Abstract
Background
Whole-genome RNA interference post-transcriptional silencing (RNAi) is a widely used method for studying the phenotypic effects of knocking down individual genes. In this study, we use a population genomic approach to characterize the rate of evolution for proteins affecting 26 RNAi knockdown phenotypes in Drosophila melanogaster.
Results
We find that only two of the 26 RNAi knockdown phenotypes are enriched for rapidly evolving proteins: innate immunity and regulation of Hedgehog signaling. Among all genes associated with an RNAi knockdown phenotype, we note examples in which the adaptively evolving proteins play a well-defined role in a given molecular pathway. However, most adaptively evolving proteins are found to perform more general cellular functions. When RNAi phenotypes are grouped into categories according to cellular function, we find that genes involved in the greatest number of phenotypic categories are also significantly more likely to have a history of rapid protein evolution.
Conclusions
We show that genes that have been demonstrated to have a measurable effect on multiple molecular phenotypes show higher rates of protein evolution than genes having an effect on a single category of phenotype. Defining pleiotropy in this way yields very different results than previous studies that define pleiotropy by the number of physical interactions, which show highly connected proteins tend to evolve more slowly than lowly connected proteins. We suggest that a high degree of pleiotropy may increase the likelihood of compensatory substitution, consistent with modern theoretical work on adaptation.
Electronic supplementary material
The online version of this article (doi:10.1186/s12862-015-0472-4) contains supplementary material, which is available to authorized users.
Keywords: Adaptation, Drosophila melanogaster, Pleiotropy, RNA interference
Background
The visibility of a protein to natural selection depends upon the phenotypic consequences of mutations to its regulatory and structural sequences. For most proteins, the phenotypic consequences of mutations first manifest at the cellular level, specifically with respect to the protein’s ability to participate in a suite of molecular interactions. This context proximally determines both the level of sequence constraint and how often a protein produces evolutionary adaptations. For over forty years, biologists have endeavored to identify variables that predict the rate of protein evolution [1, 2]. Proteome-level statistical analyses generally find that expression pattern, breadth of interactions, and the genomic context of coding sequences are all correlated with the rate of protein evolution [3]. Even the position of proteins in molecular interaction pathways (upstream or downstream) accounts for some variance in evolutionary rate [4]. It is also widely appreciated that molecular pathways involved in immunity or genome defense are often enriched for adaptively evolving proteins [5, 6]. As functional and genomic data continue to accumulate, the tools are now available to address in detail whether certain categories of pathways are more or less impacted by natural selection.
The targeted knockdown of individual genes with short interfering RNA molecules (RNAi) is routinely used to assay the relative effect of proteins on a measurable phenotype of interest [7]. While the phenotypic effects of gene knockdown are not necessarily representative of the effects of all possible point mutations [8], they are indicative of the relative importance of the protein in different molecular pathways. This study presents an evolutionary analysis of proteins found to have significant knockdown effects in 26 whole-genome RNAi experiments in Drosophila melanogaster. We ask whether groups of genes affecting a given phenotype are preferentially subject to positive natural selection, relative to a random sample from the genome. Furthermore, we identify which of these genes are most impacted by recurrent positive selection. The results indicate that both immunity and cell signaling pathways are enriched for rapidly evolving proteins and that proteins with wider pleiotropic effects are more rapidly evolving than proteins that affect a narrower range of phenotypes.
Results and discussion
Natural selection across pathways
Each RNAi experiment k yields a set of nk genes that, upon knockdown, cause a significant measurable change to the phenotype. The threshold for statistical significance of the magnitude of change is standardized across studies. Then, using population genomic data from D. melanogaster and two outgroup genomes, for each phenotype, we estimate the direction of selection statistic (DoS), which is defined as the difference between the proportion of substitutions and polymorphisms that are nonsynonymous. Under strictly neutral evolution, DoS is expected to be zero, and it is positive when the proportion of substitutions that are nonsynonymous is higher than the proportion of polymorphisms that are nonsynonymous, indicative of positive natural selection. Alternatively, DoS is negative when the proportion of polymorphisms that are nonsynonymous is higher than the proportion of substitutions that are nonsynonymous, suggestive of weak negative selection [9]. For each set of nk genes influencing a phenotype, we determine the average DoS for all genes associated with that phenotype, and using a two-tailed randomization test, we further determine whether an RNAi phenotype is enriched for genes subject to positive natural selection, compared to a random sample from the genome. We note that this test is designed to detect lineage-specific recurrent natural selection.
The average number of genes significantly influencing a single RNAi knockdown phenotype is 40 and the number ranges from only five for the “cell size regulation” phenotype to 113 for the “RTK-Ras-ERK signaling decrease” phenotype (Table 1). We find two RNAi knockdown phenotypes are affected by groups of proteins with a significantly elevated mean proportion of amino acid substitutions. One of these phenotypes involves “innate immunity”: 10 out of 13 genes involved in this RNAi knockdown phenotype have positive DoS values ranging from 0.015–0.333. Of these, four genes imd, Dredd, ird5 and Relish act upstream in the immune deficiency (IMD) pathway, which activates the NF- κB cascade to produce antimicrobial peptides as a defense response against microbial pathogens [10]. The other phenotype with genes showing a significantly elevated mean proportion of amino acid substitutions is the “hedgehog signaling decrease” phenotype. Hedgehog (Hh) signaling is a conserved cell-signaling pathway in animals, which mediates embryonic development and tissue homeostasis [11]. Of the 25 genes that have positive DoS values in the Hh signaling decrease phenotype, only two genes, Cubitus interruptus (Ci) and Fused (fu), play a specific well-characterized role in the Hh signaling complex, in particular, both act upstream in the signaling cascade. The Ci protein is a zinc finger domain encoding transcription factor, which controls the transcription of Hh target genes [12]. The Fused protein is a kinase, which forms a protein complex with Ci, and another upstream Hh pathway protein Smoothened (Smo) to regulate the downstream Hh target genes [13]. It is interesting to note that in both phenotypes, we find genes with positive DoS values play crucial upstream roles. This finding is in agreement with protein interaction data from Saccharomyces cerevisiae, and D. melanogaster, in which the upstream genes in molecular networks tend to show signatures of rapid evolution, while the downstream genes tend to be more conserved [4, 14]. Of the 26 RNAi knockdown phenotypes, 16 are related to either intracellular signal transduction or involved in cell surface receptor signaling. If rapidly evolving genes randomly associate with RNAi phenotypes, it is not unexpected to observe 1 of 16 cell signaling pathways being enriched, however previous studies of protein evolution in D. melanogaster, that rely on gene ontology annotation, do not identify any cell signaling pathway as enriched for rapidly evolving genes [15].
Table 1.
The 26 RNAi knockdown phenotypes surveyed in this study. Bolded lines indicate phenotypes that are enriched for proteins that significantly deviate from the genome average in their direction of selection (DoS) statistic
| Phenotype category | RNAi knockdown phenotype | n a | DoS |
|---|---|---|---|
| Regulation of intracellular signal transduction | Akt-TOR signaling decrease | 24 | –0.019 |
| Akt-TOR signaling increase | 22 | –0.118 | |
| Hippo signaling decrease | 99 | –0.005 | |
| Hippo signaling increase | 54 | –0.021 | |
| JAK/STAT signaling decrease | 10 | 0.044 | |
| JAK/STAT signaling increase | 6 | –0.075 | |
| RTK-Ras-ERK signaling decrease | 113 | –0.045 | |
| RTK-Ras-ERK signaling increase | 55 | –0.018 | |
| Cell surface receptor signaling pathway | Hedgehog signaling decrease | 48 | 0.043 b |
| Hedgehog signaling increase | 30 | 0.006 | |
| Notch signaling decrease | 16 | –0.018 | |
| Notch signaling increase | 18 | –0.107 | |
| Toll signaling decrease | 17 | 0.028 | |
| Toll signaling increase | 11 | 0.005 | |
| Wnt signaling activity | 90 | –0.043 | |
| Regulation of transposon integration | Blood TE activity increase | 43 | 0.011 |
| Burdock TE activity increase | 15 | –0.080 | |
| HeTA TE activity increase | 38 | 0.024 | |
| TAHRE TE activity increase | 41 | –0.002 | |
| Innate immune response | Influenza replication decrease | 18 | –0.047 |
| Innate immunity | 13 | 0.097 b | |
| M. fortuitum infection decrease | 27 | –0.111 | |
| Regulation of extent of cell growth | Cell size regulation | 5 | 0.012 |
| Cell growth and viability | 96 | –0.014 | |
| Regulation of circadian rhythm | CRY degradation | 93 | 0.006 |
| Hypoxia-inducible factor-1alpha signaling pathway | Hypoxia induced transcription | 49 | –0.025 |
| N/A | Lethals | 2853 | –0.031 |
| N/A | Whole-genome | 11148 | –0.035 |
aNumber of genes significantly affecting the RNAi knockdown phenotype
bValue is significantly greater than a random sample from the genome
The role of positively selected proteins
Across all 26 RNAi knockdown phenotypes, there are a total of 11 genes encoding proteins with significantly elevated numbers of adaptive amino acid substitutions, identified from the McDonald-Kreitman (MK) test (Table 2). Several of these proteins are considered “conserved” components of cell signaling pathways. For example, Sik3 encodes a kinase in the core Hippo pathway [16] that functions as a negative modulator of Hippo signaling. Additionally, other proteins that specifically affect Hippo pathway activity also experience recurrent positive selection, as is the case for the H3-K36 methyltransferase gene Set2, which has previously been characterized as a member of an evolutionarily conserved family of histone lysine methylation enzymes [17]. All metazoans share a common set of cell signaling pathways [18], however the degree to which constituent proteins diverge in structure, copy number, or expression pattern varies across pathways [19]. Many signaling pathways are often characterized as “conserved”, not because individual protein sequences are constrained by natural selection, but because protein homologs occupy identical pathway positions across taxa and thus, presumably, perform similar functions. These results illustrate that while signaling pathways components may be “conserved”, that does not necessarily mean the protein sequences cease to produce adaptive mutations [20]. There are notable examples of natural selection co-opting developmental signaling pathways to produce evolutionary novelties and adaptations, however these usually involve changes to the pattern of expression, not structural mutations [21–23].
Table 2.
The 11 genes experiencing recurrent positive natural selection
| Gene | n c | D N | D S | P N | P S | P FET | Phenotype(s) |
|---|---|---|---|---|---|---|---|
| nonC | 1852 | 31 | 39 | 8 | 53 | 0.000076 | Hippo decrease |
| Hedgehog increase | |||||||
| Set2 | 1408 | 27 | 37 | 17 | 73 | 0.001521 | Hedgehog increase |
| Nup153 | 1294 | 49 | 49 | 8 | 30 | 0.001623 | Hedgehog decrease |
| RTK-Ras-ERK signaling decrease | |||||||
| RTK-Ras-ERK signaling increase | |||||||
| Kib | 1076 | 5 | 26 | 0 | 62 | 0.003269 | Decreased cell viability |
| pcm | 1072 | 29 | 28 | 4 | 19 | 0.005061 | Blood TE activity |
| Cnot4 | 860 | 20 | 20 | 9 | 34 | 0.005227 | Hippo decrease |
| ZC3H3 | 414 | 9 | 8 | 6 | 31 | 0.007610 | Hippo increase |
| RTK-Ras-ERK signaling decrease | |||||||
| Nup205 | 781 | 11 | 21 | 2 | 28 | 0.007613 | Wnt increase |
| Dref | 554 | 6 | 20 | 0 | 29 | 0.007942 | Hedgehog increase |
| RTK-Ras-ERK signaling decrease | |||||||
| Sik3 | 571 | 6 | 7 | 4 | 37 | 0.008072 | Hippo decrease |
| RasGAP1 | 979 | 6 | 26 | 1 | 54 | 0.009106 | Innate immunity |
| RTK-Ras-ERK signaling increase |
For each gene, the number of codons analyzed (n c), the number of nonsynonymous (D N) and synonymous (D S) substitutions, the number of nonsynonymous (P N) and synonymous (P S) polymorphisms are given along with the P-value for Fisher’s exact test (P FET) and the RNAi knockdown phenotypes affected by the gene
Although a handful of adaptively evolving proteins in signaling pathways are exclusive to just one phenotype, many proteins also play a role in multiple cell signaling pathways. For example, the RasGap1 and Dref genes encode proteins with a history of recurrent positive selection and are involved in multiple signaling pathway phenotypes (Table 2). Both RasGap1 and Dref play a role in Ras-mediated signal transduction [24, 25], which activates multiple downstream signaling pathways. Other positively selected proteins influencing cell signaling activity perform more general cellular functions. For instance, two nucleoporin genes (Nup153, Nup205) are both positively selected. While Nup153 is involved in multiple signaling RNAi knockdown phenotypes, Nup205 is identified as significantly influencing the Wnt signaling pathway. Nucleoporin genes encode components of the nuclear pore complex and therefore play a very general role in nuclear transport; these genes have previously been shown to be adaptively evolving in D. melanogaster [26]. Interestingly, one of the knockdown phenotypes influenced by Nup153 is also influenced by the positively selected CCCH-type zinc finger gene ZC3H3; ZC3H3 encodes a necessary component linking mRNA polyadenylation with nuclear export [27]. Both groups of proteins are known to interact with viral proteins [28, 29], which may be a potential source of selective pressure.
In addition to cell signaling pathways, our analysis identifies a new candidate for positively selected proteins in the piRNA pathway. The piRNA pathway generates small RNAs that suppress transposable element (TE) activity in the germline [30]. The piRNA effector proteins Mael, Armi, Aub, and Spn-E have been previously shown to experience positive natural selection in the Drosophila phylogeny [6]. Our analysis identifies a gene pcm, which both affects TE activity and shows an increased rate of adaptive amino acid substitutions in the D. melanogaster lineage (Table 2). pcm encodes a 5′−3′ exoribonuclease that has been previously characterized as having significant sequence conservation between Drosophila, mouse, and Saccharomyces [31]. The Pcm protein is recruited by protein complexes involved in both non-sense mediated mRNA decay (NMD) and RNA interference to degrade targeted mRNAs in cytoplasmic P-bodies [32].
The effects of pleiotropy
Among the 11 genes that we infer to be subject to recurrent positive natural selection, four genes are also associated with multiple categories of RNAi knockdown phenotype (Table 2). Given that only seven of the 723 genes associated with a single category of RNAi knockdown phenotype show a history of adaptive evolution, observing four adaptively evolving genes involved in multiple categories is too many to occur by chance alone (PFET=0.0397). To explore the hypothesis that the number of RNAi phenotype categories is associated with the rate of amino acid substitution, we again use the direction of selection statistic (DoS). In total, there are 723 genes associated with a single category of RNAi knockdown phenotype, 85 genes involved with two categories, and 20 genes involved in three categories of phenotype. The mean DoS for genes associated with a single phenotypic category is −0.0302, while the mean for genes associated with two categories is −0.0248, and the mean for genes associated with three categories is 0.0936 (Fig. 1). The genes involved in three categories of RNAi knockdown phenotypes have significantly higher mean DoS than genes involved in either a single category (Mann-Whitney U test, PMWU=0.0058) or genes involved in two categories (PMWU=0.0382). Conversely, genes involved in one and two categories of RNAi knockdown phenotype do not have significantly different mean values of DoS (PMWU=0.7038). We note that this result is inconsistent with previous studies of yeast two-hybrid protein-protein interactions showing that highly interacting proteins tend to evolve more slowly [33], although more recent results are consistent with our finding [34]. This inconsistency may reflect the fact that yeast two-hybrid studies measure physical interactions among proteins, but not necessarily the number of biological processes influenced by a protein. We propose that our approach is a more accurate measure of a protein’s pleiotropic effects than are physical interaction data. Lastly, this result is not an artifact of longer genes having greater power to reject neutrality [35], since we observe no relationship between number of codons and degree of pleiotropy (r2=0.0001; P=0.926).
Fig. 1.
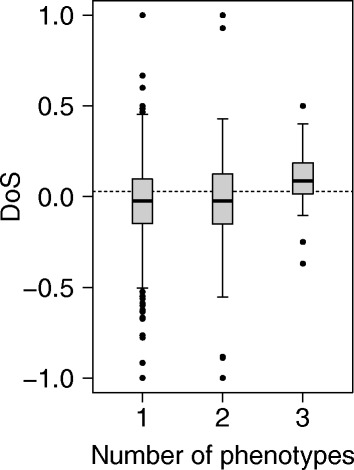
The number of phenotypic categories and the rate of protein evolution. The number of phenotypes for individual proteins is measured by the number of distinct categories of RNAi phenotypes that are significantly affected by knockdown of the corresponding gene. The box plots show the distribution of direction of selection (DoS) values for three different categories: genes that significantly affect one, two, and three different phenotypes upon RNAi knockdown. A positive DoS value indicate an excess of the proportion of substitutions in D. melanogaster that are nonsynonymous substitutions relative to the proportion of polymorphisms that are nonsynonymous. The mean DoS for genes involved in three categories of phenotype is significantly more positive compared to genes involved in either one or two phenotypic categories
We consider the number of distinct categories of RNAi knockdown phenotype as an indicator of the degree of protein pleiotropy. We find that the products of 20 genes have significant effects on three distinct categories of RNAi knockdown phenotype. We will refer to this group of genes as “highly pleiotropic”. Table 3 shows the combination of RNAi knockdown phenotypes, the number of nonsynonymous and synonymous substitutions and polymorphisms, as well as the DoS statistic for each of the highly pleiotropic genes. The most common set of phenotypic categories is “regulation of extent of cell growth”, coupled with “regulation of intracellular signal transduction” and “cell surface receptor signaling pathway”. Three of the eight genes associated with this combination of phenotypic categories encode ribosomal proteins (RpL22, RpL7, and RpS13). Across all twenty genes, there is no significant enrichment for gene ontology molecular function, the most enriched category is transcription factor binding (u-shaped, kayak, nejire, and Jra; P=0.090). Furthermore, of the 20 genes that significantly affect three different RNAi knockdown phenotypes, seven genes have opposite effects in at least two phenotypes upon knockdown. For example, Sos is a Ras-like guanine nucleotide exchange factor, which has a negative effect on the RTK-ras-ERK signaling pathway, whereas has a positive effect on the Notch signaling pathway upon knockdown (Fig. 2). Both in C. elegans and in D. melanogaster, Notch negatively regulates Ras pathway activation indicating antagonistic relationship between the Notch and the Ras signaling pathways [36]. Similarly, u-shaped, E(Pc), and zeste have opposite effects in two different phenotypes upon RNAi knockdown. u-shaped is a Zinc-finger domain containing transcription factor, which upon knockdown, significantly increases the activity of the Ras signaling pathway, whereas has a negative effect on the immune deficiency (IMD) pathway that mediates innate immunity. The protein components of the Ras pathway are known to act as suppressors of the IMD pathway, even in the absence of immune challenges, indicating antagonistic relationship between the Ras and the IMD pathways [37]. Finally, two genes, zeste and E(Pc) have the same opposite effects for the Hippo pathway and the Wnt signaling pathway upon RNAi knockdown. Both zeste and E(Pc) increase the Wnt signaling activity, but influence Hippo signaling negatively upon knockdown. Similar to previous examples of antagonism between cellular pathways, the hippo pathway components are known to negatively regulate Wnt signaling genes [38]. While there are seven genes that have opposite effects in two pathways upon RNAi knockdown, 13 “highly pleiotropic” genes have similar effects on more than two phenotypes (Fig. 2). A majority of these genes (7 out of 13) significantly decrease Ras signaling, and Hedgehog signaling pathways upon knockdown. This result is consistent with the finding that both Ras and Hedgehog signaling pathways function cooperatively in cells [39, 40].
Table 3.
Direction of selection (DoS) statistic for 20 genes involved in three different categories of RNAi knockdown phenotype
| Gene | Phenotype categoriesa | D N | D S | P N | P S | DoS |
|---|---|---|---|---|---|---|
| u-shaped | CR-IM-SR | 9 | 25 | 5 | 34 | 0.137 |
| kayak | SR-IM-IS | 5 | 11 | 5 | 26 | 0.151 |
| α COP | SR-IM-IS | 2 | 16 | 5 | 67 | 0.042 |
| E(Pc) | SR-IM-IS | 14 | 44 | 9 | 45 | 0.075 |
| Rpn6 | SR-CR-IS | 0 | 6 | 1 | 15 | –0.063 |
| CG30053 | SR-CR-IS | 3 | 10 | 12 | 8 | –0.369 |
| nejire | GR-IS-TE | 16 | 79 | 9 | 80 | 0.067 |
| Tak1 | GR-IM-IS | 6 | 13 | 3 | 9 | 0.066 |
| eIF-4a | GR-IM-IS | 14 | 44 | 9 | 45 | 0.273 |
| Rpt3 | GR-CR-IS | 2 | 7 | 0 | 16 | 0.222 |
| Jra | GR-CR-IS | 6 | 6 | 0 | 4 | 0.500 |
| CG12054 | GR-SR-IS | 0 | 10 | 4 | 12 | –0.250 |
| RpL22 | GR-SR-IS | 1 | 6 | 0 | 3 | 0.143 |
| RpL7 | GR-SR-IS | 3 | 6 | 0 | 8 | 0.333 |
| RpS13 | GR-SR-IS | 2 | 3 | 0 | 1 | 0.400 |
| Sos | GR-SR-IS | 1 | 31 | 2 | 49 | –0.008 |
| zeste | GR-SR-IS | 3 | 22 | 0 | 14 | 0.120 |
| CG2807 | GR-SR-IS | 1 | 27 | 0 | 60 | 0.036 |
| CycT | GR-SR-IS | 5 | 29 | 5 | 15 | –0.103 |
| Rpn12 | GR-SR-CR | 1 | 9 | 0 | 1 | 0.100 |
aThe two letter abbreviation codes for the phenotypic categories are: CR: Regulation of circadian rhythm, SR: Cell surface receptor signaling pathway, IS: Regulation of intracellular signal transduction, GR: Regulation of extent of cell growth, TE: Regulation of transposon integration, IM: Innate immune response
Fig. 2.
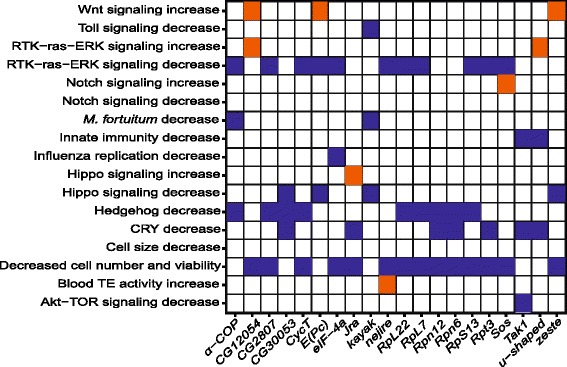
Cooperative or antagonistic pleiotropy for genes involved in three RNAi phenotypes. For the twenty highly pleiotropic genes, the tile plot shows the direction that gene knockdown has on a given phenotype. The blue tile shows negative effect on a phenotype upon knockdown; and the orange tile shows positive effect upon RNAi knockdown
Conclusions
Drosophila melanogaster represents one of most mature and powerful systems in genetics and functional genomics and is widely used as a model for studying the genetic basis of human disease [41, 42]. In particular, studies of D. melanogaster have led to significant advances in basic developmental, neurological, and immunological genetics. It is often stated that D. melanogaster is an appropriate genetic model because more than 60 % of the genes found in the D. melanogaster have human homologs [42] and that genes involved in key developmental pathways are “conserved” and functionally orthologous between humans and flies [41]. For D. melanogaster to be a viable human disease model, it is important to first understand the phenotypic effects of lineage-specific adaptations. While our results recapitulate the well-known conclusion that proteins affecting immunity and genome defense pathways are more likely to fix adaptive mutations, we also find that proteins affecting a suite of cell signaling pathways that are important for metazoan development are also fixing adaptive mutations in the D. melanogaster lineage at a significantly higher rate than the genome average. Our meta-analytical approach is conservative, such that we seek to minimize type I error in a potentially noisy data set. Less stringent criteria for statistical significance may, in fact, yield a different set of conclusions. However, our stringency adds to our confidence that the results do reflect the underlying biological realities concerning the molecular phenotypic effects of adaptive protein evolution.
In general, we refrain from speculating on the nature of the selective pressures driving the inferred adaptive evolution. However, it is important to note that the traditional MK framework used here is designed to detect recurrent bouts of adaptive evolution. One common explanation for recurrent positive selection is conflict due to an ongoing “arms race” between a host genome and either exogenous factors, such as pathogens [43], or endogenous selfish genetic elements, such as TEs or meiotic drive loci [44]. An “arms race” scenario would certainly apply to proteins involved in immunity or genome defense, as well as to proteins with general functions that interact with exogenous protein [45], such as is the case with the nucleoporins. Another potential source of recurrent positive selection is compensatory evolution [46]. Compensatory substitutions may resolve any antagonistic effects on fitness caused by an initial adaptive substitution. For instance, if strong positive selection fixes a mutation based on one aspect of the protein’s function, but that mutation also has lesser, deleterious effects on other aspects of the protein’s function, then natural selection will favor subsequent mutations that ameliorate these antagonistic effects. Our inference that proteins affecting a diverse range of molecular pathways are also more likely to experience adaptive evolution is consistent with this hypothesis. This conclusion lends support to two previous results that highlight the potential importance of compensatory evolution. The first is taken from evolutionary theory on the “cost of complexity”, which predicts that adaptive walks are characterized by initial mutations with large fitness effects, followed by mutations of smaller effect [47]. Empirical evidence also suggests that compensatory substitution is common: amino acid substitutions in D. melanogaster are observed to cluster according to their location in a protein’s tertiary structure [48], suggesting compensatory substitutions occur to preserve functional integrity. Because the MK-based framework is a widely used tool to infer the action of natural selection, the ability to distinguish “arms race” scenarios from compensatory evolution promises to bring unique new insights into the mode of protein evolution.
Methods
RNAi data
Data for 26 RNAi screens in Drosophila melanogaster are compiled from the GenomeRNAi database, release 3.0 [49]. All screens report standardized Z scores, which measure the effect that knocking down a single gene has on a phenotype, relative to that of a control gene. Across studies, we consider genes with Z<−3 or Z>3 to have significant effects on a phenotype. Positive and negative tails of Z are sampled depending on the phenotype, for example the negative tail of Z is taken for the JAK/STAT signaling decrease and the positive tail is taken for the JAK/STAT signaling increase. Off-target effects in RNAi screens may potentially overestimate the effects of single genes [50], all of the RNAi experiments cited in this study report designing dsRNA to be specific to single genes and, in some cases, knockdown effects are further validated by a variety of methods. Individual RNAi phenotypes are grouped into categories that reflect the deepest level of functional ontology that are shared by all of the phenotypes.
Population genomic data
Reference-based genome assemblies of six European and nine sub-Saharan African strains of D. melanogaster (Additional file 1: Table S1) are generated from short-read data in the NCBI short read archive [51]. Reads are mapped to the genome of the reference D. melanogaster strain y1;cn1bw1sp1 (version 5.45) using the BWA software [52]. Variants are called using the POPBAM software with default settings [53]. Gene alignments are then constructed for the longest transcript per gene from the FlyBase mRNA annotations, using the Perl script PBsnp2fa.pl (https://github.com/skingan/PBsnp2fa.pl). A total of 13329 alignments were initially constructed. Ancestral and derived states are inferred by aligning to the genomes of both D. simulans strain MD063 [54] and D. yakuba strain Tai18E2 [55]. Requiring sequence alignment to both D. simulans and D. yakuba limits the data set to 11148 total gene alignments (2839 gene alignments are dropped) and it is likely that very rapidly evolving genes may not appear in the final data set.
Tests of natural selection
To determine the relative effects of natural selection across different RNAi knockdown phenotypes, we perform two analyses. First, we ask whether the genes associated with each RNAi knockdown phenotype, as a group, are enriched for amino acid substitutions (indicating adaptive evolution). We ask whether the genes significantly affecting each phenotype have an increased number of nonsynonymous substitutions compared to nonsynonymous polymorphisms using the direction of selection (DoS) statistic [9]. The DoS statistic is defined as the difference between the proportion of nonsynonymous substitutions (DN) to the sum of synonymous substitutions (DS) and nonsynonymous substitutions (DN) and the proportion of nonsynonymous polymorphisms (PS) to the sum of synonymous polymorphisms (PS) and nonsynonymous polymorphisms (PN), given as DoS=DN/(DS+DN)−PN/(PS+PN). Statistical significance of DoS is assessed by a bootstrap procedure, in which a null distribution is calculated by selecting a random sample of N genes from the genome, where N is the number of genes in the phenotype to be evaluated. Significance is assessed for DoS using a two-tailed approach, therefore empirical values are considered significant if they fall outside 0.975 quantile of the null distribution. For each phenotype, 10000 bootstrap replicates are performed using the statistical programming language R.
Our second analysis uses single locus MK tests to evaluate individual gene alignments for signatures of positive natural selection. The MK test considers the null hypothesis that the ratio of nonsynonymous (DN) and synonymous (DS) substitutions between D. melanogaster and D. simulans is equal to the ratio of nonsynonymous (PN) and synonymous (PS) polymorphisms within D. melanogaster [56]. Given that the ratio of PN/PS forms the expectation for the ratio of DN/DS, we calculate probability of obtaining DN higher than the observed value using a one-tailed Fisher’s exact test (FET). Gene alignments with fewer than six sites in any of the marginal counts are considered to have zero power [35]. Of the original 11148 MK tests, by the above criterion, 4063 tests are determined to have zero power and are subsequently removed [57] leaving 7085 valid tests. It is likely that removing low-power tests results in the elimination of genes experiencing a low rate of neutral mutation, but since there is no predictable relationship between neutral mutation rate and the distribution of fitness effects [58], there is no concrete a priori reason to believe this procedure will systematically bias our analysis of natural selection. From the remaining valid MK tests, using the Perl script mk-fdr.pl (https://github.com/dgarriga/mk-fdr) the proportion that are truly null is estimated to be 0.978, using a method designed to analyze P-value distributions from conservative tests [54]. At the 5 % level of significance, this corresponds to a false discovery rate of 40.9 %. However, we only consider tests with PFET<0.01 to be statistically significant, which corresponds to a false discovery rate of 22.7 %. Finally, it should be noted that we observe a significant negative correlation between the number of codons in a gene and the MK test P-value (r2=0.01334; P≪0.001).
Acknowledgments
We are grateful to Sarah B. Kingan and Amanda Larracuente for insightful discussion and comments on previous drafts of this manuscript. We would also like to thank Anthony J. Geneva, who contributed analyses to previous drafts of this manuscript. This work was made possible by National Institutes of Health grant R01-ODO1054801 to DG and National Science Foundation grant DEB-1209536 to DG and JPV.
Additional file
Supplementary Tables. Table S1. The 26 RNAi knockdown phenotypes surveyed in this study. We identify phenotypes that are enriched for proteins that significantly deviate from the genome average in their direction of selection (DoS). Table S2. Details of the 15 Drosophila melanogaster strains used in this study, including database accession numbers and identifiers [51], percent of the reference genome with reads mapped from that strain, and the average read depth across the entire genome assembly. (PDF 103 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JPV compiled the RNAi and population genomic data sets, performed the M-K tests and drafted the manuscript. DG conceived of the study, performed statistical analyses and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Kimura M, Ohta T. On some principles governing molecular evolution. Proc Natl Acad Sci USA. 1974;71:2848–852. doi: 10.1073/pnas.71.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson AC, Carlson SS, White TJ. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- 3.Pal C, Papp B, Lercher MJ. An integrated view of protein evolution. Nat Rev Genet. 2006;7:337–48. doi: 10.1038/nrg1838. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Ponce D, Guirao-Rico S, Orengo DJ, Segarra C, Rozas J, Aguadé M. Molecular population genetics of the insulin/TOR signal transduction pathway: a network-level analysis in Drosophila melanogaster. Mol Biol Evol. 2012;29:123–32. doi: 10.1093/molbev/msr160. [DOI] [PubMed] [Google Scholar]
- 5.Sackton TB, Lazzaro BP, Schlenke TA, Evans JD, Hultmark D, Clark AG. Dynamic evolution of the innate immune system in Drosophila. Nat Genet. 2007;39:1461–1468. doi: 10.1038/ng.2007.60. [DOI] [PubMed] [Google Scholar]
- 6.Obbard DJ, Gordon KHJ, Buck AH, Jiggins FM. The evolution of RNAi as a defence against viruses and transposable elements. Philos T Roy Soc B. 2009;364:99–115. doi: 10.1098/rstb.2008.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohr S, Bakal C, Perrimon N. Genomic screening with RNAi: results and challenges. Ann Rev Biochem. 2010;79:37–64. doi: 10.1146/annurev-biochem-060408-092949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern DL. Perspective: evolutionary developmental biology and the problem of variation. Evolution. 2000;54:1079–1091. doi: 10.1111/j.0014-3820.2000.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 9.Stoletzki N, Eyre-Walker A. Estimation of the neutrality index. Mol Biol Evol. 2011;28:63–70. doi: 10.1093/molbev/msq249. [DOI] [PubMed] [Google Scholar]
- 10.Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–14. doi: 10.1016/S1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 11.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–29. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 12.Müller B, Basler K. The repressor and activator forms of Cubitus interruptus control Hedgehog target genes through common generic Gli-binding sites. Development. 2000;127:2999–3007. doi: 10.1242/dev.127.14.2999. [DOI] [PubMed] [Google Scholar]
- 13.Aikin RA, Ayers KL, Therond PP. The role of kinases in the Hedgehog signalling pathway. EMBO Rep. 2008;9:330–6. doi: 10.1038/embor.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maslov S, Sneppen K, Eriksen KA, Yan KK. Upstream plasticity and downstream robustness in evolution of molecular networks. BMC Evol Biol. 2004;4:9. doi: 10.1186/1471-2148-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langley CH, Stevens K, Cardeno C, Lee YCG, Schrider DR, Pool JE, et al. Genomic variation in natural populations of Drosophila melanogaster. Genetics. 2012;192:533–98. doi: 10.1534/genetics.112.142018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wehr MC, Holder MV, Gailite I, Saunders RE, Maile TM, Ciirdaeva E, et al. Salt-inducible kinases regulate growth through the Hippo signalling pathway in Drosophila. Nat Cell Biol. 2013;15:61–71. doi: 10.1038/ncb2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stabell M, Larsson J, Aalen RB, Lambertsson A. Drosophila dSet2 functions in H3-K36 methylation and is required for development. Biochem Biophys Res Commun. 2007;359:784–9. doi: 10.1016/j.bbrc.2007.05.189. [DOI] [PubMed] [Google Scholar]
- 18.Gerhart J. 1998 warkany lecture: signaling pathways in development. Teratology. 1999;60:226–39. doi: 10.1002/(SICI)1096-9926(199910)60:4<226::AID-TERA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 19.Pires-daSilva A, Sommer RJ. The evolution of signalling pathways in animal development. Nat Rev Genet. 2003;4:39–49. doi: 10.1038/nrg977. [DOI] [PubMed] [Google Scholar]
- 20.Serra F, Arbiza L, Dopazo J, Dopazo H. Natural selection on functional modules, a genome-wide analysis. PLoS Comput Biol. 2011;7:1001093. doi: 10.1371/journal.pcbi.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keys DN, Lewis DL, Selegue JE, Pearson BJ, Goodrich LV, Johnson RL, et al. Recruitment of a hedgehog regulatory circuit in butterfly eyespot evolution. Science. 1999;283:532–4. doi: 10.1126/science.283.5401.532. [DOI] [PubMed] [Google Scholar]
- 22.Kuraku S, Usuda R, Kuratani S. Comprehensive survey of carapacial ridge-specific genes in turtle implies co-option of some regulatory genes in carapace evolution. Evol Dev. 2005;7:3–17. doi: 10.1111/j.1525-142X.2005.05002.x. [DOI] [PubMed] [Google Scholar]
- 23.Moczek AP, Nagy LM. Diverse developmental mechanisms contribute to different levels of diversity in horned beetles. Evol Dev. 2005;7:175–85. doi: 10.1111/j.1525-142X.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 24.Gaul U, Mardon G, Rubin GM. A putative Ras GTPase activating protein acts as a negative regulator of signaling by the Sevenless receptor tyrosine kinase. Cell. 1992;68:1007–1019. doi: 10.1016/0092-8674(92)90073-L. [DOI] [PubMed] [Google Scholar]
- 25.Huang AM, Rubin GM. A misexpression screen identifies genes that can modulate RAS1 pathway signaling in Drosophila melanogaster. Genetics. 2000;156:1219–1230. doi: 10.1093/genetics/156.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Presgraves DC, Stephan W. Pervasive adaptive evolution among interactors of the Drosophila hybrid inviability gene, Nup96. Mol Biol Evol. 2007;24:306–14. doi: 10.1093/molbev/msl157. [DOI] [PubMed] [Google Scholar]
- 27.Hurt JA, Obar RA, Zhai B, Farny NG, Gygi SP, Silver PA. A conserved CCCH-type zinc finger protein regulates mRNA nuclear adenylation and export. J Cell Biol. 2009;185:265–77. doi: 10.1083/jcb.200811072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varadarajan P, Mahalingam S, Liu P, Ng SB, Gandotra S, Dorairajoo DS, et al. The functionally conserved nucleoporins Nup124p from fission yeast and the human Nup153 mediate nuclear import and activity of the Tf1 retrotransposon and HIV-1 Vpr. Mol Biol Cell. 2005;16:1823–1838. doi: 10.1091/mbc.E04-07-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozaki T, Takahama M, Misawa T, Matsuura Y, Akira S, Saitoh T. Role of zinc-finger anti-viral protein in host defense against Sindbis virus. Int Immunol. 2015;27:357–64. doi: 10.1093/intimm/dxv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–7. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 31.Till DD, Linz B, Seago JE, Elgar SJ, Marujo PE, de Lourdes Elias M, et al. Identification and developmental expression of a 5’-3’ exoribonuclease from Drosophila melanogaster. Mech Develop. 1998;79:51–5. doi: 10.1016/S0925-4773(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 32.Rehwinkel J, Behm-Ansmant I, Gatfield D, Izaurralde E. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA. 2005;11:1640–1647. doi: 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn MW, Kern AD. Comparative genomics of centrality and essentiality in three eukaryotic protein-interaction networks. Mol Biol Evol. 2005;22:803–6. doi: 10.1093/molbev/msi072. [DOI] [PubMed] [Google Scholar]
- 34.Luisi P, Alvarez-Ponce D, Pybus M, Fares MA, Bertranpetit J, Laayouni H. Recent positive selection has acted on genes encoding proteins with more interactions within the whole human interactome. Genome Biol Evol. 2015;7:1141–1154. doi: 10.1093/gbe/evv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andolfatto P. Controlling type-I error of the McDonald-Kreitman test in genomewide scans for selection on noncoding DNA. Genetics. 2008;180:1767–1771. doi: 10.1534/genetics.108.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundaram MV. The love-hate relationship between Ras and Notch. Gene Dev. 2005;19:1825–1839. doi: 10.1101/gad.1330605. [DOI] [PubMed] [Google Scholar]
- 37.Ragab A, Buechling T, Gesellchen V, Spirohn K, Boettcher AL, Boutros M. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2011;30:1123–1136. doi: 10.1038/emboj.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.di Magliano MP, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Gene Dev. 2006;20(22):3161–173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lauth M. RAS and Hedgehog– partners in crime. Front Biosci (Landmark Ed) 2011;16:2259–270. doi: 10.2741/3852. [DOI] [PubMed] [Google Scholar]
- 41.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 42.Wangler MF, Yamamoto S, Bellen HJ. Fruit flies in biomedical research. Genetics. 2015;199(3):639–53. doi: 10.1534/genetics.114.171785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daugherty MD, Malik HS. Rules of engagement: molecular insights from host-virus arms races. Ann Rev Genet. 2012;46:677–700. doi: 10.1146/annurev-genet-110711-155522. [DOI] [PubMed] [Google Scholar]
- 44.Werren JH. Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc Natl Acad Sci USA. 2011;108:10863–10870. doi: 10.1073/pnas.1102343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daub JT, Hofer T, Cutivet E, Dupanloup I, Quintana-Murci L, Robinson-Rechavi M, et al. Evidence for polygenic adaptation to pathogens in the human genome. Mol Biol Evol. 2013;30(7):1544–1558. doi: 10.1093/molbev/mst080. [DOI] [PubMed] [Google Scholar]
- 46.Akashi H, Osada N, Ohta T. Weak selection and protein evolution. Genetics. 2012;192:15–31. doi: 10.1534/genetics.112.140178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orr HA. The population genetics of adaptation: the adaptation of DNA sequences. Evolution. 2002;56:1317–1330. doi: 10.1111/j.0014-3820.2002.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 48.Callahan B, Neher RA, Bachtrog D, Andolfatto P, Shraiman BI. Correlated evolution of nearby residues in Drosophilid proteins. PLoS Genet. 2011;7:1001315. doi: 10.1371/journal.pgen.1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt EE, Pelz O, Buhlmann S, Kerr G, Horn T, Boutros M. GenomeRNAi: a database for cell-based and in vivo RNAi phenotypes, 2013 update. Nucleic Acids Res. 2013;41:1021–1026. doi: 10.1093/nar/gks1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y, Creanga A, Lum L, Beachy PA. Prevalence of off-target effects in Drosophila RNA interference screens. Nature. 2006;443:359–63. doi: 10.1038/nature05179. [DOI] [PubMed] [Google Scholar]
- 51.Pool J, Corbett-Detig R, Sugino R, Stevens K, Cardeno C, Crepeau M, et al. Population genomics of sub-Saharan Drosophila melanogaster: African diversity and non-African admixture. PLoS Genet. 2012;8:1003080. doi: 10.1371/journal.pgen.1003080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrigan D. POPBAM: tools for evolutionary analysis of short read sequence alignments. Evol Bioinform. 2013;9:343–53. doi: 10.4137/EBO.S12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garrigan D, Kingan SB, Geneva AJ, Andolfatto P, Clark AG, Thornton KR, et al. Genome sequencing reveals complex speciation in the Drosophila simulans clade. Genome Res. 2012;22:1499–1511. doi: 10.1101/gr.130922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–18. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- 56.McDonald JH, Kreitman M. Adaptive protein evolution at the Adh locus in Drosophila. Nature. 1991;351:652–4. doi: 10.1038/351652a0. [DOI] [PubMed] [Google Scholar]
- 57.Dialsingh I, Austin SR, Altman NS. Estimating the proportion of true null hypotheses when the statistics are discrete. Bioinformatics. 2015;31:2303–309. doi: 10.1093/bioinformatics/btv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eyre-Walker A, Keightley PD. The distribution of fitness effects of new mutations. Nat Rev Genet. 2007;8:610–8. doi: 10.1038/nrg2146. [DOI] [PubMed] [Google Scholar]


