 10.1001/jama.283.24.3255. [DOI] [PubMed] [Google Scholar]
- 8.Chan KA, Andrade SE, Boles M, et al. Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. Lancet. 2000;355(9222):2185–2188. doi: 10.1016/S0140-6736(00)02400-4. [DOI] [PubMed] [Google Scholar]
- 9.Scranton RE, Young M, Lawler E, Solomon D, Gagnon D, Gaziano JM. Statin use and fracture risk: study of a US veterans population. Arch Intern Med. 2005;165(17):2007–2012. doi: 10.1001/archinte.165.17.2007. [DOI] [PubMed] [Google Scholar]
- 10.Wang PS, Solomon DH, Mogun H, Avorn J. HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA. 2000;283(24):3211–3216. doi: 10.1001/jama.283.24.3211. [DOI] [PubMed] [Google Scholar]
- 11.Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA. 2000;283(24):3205–3210. doi: 10.1001/jama.283.24.3205. [DOI] [PubMed] [Google Scholar]
- 12.Van Staa TP, Wegman S, de Vries F, Leufkens B, Cooper C. Use of statins and risk of fractures. JAMA. 2001;285(14):1850–1855. doi: 10.1001/jama.285.14.1850. [DOI] [PubMed] [Google Scholar]
- 13.LaCroix AZ, Cauley JA, Pettinger M, et al. Statin use, clinical fracture, and bone density in postmenopausal women: results from the Women’s Health Initiative Observational Study. Ann Intern Med. 2003;139(2):97–104. doi: 10.7326/0003-4819-139-2-200307150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lupattelli G, Scarponi AM, Vaudo G, et al. Simvastatin increases bone mineral density in hypercholesterolemic postmenopausal women. Metabolism. 2004;53(6):744–748. doi: 10.1016/j.metabol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone-mineral density in postmenopausal women. Lancet. 2000;355(9222):2218–2219. doi: 10.1016/s0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Zhu L-P, Yang X-L, Huang H-L, Ye D-Q. HMG-CoA reductase inhibitors (statins) and bone mineral density: a meta-analysis. Bone. 2013;54(1):151–156. doi: 10.1016/j.bone.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 17.El-Sohemy Statin drugs and the risk of fracture. JAMA. 2000;284(15):1921–1922. [PubMed] [Google Scholar]
- 18.Reid IR, Hague W, Emberson J, et al. Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial. Long-term Intervention with Pravastatin in Ischaemic Disease. Lancet. 2001;357(9255):509–512. doi: 10.1016/s0140-6736(00)04042-3. [DOI] [PubMed] [Google Scholar]
- 19.Bauer DC, Mundy GR, Jamal SA, et al. Use of statins and fracture: results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med. 2004;164(2):146–152. doi: 10.1001/archinte.164.2.146. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem. 2009;55(2):305–312. doi: 10.1373/clinchem.2008.120642. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Am J Cardiol. 2010;106(2):204–209. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Saito T, Kobayashi R, et al. C-reactive protein predicts incident fracture in community-dwelling elderly Japanese women: the Muramatsu study. Osteoporos Int J. 2011;22(7):2145–2150. doi: 10.1007/s00198-010-1425-9. [DOI] [PubMed] [Google Scholar]
- 24.Pasco JA, Kotowicz MA, Henry MJ, et al. High-sensitivity C-reactive protein and fracture risk in elderly women. JAMA. 2006;296(11):1353–1355. doi: 10.1001/jama.296.11.1353. [DOI] [PubMed] [Google Scholar]
- 25.Schett G, Kiechl S, Weger S, et al. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch Intern Med. 2006;166(22):2495–2501. doi: 10.1001/archinte.166.22.2495. [DOI] [PubMed] [Google Scholar]
- 26.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 27.Helin-Salmivaara A, Korhonen MJ, Lehenkari P, et al. Statins and hip fracture prevention--a population based cohort study in women. PloS One. 2012;7(10):e48095. doi: 10.1371/journal.pone.0048095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue J, Zhang X, Dong B, Yang M. Statins and bone health in postmenopausal women: a systematic review of randomized controlled trials. Menopause. 2010;17(5):1071–1079. doi: 10.1097/gme.0b013e3181d3e036. [DOI] [PubMed] [Google Scholar]
- 29.Dormuth CR, Patrick AR, Shrank WH, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119(15):2051–2057. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glynn RJ, Schneeweiss S, Wang PS, Levin R, Avorn J. Selective prescribing led to overestimation of the benefits of lipid-lowering drugs. J Clin Epidemiol. 2006;59(8):819–828. doi: 10.1016/j.jclinepi.2005.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.