Abstract
Deschampsia antarctica Desv. (Poaceae) (2n = 26) is one of the two vascular plants adapted to the harshest environment of the Antarctic. Although the species is a valuable model for study of environmental stress tolerance in plants, its karyotype is still poorly investigated. We firstly conducted a comprehensive molecular cytogenetic analysis of D. antarctica collected on four islands of the Maritime Antarctic. D. antarctica karyotypes were studied by Giemsa C- and DAPI/C-banding, Ag-NOR staining, multicolour fluorescence in situ hybridization with repeated DNA probes (pTa71, pTa794, telomere repeats, pSc119.2, pAs1) and the GAA simple sequence repeat probe. We also performed sequential rapid in situ hybridization with genomic DNA of D. caespitosa. Two chromosome pairs bearing transcriptionally active 45S rDNA loci and five pairs with 5S rDNA sites were detected. A weak intercalary site of telomere repeats was revealed on the largest chromosome in addition to telomere hybridization signals at terminal positions. This fact confirms indirectly the hypothesis that chromosome fusion might have been the cause of the unusual for cereals chromosome number in this species. Based on patterns of distribution of the examined molecular cytogenetic markers, all chromosomes in karyotypes were identified, and chromosome idiograms of D. antarctica were constructed. B chromosomes were found in most karyotypes of plants from Darboux Island. A mixoploid plant with mainly triploid cells bearing a Robertsonian rearrangement was detected among typical diploid specimens from Great Jalour Island. The karyotype variability found in D. antarctica is probably an expression of genome instability induced by environmental stress factors. The differences in C-banding patterns and in chromosome distribution of rDNA loci as well as homologous highly repeated DNA sequences detected between genomes of D. antarctica and its related species D. caespitosa indicate that genome reorganization involving coding and noncoding repeated DNA sequences had occurred during the divergence of these species.
Introduction
The Antarctic Hairgrass, Deschampsia antarctica Desv. (Poaceae) (2n = 26), is one of the only two flowering plant species found in Antarctica [1, 2]. D. antarctica has successfully adapted to the harshest environmental conditions (extremely low temperatures, drought, high salinity and flooding, high level of UV radiation, low precipitation). The Antarctic Hairgrass shows tolerance to extreme cold and dry conditions [2–6], ability to become established rapidly [7], and it can photosynthesize at freezing point [8]. The successful Antarctic vegetation of D. antarctica must have one or various mechanisms which allow the species to live in the extreme environments [3, 5]. One of these mechanisms might be the genomic variability that under environmental stress promotes increasing genome diversity in wild grasses and, consequently, increasing the adaptive potential of populations [9]. The Antarctic Hairgrass could serve as a model for the study of regulation of genome activity as well as for the investigation of the mechanisms responsible for plant adaptation to freezing tolerance [6]. D. antarctica might also be a useful source of genes associated with stress tolerance and environmental adaptation allowing the development of breeding strategies in agronomical valuable crops [10–11]. Besides, extracts from D. antarctica are known to display protective effects against ultraviolet radiation used for pharmaceutical purposes [12].
The evident importance of D. antarctica genome as a unique recourse for such studies has generated a number of research works mainly performed at the morphological, physiological, biochemical or molecular levels [13–14]. In particular, the chloroplast genome has been sequenced and plastid transcriptome profiles of the coding/noncoding genes have been investigated [11, 15]. The Lip3F9 polypeptide with lipase activity from D. antarctica has been isolated and characterized [16]. A multi-gene family from D. antarctica encoding ice recrystallization inhibition proteins whose transcript levels are responsive to cold acclimation, has also been characterized [5–6]. However, little nuclear genomic information is still available for this species [6, 15–16].
The results of previous molecular genetic studies with the use of RAPD- and AFLP-analyses showed different levels of genetic diversity in D. antarctica populations from various regions of Maritime Antarctic [14, 17–21]. It was also shown that environmental stress factors can lead to physiological stress followed by structural variations in plant genomes including chromosome rearrangements, mixoploidy and aneuploidy [14, 22–25]., The comparative molecular cytogenetic analysis of populations of D. antarctica growing in different areas of the Maritime Antarctic has not been studied yet as the karyotype of D. antarctica is still poorly investigated. Only the chromosome number 2n = 26 = 2(5m+3sm+4st+1t) with one pair of satellite (SAT) chromosomes had been determined by simple monochrome staining [1, 26]. A detailed knowledge of karyotype structure of D. antarctica is needed for further chromosome mapping of genes associated with stress tolerance as well as comparative cytogenetic studies within the genus Deschampsia for a better understanding of organization, structure, and evolution of this genus.
In the present paper, a comprehensive molecular cytogenetic analysis of D. antarctica specimens from four islands of the west coast of the Antarctic Peninsula was carried out for the first time. In karyotypes of D. antarctica, Giemsa C-, DAPI/C-banding patterns and AgNOR staining were studied. Chromosome distribution of 45S and 5S rDNA, telomere repeats, satellite DNA sequences of cereals pSc119.2 and pAs1, the (GAA)n microsatellite sequence as well as homologous highly repeated sequences of genomic DNA of the related species D. caespitosa were investigated by multicolour fluorescence in situ hybridization (FISH) and sequential rapid genomic in situ hybridization (rapid GISH).
Materials and Methods
Ethics Statement
This study including sample collection and experimental research conducted on these materials was according to the law on activities and environmental protection to Antarctic approved by the Ministry of Education and Science of Ukraine.
Plant material
The seeds of D. antarctica growing under natural conditions were collected in the vicinity of the Ukrainian Antarctic Station “Academician Vernadsky”: Galindez Island (northwestern corner of the island at Caroline point: 65°14.955´ S, 64°15.181´ W), Skua Island (northwestern corner of the island: 65°15.302´ S, 64°16.493´ W), Great Jalour Island (northeastern part of the island: 65°14.039´S, 64°9.761´W) and Darboux Island (northern end of the island in the vicinity of a rocky grotto: 65°23.707´ S, 64°12.905´ W). The habitats of D. antarctica were small (up to 1 km2) rocky islands partly covered with snow. The plant material was obtained from the research Antarctic expeditions (seasons 2005–2008) organized by the National Antarctic Scientific Center of Ukraine. The accessions of D. caespitosa were collected (natural population, Kyiv region, Ukraine) and identified by Dr. I.Yu. Parnikoza, Institute of Molecular Biology and Genetics of National Academy of Sciences of Ukraine (Kyiv, Ukraine). The identification of the species was performed according to the Manual of vascular plants of Ukraine [27].
For obtaining aseptic roots of D. antarctica, dry seeds were sterilized and germinated as described earlier [28]. The obtained aseptic plants were cultivated on B5 agar nutrient medium [29] supplemented with 0.1 mg/L α-naphthaleneacetic acid (NAA). To produce plant material in sufficient quantity, the plants were in vitro propagated through fragmentation of the obtained root mat.
Fixation
For cell cycle synchronization and accumulation of mitotic divisions, root tips were incubated in ice water for 24 hours at 0°C and then fixed in the ethanol:glacial acetic acid fixative (3:1) for 48 h at room temperature. Fixed roots were stored in the fixative at -20°C before use.
Chromosome spread preparation
For C-banding, chromosomes spreads were prepared as described previously [30].
For in situ hybridization and DAPI staining, before chromosome spread preparation, the roots were stained in 1% acetocarmine solution in 45% acetic acid for 40 min, the tip caps with root meristem were cut on the object-plate, the meristem was macerated in a drop of 45% acetic acid, and then squashed chromosome preparations were made. The cover slips were removed after freezing, and the preparations were dehydrated and stored in 96% ethanol at -20°C.
C-banding
The C-banding procedure was carried out according to the technique described previously [31].
Fluorescence in situ hybridization
Following probes were used for FISH:
pTa71 containing a 9 kb long repeated DNA sequence of common wheat encoding 18S, 5.8S and 26S rRNA genes including spacers [32];
pTa794 containing a 420 bp long repeated DNA sequence of wheat containing the 5S rRNA gene and the intergenic spacer [33];
pAs1 containing a 1 kb repeated DNA sequence isolated from Aegilops tauschii [34];
pSc119.2 containing a120 bp long repeated DNA sequence isolated from rye [35];
the Arabidopsis-type telomere repeat probe (TTTAGGG)n generated by PCR according to [36];
the (GAA)n-oligonucleotide probe labelled at the 3’-end with fluorescein-12-dUTP (Roche Diagnostics, Mannheim, Germany) was synthesized using a synthesizer ABI 394 (Applied BioSystems, Redwood City, USA).
Dual and multicolour FISH assays were performed using combinations of DNA probes labelled directly with different fluorochromes including SpectrumAqua, SpectrumGreen, SpectrumGold, SpectrumRed (Abbott Molecular, Wiesbaden, Germany) and also labelled indirectly with biotin-16-dUTP (Roche Diagnostics, Mannheim, Germany) or digoxigenin-11-dUTP (Roche Diagnostics, Mannheim, Germany) by nick translation according to manufacturers’ protocols. FISH procedure was conducted as described previously [37].
DAPI staining
After FISH, chromosome slides were stained with 0.1 μg/ml DAPI (4',6-diamidino-2-phenylindole (DAPI, (Serva, Heidelberg, Germany)) in Vectashield mounting medium (Vector laboratories, Peterborough, UK).
Rapid GISH procedure
Genomic DNA of D. caespitosa was isolated from young leaves using CTAB (cetyltrimethylammonium bromide) standard protocol [38] and labelled with SpectrumAqua (Abbott Molecular, Wiesbaden, Germany) by nick translation according to the manufacturer’s instructions. After FISH procedures and documentation of the hybridization patterns, the chromosome slides were washed for 5 minutes in distilled water, dehydrated through the ethanol series, denatured in 70% formamide in 2 × SSC at 73°C for 3 min, dehydrated through the cold ethanol series and air dried. The labelled genomic DNA probe (80 ng) was dissolved in hybridization mixture (10% w/v dextran sulfate, 50% v/v formamide, 1% v/v Tween and 2 × SSC) (with the total volume 15 μL), denatured at 85°C for 5 min, chilled on ice and placed onto the slide. After the hybridization for 40 minutes at 37°C in a moist chamber, the slides were washed and DAPI stained as described previously [37].
Ag-NOR staining
Ag-NOR staining was performed according to Howell and Black [39]. After Ag-NOR staining slides were mounted in 0.1 μg/ml of DAPI (4',6-diamidino-2-phenylindole) (Serva, Heidelberg, Germany) in Vectashield medium (Vector laboratories, Peterborough, UK) for chromosome visualization.
Chromosome analysis
The slides were examined using Olympus BX61 epifluorescence microscope (Olympus, Tokyo, Japan). Images were taken with monochrome CCD camera (Cool Snap, Roper Scientific Inc., Tucson, USA) and sequentially collected in grayscale channels. Then, they were pseudocoloured and processed with Adobe Photoshop 10.0 (Adobe Systems Inc., USA) software. At least five specimens of D. antarctica from each island and fifteen metaphase plates from each specimen were analyzed.
The chromosome images were measured using an image analysis system “Videotest-Karyo 1.5” (IstaVideotest, St Petersburg, Russia). Based on the results of measurements of 10 metaphase plates from 5 individual plants, relative chromosome length (100 x chromosome length/total haploid length) and centromeric index (100 x length of short arm/total chromosome length) were calculated. The chromosome pairs were classified according to their centromeric position [40] in the decreasing order of size.
Results
C- and DAPI/C-banding analysis
D. antarctica specimens with typical diploid karyotypes (2n = 26) were found on all four islands (Galindez, Darboux, Skua and Great Jalour) of the Maritime Antarctic (Figs 1 and 2). Chromosomes in the karyotypes were subdivided into four groups based on their centromeric position and morphology. The first group comprised six metacentric chromosomes, the second group–two submetacentric chromosomes, the third group–three subtelocentric, and the fourth group–two telocentric chromosomes. Accordingly, the karyotype formula was 2n = 26 = 2(6m+2sm+3st+2t).
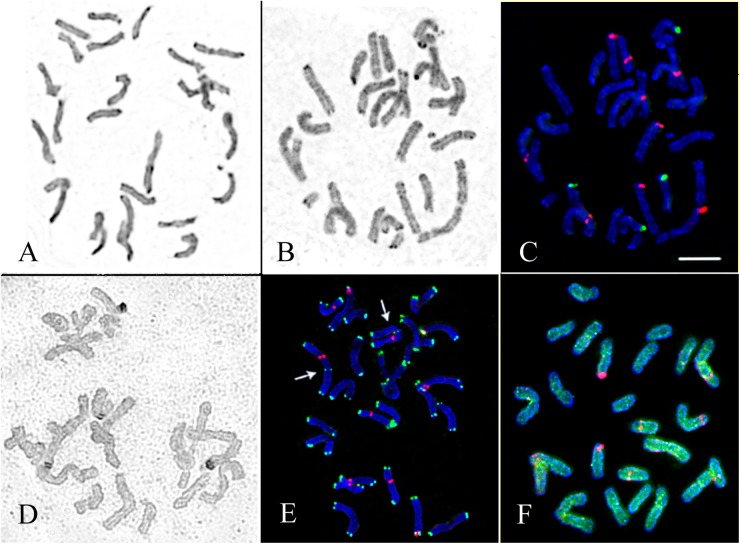
Fig 1. Chromosome spreads of D. antarctica.
(A) Giemsa C-banded chromosomes of the specimen from Galindez Island. (B) Inverted image of the DAPI/C-banded karyotype and (C) localization of 45S (green) and 5S (red) rDNA sites on chromosomes of the specimen from Galindez Island. (D) Ag-NOR staining patterns (dark segments) of chromosomes of the specimen from Skua Island. (E) Localization of telomeric repeats (green), 45S (green) and 5S (red) rDNA loci and in the karyotype of the specimen from Skua Island. Arrows point to the intercalary loci of telomere repeats detected on the largest chromosome pair. (F) Distribution of 5S rDNA sites (red) and GAA microsatellite sequence (green) on chromosomes of the specimen from Skua Island. Scale bar—5 μm.
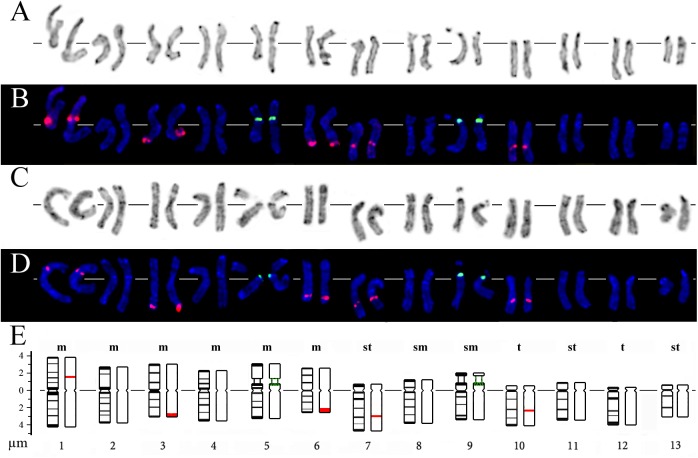
Fig 2. Karyograms and idiograms of D. antarctica chromosomes.
Karyograms after (A) Giemsa C-banding and (B) FISH with 45S (green) and 5S (red) rDNA probes (the same metaphase plate as in Fig 1A). Karyograms after (C) DAPI/C-banding (inverted image) and (D) FISH with 45S (green) and 5S (red) rDNA (the same metaphase plate as in Fig 1B and 1C). (E) Idiograms of D. antarctica showing relative sizes and positions of DAPI/C-bands (black segments), 45S (green) and 5S rDNA (red).
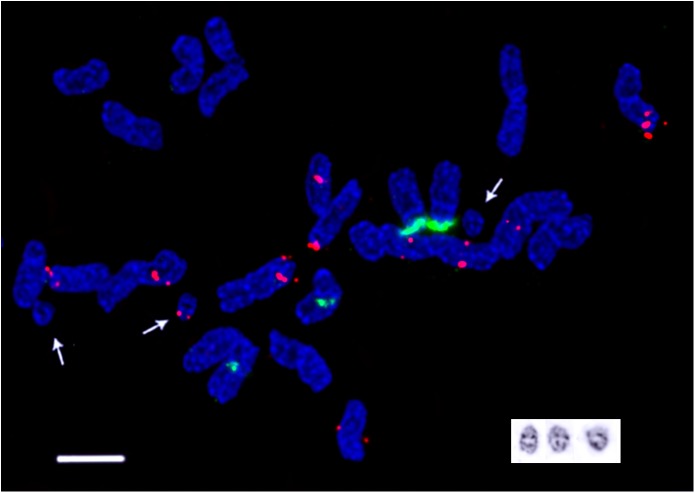
In karyotypes of most of the specimens from Darboux Island, 1–3 supernumerary chromosomes (B chromosomes) were observed (Fig 3). Also, a mixoploid plant was detected among the specimens from Great Jalour Island. The analysis of 82 metaphases from six roots of the plant showed that the chromosome number ranged from 27 to 54, and 67 metaphases had 38 chromosomes (modal number).
Fig 3. Chromosome spread of D. antarctica specimen from Darboux Island.
Chromosome localization of 45S (green) and 5S (red) rDNA sites and inverted image of DAPI/C-banded B-chromosomes (bottom right). Arrows point to the B-chromosomes. Scale bar—5 μm.
C-banding analysis of D. antarctica showed similar distribution of Giemsa C-bands in karyotypes of the specimens from different islands: large C-bands were located in the pericentromeric and telomeric regions of chromosomes, while a number of small and also faint and inconsistent C-bands were detected in the interstitial chromosome regions (Figs 1A and 2A).
After FISH procedures, chromosome staining with DAPI revealed C-like banding patterns (DAPI/C-banding patterns) (Figs 1B and 2C) which became more evident after several sequential FISH procedures.
Visual analysis of Giemsa C- and DAPI/C-banding patterns of the specimens indicated small variations in size and number of the intercalary and telomeric bands as well as C-bands adjacent to the secondary constriction regions of the satellite chromosomes. Bs, found in karyotypes of the specimens from Darboux Island, possessed distinct heterochromatic bands in their telomeric regions (Fig 3).
Chromosome localization of 45S and 5S rDNA sites
FISH-analysis showed that 45S rDNA loci were localized on two chromosome pairs: in the proximal region of the short arm of a metacentric chromosome pair and in the distal region of the short arm of a submetacentric chromosome pair. The secondary constriction of the submetacentric chromosome was well-defined, and the 45S rDNA site was larger (though the satellite was smaller) compared with the other SAT chromosome pair (Figs 1A, 1B, 1C, 2 and 3). We observed 5S rDNA sites on five chromosome pairs: in the proximal region of the short arm of the largest metacentric chromosome, in the subtelomeric regions of the long arms of two other metacentric chromosomes, in the proximal regions of the long arms of a pair of subtelocentric chromosomes and a pair of telocentric chromosomes. 5S rDNA loci were smaller and more polymorphic in size than 45S rDNA loci (Figs 1C, 1E, 1F, 2B and 2D). Besides, weak 5S rDNA sites were detected in the subtelomeric regions of one of the Bs found in some kariotypes of D. antarctica from Darboux Island (Fig 3).
Detection of active nucleolus organizer regions by Ag-NOR staining
In diploid metaphase spreads, Ag-NOR staining revealed four stained regions in two chromosome pairs indicating that the NORs were transcriptionally active in both SAT chromosomes. Two Ag-NORs are clearly more intense than the other two (Fig 1D).
Chromosome localization of telomere repeats
In FISH assays, the sites of telomere repeat sequences were detected in the terminal regions of all chromosomes. Additionally, in karyotypes of all the specimens, a polymorphic interstitial hybridization signal of the telomere repeats was localized in the proximal position of the long arm of the largest chromosome (Figs 1E, 4A and 4C).
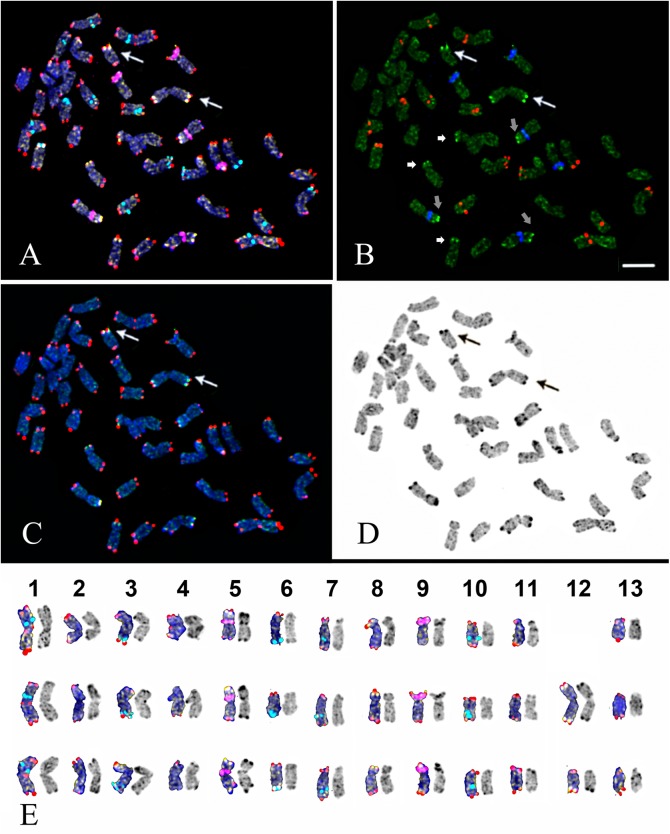
Fig 4. Chromosome spread of the triploid D. antarctica specimen from Great Jalour Island.
(A) The merged fluorescent image: multicolour FISH with telomeric repeats (red), 45S (purple) and 5S (light blue) rDNA probes and sequential rapid GISH with genomic DNA of D. caespitoza (yellow). (B) Localization of chromosome markers revealed by rapid GISH (light green) in relation to 45S (blue) and 5S (red) rDNA loci. Arrows indicate the brightest green signals detected on chromosomes 5 (grey arrows), 8 (short arrows) and 12 (long arrows). (C) Chromosome markers revealed by rapid GISH (yellow) in relation to telomeric repeat signals (red). (D) Inverted image of DAPI/C-banded chromosomes. Long arrows indicate chromosome 12 and a Robertsonian translocation between two homologous chromosomes 12. (E) Karyograms of metaphase chromosomes after multicolour FISH (left) and DAPI/C-banding (inverted image) (right). Scale bar—5 μm.
FISH mapping of pAs1, pSc119.2 and GAA-oligonucleotide probes
FISH analysis revealed dispersed hybridization sites of the GAA microsatellite sequence along the chromosomes of D. antarctica (Fig 1F). Hybridization signals of pAs1 or pSc119.2 probes were not visualized.
Chromosome identification and karyotype analysis
Based on chromosome morphology, Giemsa C- and DAPI/C-banding patterns and also results of FISH analysis, all chromosomes in the karyotypes of D. antarctica were identified. Chromosome idiograms of typical diploid D. antarctica including Giemsa C- and DAPI/C-banding patterns as well as 45S and 5S rDNA localization were constructed (Fig 2E).
The analysis of DAPI/C-banding patterns of the 38-chromosome karyotype of mixoplod D. antarctica from Great Jalour Island allowed us to conclude that it was a triploid possessing a chromosome rearrangement (presumably chromosome fusion) (Fig 4D and 4E). FISH analysis showed that the rearranged chromosome did not possess any chromosome markers. Accordingly, we performed a rapid GISH procedure using labelled genomic DNA of the related species D. caespitosa as a probe. The hybridization procedure revealed bright signals in the subtelomeric regions of the short arms of chromosomes 5, 8 and 12. Two of the homologous telocentric chromosomes 12 were involved in the Robertsonian rearrangement (centric fusion translocation) (Fig 4 arrows).
The Robertsonian translocation t(12; 12) was observed in most of the cells of the mixoploid plant. Two tetraploid metaphases comprised four haploid chromosome sets (4n = 52) without translocations. Also, two chromosome translocations t(12; 12) together with four haploid chromosome sets were found in one metaphase having 54 chromosomes. The number of chromosomes in the other metaphase plates varied from 27 to 48, but it was not possible to determine whether or not the cell was aneuploid as some of the chromosomes could be lost during slide preparation (Table 1).
Table 1. Cytogenetic analysis of D. antarctica specimens from Great Jalour Island.
| Number of plants | Genotype | Number of roots | Number of chromosomes | Number of metaphases | Number of t(12; 12) | Ploidy level |
|---|---|---|---|---|---|---|
| 4 | Typical diploid | 7 | 26 | 67 | 0 | 2x = 26 |
| 1 | Mixoploid | 1 | 27 | 1 | 1 | 2x = 26+t(12; 12) |
| 36 | 1 | 1 | ||||
| 38 | 9 | 1 | 3x = 37+t(12; 12) | |||
| 48 | 1 | 1 | ||||
| 54 | 1 | 2 | 4x = 52+2t(12; 12) | |||
| 1 | 32 | 1 | 1 | |||
| 35 | 1 | 0 | ||||
| 36 | 1 | 1 | ||||
| 38 | 10 | 1 | 3x = 37+t(12; 12) | |||
| 45 | 1 | 1 | ||||
| 52 | 2 | 0 | 4x = 52 | |||
| 1 | 33 | 1 | 1 | |||
| 38 | 13 | 1 | 3x = 37+t(12; 12) | |||
| 1 | 34 | 1 | 1 | |||
| 36 | 1 | 1 | ||||
| 38 | 12 | 1 | 3x = 37+t(12; 12) | |||
| 1 | 37 | 1 | 1 | |||
| 38 | 11 | 1 | 3x = 37+t(12; 12) | |||
| 1 | 36 | 1 | 1 | |||
| 38 | 12 | 1 | 3x = 37+t(12; 12) |
Discussion
Deschampsia P. Beauv. is a cosmopolitan genus that involves a group of taxa with a wide geographical distribution [26, 41]. The high morphological diversity makes the taxonomy of the genus very difficult [26]. Most of Deschampsia species have an unusual for cereals basic chromosome number x = 13 with the exception of D. setacea (2n = 2x = 14), D. atropurpurea (2n = 2x = 14) and D. fluxuosa (2n = 4x = 28) which present a basic number of x = 7 [26]. A molecular phylogenetic study of Deschampsia based on the analysis of nuclear ribosomal internal transcribed spacer (ITS) and plastid trnL intron sequences showed that D. antropurpurea and D. fluxuosa should be excluded from the genus Deschampsia, while the core of the genus Deschampsia was mainly represented by the subspecies of D. caespitosa (D. caespitosa complex) [42]. The D. caespitosa complex is the most studied taxon in the genus with a widespread distribution including extreme polar environments [41, 42]. In D. caespitosa, different karyotypes were described [41–43], and also inter- and intraindividual variability in chromosome number (2n = 15–28) as well as different ploidy levels (2x = 26; 3x = 39; 4x = 52) were reported [41–44].
D. antarctica, according to the phylogenetic studies based on the analysis of nuclear ribosomal ITS and plastid sequences, was included in the core of the genus Deschampsia [45, 46]. However, in order to clarify the phylogenetic relationships of this species within the genus, the need for obtaining data of different geographical origins and ploidy levels has been pointed out [26, 46].
In the present study, a cytogenetic analysis of D. antarctica specimens from four localities of Maritime Antarctic also revealed 13 chromosome pairs in typical diploid karyotypes. However, morphological analysis of chromosomes detected minor differences in the karyotype formula (2n = 26 = 2(6m+2sm+3st+2t)) compared to the earlier described one (2n = 26 = 2(5m+3sm+4st+t)) [26]. This is probably due to the fact that we have used chromosome specific markers for more precise identification of homologous pairs.
Apart from the chromosome number, similar features in the karyotype structure of D. antarctica and D. caespitosa were revealed [26, 41], in particular, their karyotypes contain six pairs of metacentric chromosomes with one pair being almost twice as long as the others and seven pairs of acrocentric chromosomes [26, 42]. The appearance of such a long metacentric chromosome in karyotypes of these species could be a result of a translocation (or a centric fusion) between two normal sized acrocentric chromosomes, giving rise to the unusual for cereals chromosome number 2n = 26 [41]. The additional intercalary signal of telomere repeats detected in the largest chromosome 1 indirectly confirms this assumption because intercalary sites of telomere repeats have been considered to be the remnants of ancestral chromosome fusions (or other rearrangements) produced during the evolution of karyotypes [47].
In karyotypes of the most of D. antarctica specimens from Darboux Island, we found supernumerary chromosomes (B chromosomes). Due to the dispensable nature of Bs, they can be present or absent among individuals of the same population in a species [48–49]. It should be noticed that B chromosomes were previously detected in karyotypes of other species of the D. caespitosa complex [14]. Also, the correlation between the appearance of Bs in a karyotype and environmental conditions was found [48, 50–51]. Interestingly, the Bs were found only in karyotypes of D. antarctica from the southernmost Darboux Island (correspondingly, exposed to the harshest environmental conditions). Nevertheless, there is a strong possibility that Bs can exist in karyotypes of D. antarctica established on the other islands as not all the clusters (populations) of plants were studied.
Furthermore, apart from typical diploid specimens of D. antarctica, a mixoploid plant with mainly triploid cells bearing the Robertsonian rearrangement between homologous chromosomes was found among the samples from Great Jalour Island. In harsh conditions of Maritime Antarctic, where the areas available for colonization by the species are restricted to small isolated clusters among rocks and rock cracks, one of the types of propagation (sexual or asexual) could gain an advantage over the other one. The chromosome rearrangement could appear in the population under the influence of some stress factors. The meiotic mutations and/or apomictic propagation had maintained it as a triploid seed, and after the seed germination, mixoploidy was detected in the roots. Our observations are in agreement with early reported suggestions that the presence of mixoploid plants in D. antarctica populations is maintained by its capacity of vegetative and/or apomictic propagation [14; 52]. It has been shown that apomixis is accompanied with phenomena of poly-, aneu-, and mixoploidy in flowering plants [53, 54]. Also, a high level of aneuploidy has been found in plant species propagated asexually [55].
The karyotype variability revealed in the present study might be a manifestation of the cytogenetic abnormalities (mixoploidy, polyploidy, chromosome rearrangements and appearance of B chromosomes) which are especially common in plants growing under various environmental stresses [22, 24–25]. Environmental stress-induced inter and intra individual variability in chromosome number, aneuploidy and aneusomaty were early found in D. caespitosa [42, 44, 46]. Interestingly, in D. antarctica, which is the only Deschampsia species adapted to the harsh environment of the Antarctic, we observed almost the full range of karyotype variability found in the whole genus.
Although monochrome staining detected evident karyotype similarities in D. antarctica and D. caespitosa, detailed molecular cytogenetic analysis revealed structural differences between them. Particularly, Giemsa C-banding analysis showed a smaller amount of C heterochromatin in karyotypes of D. antarctica specimens compared to D. caespitosa [41]. The observed C-banding patterns in D. antarctica karyotype were more like the C-banding patterns of D. setacea (2n = 14) and D. fluxuosa (2n = 28) [41] as well as some diploid Avena L. species (2n = 14) [56] than in D. caespitosa (2n = 26) which mostly had large telomeric C-positive bands in the long arms of the chromosomes but lacked diffuse heterochromatin [41]. Although we found very few published studies on Giemsa C-banding analysis within the genus Deschampsia [41], and further investigation is needed, these observations indicate that genome reorganization involving repeated DNA sequences had occurred during the divergence of D. antarctica and its related species D. caespitosa.
Analysis of C-banding patterns in karyotypes of the examined specimens of D. antarctica from different islands revealed a low level of polymorphism between the studied populations in the number and size of intercalary, pericentromeric and telomeric C-bands as well as the bands adjacent to the secondary constriction regions of the SAT chromosomes. This may be due to the narrow distribution area of the species. Nevertheless, further detailed investigation of the intraspecific variation in C-banding patterns is needed in order to investigate the possible influence of the harsh environments of Antarctica's interior on C-heterochromatin content in this extremophile.
After FISH, that includes denaturation and renaturation of chromosome DNA, DAPI staining displays AT-rich heterochromatin stains as DAPI(+) bands and reveals Giemsa C-banding-like patterns (DAPI/C-banding patterns) in karyotypes of vascular plants [57]. DAPI/C-banding, together with localization of rDNA loci, provides a useful tool to detect chromosome variations among populations and closely related species [58–59]. Such approach allowed us to identify all the chromosome pairs in D. antarctica karyotypes and construct chromosome idiograms of the species using Giemsa C- and DAPI/C-banding patterns as well as 45S and 5S rDNA localization. The obtained results are necessary for karyotype comparisons and clarifying phylogenetic relationships within the genus Deschampsia.
FISH analysis revealed differences in chromosome localization of rDNA loci between karyotypes of D. antarctica and D. caespitosa. In D. antarctica, we detected 45S rDNA sites in two chromosome pairs. Only one of the SAT chromosome pairs possessed a well-defined secondary constriction, and this was probably the reason why monochrome staining had previously revealed only one SAT chromosome pair in D. antarctica karyotype [26]. In comparison, the karyotype of D. caespitosa was shown to comprise three SAT chromosome pairs bearing 45S rDNA [43]. The absence of one pair of SAT chromosomes with proximal 45S rDNA in D. antarctica karyotype could be due to chromosome rearrangements occurred during the speciation process. Using Ag-NOR staining specific to transcriptionally active NORs [60–61], we found that the NORs of both SAT chromosomes in D. antarctica were functionally active. However, the transcriptional activity of the NORs in D. caespitosa as well as in other Deschampsia species has not been studied yet, and further investigation of the NORs origin is required.
The number and chromosome localization of 5S rDNA sites in D. antarctica karyotype found in the present study, differed from the distribution of 5S rDNA in D. caespitosa described earlier. In D. caespitosa karyotype, 5S rDNA were localized in four chromosome pairs, one of which had 5S rDNA bands in both arms [43]. In D. antarctica, ten 5S rDNA loci were found on five chromosome pairs. Besides, 5S rDNA site was detected in one of the Bs found in karyotypes of D. antarctica specimens from Darboux Island. It was previously shown that B chromosomes detected in karyotypes of some Poaceae species contained ribosomal genes as well as pSc119.2 tandem repeats [24, 62–65]. In agreement with these observations, our results showed that in karyotypes of D. antarctica specimens from Darboux Island there were at least two types of B chromosomes which possessed distinct DAPI/C-positive bands and one of them contained 5S rDNA sequences.
Thus, the revealed differences in C-heterochromatin content and in numbers and distribution of rDNA sites between genomes of D. antarctica and D. caespitosa showed that genome reorganization involving coding and noncoding repeated DNA sequences occurred during the divergence of the species.
Vascular plants are known to be enriched with repetitive DNAs which may constitute up to 95% (in Allium cepa) of the total genomic DNA and play an important role in the speciation processes [66–68]. Knowledge of the distribution, genomic organization and chromosome localization of highly repeated DNA sequences in plants as well as in their ancestors provides information about their reorganization during evolution [69]. It should be noticed that phylogenetic position of the genus Deschampsia within the family Poaceae is still controversial. Based on classical morphological traits, the genus was considered to belong to the Avenea tribe [41, 70]. However, recent molecular genetic studies have shown alternative phylogenetic positions of Deschampsia (i.e., Aveneae or Poeae tribes) depending on the target sequences used for examination or the parameters used for grouping [15, 45, 71–73]. Accordingly, we performed FISH analysis of D. antarctica using several DNA probes specific for members of the family Poaceae (that includes both Aveneae and Poeae tribes).
Recently, microsatellite DNA sequences have become one of the most widely used molecular markers for genetic studies as they are major components of many plant genomes. The GAA-microsatellite sequence was found to distribute in genomes of different cereals [74], and FISH with the GAA microsatellite sequence as a probe produced banding patterns similar to those obtained by N-banding in chromosomes of some cereals [75]. In the present study, FISH analysis revealed dispersed hybridization sites of the GAA microsatellite along the chromosomes of D. antarctica, and no distinct clusters of the sequence, which could be used for chromosome identification, were detected.
The repetitive DNA sequence originally isolated from rye, pSc119.2, was found in the genomes of many species of the tribe Triticeae [35, 76–77]. Also, FISH analysis revealed dispersal distribution of the pSc119.2 probe along the chromosomes of Avena sativa [78]. The clone pAs1 (Afa-family repeat isolated from Ae. tauschii) is widely spread in the members of Hordeinae and Triticinae [34, 74, 79–80], but the presence of these repeats in genomes of the members of the Aveneae or Poeae has not been studied yet. According to our results, FISH analysis did not reveal distinct hybridization sites of both satellite DNA sequences pAs1 and pSc119.2 on chromosomes of D. antarctica.
The obtained data suggest that either the repeats related to pSc119.2 or pAs1 had been lost in D. antarctica genome or they had diverged significantly and, therefore, they can not be detected by FISH. Alternatively, they could appear in the phylogenetic lineages Hordeinae and Triticinae after their divergence from the lineages Aveneae and/or Poeae. For a better understanding of the phylogenetic position of the genus Deschampsia within the family Poaceae as well as clarifying the relationships within the genus, further molecular cytogenetic studies of Deschampsia species are required.
Conclusions
The karyotype variability found in D. antarctica specimens collected on four islands of the Maritime Antarctic is probably an expression of genome instability induced by environmental stress factors. The revealed intercalary site of telomere repeats confirms indirectly the hypothesis that chromosome fusion might have been the cause of the unusual for cereals chromosome number in this species. The differences in C-banding patterns and in chromosome distribution of rDNA loci as well as homologous highly repeated DNA sequences detected between genomes of D. antarctica and its related species D. caespitosa indicate that genome reorganization involving coding and noncoding repeated DNA sequences had occurred during the divergence of these species. The molecular cytogenetic analysis of chromosomes of D. antarctica performed in this study is important for further comparative cytogenetic studies of Deschampsia species and clarifying the relationships within the genus. Also, the obtained data provide a basis for genetic and biotechnological applications and would be useful for breeding cold tolerance in crops.
Acknowledgments
The authors acknowledge the support of the National Antarctic Scientific Center of the Ministry of Education and Science of Ukraine. The research was performed as part of the National targeted scientific and technological program of research in Antarctic for 2011–2020. We thank Dr. Prof. Polyschuk V.P. and Dr. Dykyy I.V. for collecting D. antartctica seeds. We also thank Dr. Surzhikov S.A. for providing us with the (GAA)n-oligonucleotide probe.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Russian Foundation of Basic Research [grants no. 15-04-05574; 14- 08-01167] and Fundamental Research Program of the Russian Academy of Sciences “Dynamics of Plant, Animal and Human Genofonds” [grant no. 0103-2015-0117]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moore DM. Chromosome numbers of Falkland Islands angiosperms. BAS Bulletin. 1967;14: 69–82. [Google Scholar]
- 2. Alberdi M, Bravo LA, Gutiérrez A, Gidekel M, Corcuera LJ. Ecophysiology of Antarctic vascular plants. Physiol Plant. 2002;115: 479–486. [DOI] [PubMed] [Google Scholar]
- 3. Bravo LA, Ulloa N, Zuniga GE, Casanova A, Corcuera LJ, Alberdi M. Cold resistance in Antarctic angiosperms. Physiol Plant. 2001;111: 55–65. [Google Scholar]
- 4. Bravo LA, Griffith M. Characterization of antifreeze activity in Antarctic plants. J Exp Bot. 2005;56: 1189–96. [DOI] [PubMed] [Google Scholar]
- 5. John UP, Polotnianka RM, Sivakumaran KA, Chew O, Macin L, Kuiper MJ, et al. Ice recrystallization inhibition proteins (IRIPs) and freeze tolerance in the cryophilic Antarctic hair grass Deschampsia antarctica E. Desv. Plant Cell Environ. 2009;32: 336–348. 10.1111/j.1365-3040.2008.01925.x [DOI] [PubMed] [Google Scholar]
- 6. Chew O, Lelean S, John UP, Spangenberg GC. Cold acclimation induces rapid and dynamic changes in freeze tolerance mechanisms in the cryophile Deschampsia antarctica E. Desv. Plant Cell Environ. 2012;35: 829–837. 10.1111/j.1365-3040.2011.02456.x [DOI] [PubMed] [Google Scholar]
- 7. Greene DM, Holtom A Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv.: III. Distribution, habitats and performance in the Antarctic botanical zone. BAS Bulletin. 1971;26: 1–29. [Google Scholar]
- 8. Xiong FS, Ruhland CT, Day TA. Photosynthetic temperature response of the Antarctic vascular plants Colobanthus quitensis and Deschampsia antarctica . Physiol Plant. 1999;106: 276–286. [Google Scholar]
- 9. Henry RJ. Genomics strategies for germplasm characterization and the development of climate resilient crops. Front Plant Sci. 2014;5: 68 10.3389/fpls.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. John UP, Spangenberg G. Xenogenomics: genomic bioprospecting in indigenous and exotic plants through EST discovery, cDNA microarray-based expression profiling and functional genomics. Comp Funct Genomics. 2005;6: 230–235. 10.1002/cfg.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee J, Noh EK, Choi HS, Shin SC, Park H, Lee H. Transcriptome sequencing of the Antarctic vascular plant Deschampsia antarctica Desv. under abiotic stress. Planta. 2013;237: 823–836. 10.1007/s00425-012-1797-5 [DOI] [PubMed] [Google Scholar]
- 12. Pereira BK, Rosa RM, da Silva J, Guecheva TN, Oliveira IM, Ianistcki M, et al. Protective effects of three extracts from Antarctic plants against ultraviolet radiation in several biological models. J Photochem Photobiol B. 2009;96: 117–129. 10.1016/j.jphotobiol.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 13. Ruhland CT, Xiong FS, Clark WD, Day TA. The influence of ultraviolet-B radiation on growth, hydroxycinnamic acids and flavonoids of Deschampsia antarctica during Springtime ozone depletion in Antarctica. Photochem Photobiol. 2005;81:1086–1093. [DOI] [PubMed] [Google Scholar]
- 14. Parnikoza I, Kozeretska I, Kunakh V. Vascular plants of the Maritime Antarctic: origin and adaptation. Am J Plant Sci. 2011;2: 381–395. [Google Scholar]
- 15. Lee J, Kang Y, Shin SC, Park H, Lee H. Combined analysis of the chloroplast genome and transcriptome of the Antarctic vascular plant Deschampsia antarctica Desv. PLoS ONE. 2014. March 19;9(3):e92501 10.1371/journal.pone.0092501 eCollection 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabert C, Gutiérrez-Moraga A, Navarrete A, Navarrete-Campos D, Bravo L, Gidekel M. Expression of a Deschampsia antarctica Desv. polypeptide with lipase activity in a Pichia pastoris vector. Int J Mol Sci. 2014;15: 2359–2367. 10.3390/ijms15022359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holderegger R, Stehlic I, Lewis RI, Smith Abbott RJ. Population of Antarctic hairgrass (Deschampsia antarctica) show low genetic diversity. Arct. Antarct. Alp. Res. 2003;35: 214–217. [Google Scholar]
- 18. Chwedorzewska K, Bednarek PT, Puchalski J. Molecular variation of Antarctic grass Deschampsia antarctica Desv. from King George Island (Antarctica). Acta Soc. Bot. Pol. Pol. Tow. Bot. 2004;73: 23–29. [Google Scholar]
- 19. Van der Wouw M, Van Dijk P, Huiskes ADHL. Regional Genetic Diversity Patterns in Antarctic Hair-grass (Deschampsia antarctica Desv.). J Biogeogr. 2007;35: 365–376. [Google Scholar]
- 20. Chwedorzewska KJ. Bednarek PT. Genetic variability in the Antarctic hairgrass Deschampsia antarctica Desv from Maritime Antarctic and subantarctic sites. Pol J Ecol. 2008;56: 209–216. [Google Scholar]
- 21. Volkov RA, Kozeretska IA, Kyryachenko SS, Andreev IO, Maidanyuk DN, Parnikoza IYu, et al. Molecular evolution and variability of ITS1-ITS2 in populations of Deschampsia antarctica from two regions of the Maritime Antarctic. Polar Sci. 2010;4: 469–478. [Google Scholar]
- 22. Madlung A, Comal L. The effect of stress on genome regulation and structure. Ann Bot. 2004;94: 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chinnusamy V, Zhu JK. Epigenetic regulation of stress response in plants. Curr Opin Plant Biol. 2009;12: 133–139. 10.1016/j.pbi.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belyayev A, Raskina O. Chromosome evolution in marginal populations of Aegilops speltoides: causes and consequences. Ann Bot. 2013;111: 531–538. 10.1093/aob/mct023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Houben A, Banaei-Moghaddam AM, Klemme S. Biology and evolution of B chromosomes In: Greilhuber J, Dolezel J, Wendel JF, editors. Plant Genome Diversity. Vienna: Springer; 2013. pp.149–165. [Google Scholar]
- 26. Cardone S, Sawatani P, Rush P, Garcнa A, Poggio L, Schrauf G. Karyological studies in Deschampsia antarctica Desv. (Poaceae). Polar Biol. 2009;32: 427–433. [Google Scholar]
- 27. Dobrochaeva DN, Kotov MI, Prokudin YuN, Barbarych AI. Manual of vascular plants of Ukraine 2nd ed. Kyiv: Fitosotsiotsentr; 1999. [Google Scholar]
- 28. Zagrichuk OM, Drobyk NM, Kozeretska IA, Parnikoza IU, Kunah VA. Introduction to culture in vitro Deschampsіa antarctіca Desv. (Poaceae) from two coastal areas of Antarctica. UAJ. 2011/2012;10–11: 289–295. [Google Scholar]
- 29. Gamborg OL, Eveleigh DE. Culture methods and detection of glucanases in suspension cultures of wheat and barley. Can J Biochem. 1968;46: 417–421. [DOI] [PubMed] [Google Scholar]
- 30. Yurkevich OY, Naumenko-Svetlova AA, Bolsheva NL, Samatadze TE, Rachinskaya OA, Kudryavtseva AV, et al. Investigation of genome polymorphism and seed coat anatomy of species of section Adenolinum from the genus Linum . Genet Resour Crop Evol. 2013;60: 661–676. [Google Scholar]
- 31. Muravenko OV, Samatadze TE, Popov KV, Amosova AV, Zelenin AV. Comparative study of genomes of two species of flax by C-banding of chromosomes. Russ J Genet. 2001;37: 332–335. [PubMed] [Google Scholar]
- 32. Gerlach WL, Bedbrook JR. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979;7: 1869–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gerlach WL, Dyer TA. Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Res. 1980;8: 4851–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rayburn AL, Gill BS. Molecular identification of the D-genome chromosomes of wheat. J Hered. 198677: 253–255. [Google Scholar]
- 35. Bedbrook JR, Jones J, O'Dell M, Thompson R, Flavell RB. A molecular description of telomeric heterochromatin in Secale species. Cell. 1980;19: 545–560. [DOI] [PubMed] [Google Scholar]
- 36. Cox AV, Bennett ST, Parokonny AS, Kenton A, Callimassia MA, Bennett MD. Comparison of plant telomere locations using a PCR-generated synthetic probe. Ann Bot. 1993;72: 239–247. [Google Scholar]
- 37. Muravenko OV, Yurkevich OY, Bolsheva NL, Samatadze TE, Nosova IV, Zelenina DA, et al. Comparison of genomes of eight species of sections Linum and Adenolinum from the genus Linum based on chromosome banding, molecular markers and RAPD analysis. Genetica. 2009;135: 245–255. 10.1007/s10709-008-9273-7 [DOI] [PubMed] [Google Scholar]
- 38. Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19: 11–15. [Google Scholar]
- 39. Howell WM, Black DA. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980;36: 1014–1015. [DOI] [PubMed] [Google Scholar]
- 40. Levan A, Fredga K, Sandberg A. Nomenclature for centromeric position on chromosomes. Hereditas. 1964;52: 201–222. [Google Scholar]
- 41. Garsia-Suarez R, Alonso-Blanco C, Fernandez-Carvajal MC, Fernandez-Prieto JA, Roca F, Giraldez R. Diversity and systematics of Deschampsia sensu lato (Poaceae), inferred from karyotypes, protein electrophoresis, total genomic DNA hybridization and chloroplast DNA analysis. Plant Syst Evol. 1997;205: 99–110. [Google Scholar]
- 42. Kawano S. Cytogeography and evolution of the Deschampsia caespitosa complex. Can J Bot. 1963;41:719–742. [Google Scholar]
- 43. Winterfeld G, Roser M. Disposition of ribosomal DNAs in the chromosomes of perennial oats (Poaceae: Aveneae). Bot J Linn Soc. 2007;155: 193–210. [Google Scholar]
- 44. Nkongolo KK, Deck A, Michael P. Molecular and cytological analyses of Deschampsia caespitosa populations from Northern Ontario (Canada). Genome. 2001;44: 818–825. [DOI] [PubMed] [Google Scholar]
- 45. Souto DPF, Catalano SA, Tosto D, Bernasconi P, Sala A, Wagner M, et al. Phylogenetic relationships of Deschampsia antarctica (Poaceae): Insights from nuclear ribosomal ITS. Plant Syst Evol. 2006;261: 1–9. [Google Scholar]
- 46. Chiapella J. A molecular phylogenetic study of Deschampsia (Poaceae: Aveneae) inferred from nuclear ITS and plastid trnL sequence data: support for the recognition of Avenella and Vahlodea . Taxon. 2007;56: 55–64. [Google Scholar]
- 47. Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E. Telomeric repeats far from the ends: Mechanisms of origin and role in evolution. Cytogenet Genome Res. 2008;122: 219–228. 10.1159/000167807 [DOI] [PubMed] [Google Scholar]
- 48. White MJD. The chromosomes 6 th ed. London: Chapman & Hall Press; 1973. [Google Scholar]
- 49. Manoj K, Friebe B, Koul AK, Gill BS. Origin of an apparent B chromosome by mutation, chromosome fragmentation and specific DNA sequence amplification. Chromosoma. 2002;111: 332–340. [DOI] [PubMed] [Google Scholar]
- 50. Camacho JP, Sharbel TF, Beukeboom LW. B chromosome evolution. Philos Trans R Soc Lond B Biol Sci. 2000;355: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Butorina AK, Bogdanova EV. Adaptive significance and possible origin of B-chromosomes in Picea Glauca (moench.) voss = P.canadensis B.S.P. Tsitologiia. 2001;43: 809–814. [PubMed] [Google Scholar]
- 52. Kravets AA, Taran NY, Storozhenko VA. Plasticity of morphogenesis and features of reproduction plants Colobanthus quitensis and Deschampsia antarctica in Antarctic region. UAJ. 2011/2012;10–11: 302–305. [Google Scholar]
- 53. Yudanova SS. Mixoploidy and apozygoty in sugar beet. Sugar Tech. 2003;5: 173–175. [Google Scholar]
- 54. Kashin AS. Genesis of cells of apical meristems and realization of gametophytic apomixis in flowering plants. Ontogenez. 2012;43: 121–135. [PubMed] [Google Scholar]
- 55. Kozyrenko MM, Artyukova EV, Lauve LS, Boltenkov EV. Genetic variability of callus cultures of some Iris species. Biotekhnologiya. 2002;4: 38–48. [Google Scholar]
- 56. Badaeva ED, Shelukhina OY, Diederichsen A, Loskutov IG, Pukhalskiy VA. Comparative cytogenetic analysis of Avena macrostachya and diploid C-genome Avena species. Genome. 2010;53:125–37. 10.1139/g09-089 [DOI] [PubMed] [Google Scholar]
- 57. Barros e Silva AE, Guerra M. The meaning of DAPI bands observed after C-banding and FISH procedures. Biotech Histochem. 2010;85: 115–125. 10.1080/10520290903149596 [DOI] [PubMed] [Google Scholar]
- 58. Lavania UC, Kushwaha J, Lavania S, Basu S. Chromosomal localization of rDNA and DAPI bands in solanaceous medicinal plant Hyoscyamus niger L. J Genet. 2010;89: 493–496. [DOI] [PubMed] [Google Scholar]
- 59. Gaiero P, Mazzella C, Vaio M, Barros e Silva AE, Santiñaque FF, López-Carro B, Folle GA, Guerra M. An unusually high heterochromatin content and large genome size in the palm tree Trithrinax campestris (Arecaceae). Austral. J. Bot. 2012;60: 378–382. [Google Scholar]
- 60. Miller DA, Dev VG, Tantravahi R, Miller OJ. Suppression of human nucleolus organizer activity in mouse-human somatic hybrid cell. Exp Cell Res. 1976;101: 235–243. [DOI] [PubMed] [Google Scholar]
- 61. Miller DA, Tantravahi R, Dev VG, Miller OJ. Frequency of satellite association of human chromosomes is correlated with amount of Ag-staining of the nucleolus organizer region. Am J Hum Genet. 1977;29: 390–502. [PMC free article] [PubMed] [Google Scholar]
- 62. Friebe B, Jiang J, Gill B. Detection of 5S rDNA and other repeated DNA on supernumerary B-chromosomes of Triticum species (Poaceae). Plant Syst Evol. 1995;196: 131–139. [Google Scholar]
- 63. Muravenko OV, Bolsheva NL, Yurkevich OYu, Nosova IV, Rachinskaya OA, Samatadze TE, et al. Karyogenomics of species of the genus Linum L. Russ J Genet. 2010;46: 1339–1342. [PubMed] [Google Scholar]
- 64. Ruban A, Fuchs J, Marques A, Schubert V, Soloviev A, Raskina O, et al. B chromosomes of Aegilops speltoides are enriched in organelle genome-derived sequences. PLoS One. 2014. February 26;9(2): e90214 10.1371/journal.pone.0090214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bolsheva NL, Zelenin AV, Nosova IV, Amosova AV, Samatadze TE, Yurkevich OYu, et al. The diversity of karyotypes and genomes within section Syllinum of the genus Linum (Linaceae) revealed by molecular cytogenetic markers and RAPD analysis. PLoS One. 2015. April 2;10(4):e0122015 10.1371/journal.pone.0122015 eCollection 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Flavell RB, Bennett MD, Smith JB, Smith DB. Genome size and the proportion of repeated nucleotide sequence DNA in plants. Biochem Genet. 1974;12: 257–269. [DOI] [PubMed] [Google Scholar]
- 67. Bennett MD. Plant genome values: How much do we know? Proc Natl Acad Sci U S A. 1998;95: 2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mehrotra S, Goyal V. Repetitive sequences in plant nuclear DNA: types, distribution, evolution and function. Genomics Proteomics Bioinformatics. 2014;12: 164–171. 10.1016/j.gpb.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Flavell RB, O'Dell M, Hutchinson J. Nucleotide sequence organization in plant chromosomes and evidence for sequence translocation during evolution. Cold Spring Harb Symp Quant Biol. 1981;45: 501–508.* [DOI] [PubMed] [Google Scholar]
- 70. Moore DM. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarcica Desv. II. Taxonomy, distribution and relationships. BAS Bulletin. 1970;23: 633–680. [Google Scholar]
- 71. Saarela JM, Liu Q, Peterson PM, Soreng RJ, Paszko B. Phylogenetics of the grass ‘Aveneae-type plastid DNA clade’ In: Seberg O, Petersen G, Barfod AS, Davis JI, editors. Diversity, phylogeny, and evolution in the monocotyledons. Aarhus: Aarhus University Press; 2010. pp. 557–588. [Google Scholar]
- 72. Rodionov AV, Kotseruba VV, Kim ES, Punina EO, Nosov NN. Grass genome and chromosome sets evolution. Tsitologiia. 2013;55: 225–229. [PubMed] [Google Scholar]
- 73. Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, et al. A worldwide phylogenetic classification of the Poaceae (Gramineae). J Syst Evol. 2015;53: 117–137. [Google Scholar]
- 74. Cabral AL, Karaoglu H, Park RF. The use of microsatellite polymorphisms to characterize and compare genetic variability in Avena strigosa and A.barbata . Genet Resour Crop Evol. 2012;60: 1153–1163. [Google Scholar]
- 75. Pedersen C, Rasmussen SK, Linde-Laursen I. Genome and chromosome identification in cultivated barley and related species of the Triticeae (Poaceae) by in situ hybridization with the GAA-satellite sequence. Genome. 1996;39: 93–104. [DOI] [PubMed] [Google Scholar]
- 76. Badaeva ED, Amosova AV, Samatadze TE, Zoshchuk SA, Shostak NG, Chikida NN, et al. Genome differentiation in Aegilops. 4. Evolution of the U-genome cluster. Plant Syst Evol. 2004;246: 45–76. [Google Scholar]
- 77. Contento A, Heslop-Harrison JS, Schwarzacher T. Diversity of a major repetitive DNA sequence in diploid and polyploid Triticeae . Cytogenet Genome Res. 2005;109: 34–42. [DOI] [PubMed] [Google Scholar]
- 78. Katsiotis A, Hagidimitriou M, Heslop-Harrison JS. The close relationship between the A and B genomes in Avena L. (Poaceae) determined by molecular cytogenetic analysis of total genomic, tandemly and dispersed repetitive DNA sequences. Ann Bot. 1997;79: 103–109. [Google Scholar]
- 79. Cabrera A, Friebe B, Jiang J, Gill BS. Characterization of Hordeum chilense chromosomes by C-banding and in situ hybridization using highly repeated DNA probes. Genome. 1995;38: 435–442. [DOI] [PubMed] [Google Scholar]
- 80. Nagaki K., Tsujimoto H, Sasakuma T. Dynamics of tandem repetitive Afa-family sequences in Triticeae, wheat-related species. J Mol Evol. 1998;47: 183–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.