Summary
Purpose
This phase I study explored gefitinib (G) and capecitabine (C) in metastatic breast cancer (MBC).
Methods
Sequential cohorts (n=3) received G and escalating C on a 14 day on/7 day off schedule, with a validation cohort (n=10) at the maximum tolerated dose (MTD). Dose limiting toxicity (DLT) was defined in cycle 1. The primary endpoint was safety; secondary endpoints included response and adherence.
Results
19 patients were treated for a median of 5 cycles. No patients in sequential cohorts experienced DLT; C MTD was 2000 mg/m2/day when paired with daily G 250 mg. In the validation cohort, 4 experienced serious toxicities, including diarrhea, mucositis, and palmarplantar dysesthesia. At the MTD, 6 (46%) required a C dose reduction, and 3 (23%) came off study for toxicity. One partial response was observed (8%, 95% CI 0.2–38.5%); 5 had stable disease > 24 weeks (26%, 95% CI 9–51%). Patients missed few drug doses, with the suggestion of overadherence to therapy.
Conclusions
In this phase I study of G and C in MBC, a C MTD was identified, and significant toxicity was observed. 8% demonstrated a response, with 26% maintaining stable disease. The possibility of overadherence, as suggested in this study, may have implications for other trials of oral antineoplastic therapy.
Keywords: Adherence, Breast cancer, metastatic, Capecitabine, EGFR, Gefitinib, Phase 1
Introduction
Much of contemporary breast cancer treatment capitalizes on the identification of specific targets for drug therapy, including hormone receptors, human epidermal growth factor receptor (HER2), and vascular endothelial growth factor (VEGF). Epidermal growth factor receptor (EGFR) has also been identified as a relevant neoplastic target, as it appears to play a significant role in multiple cellular processes required for tumor growth and survival. Tumor cells may contain abnormal levels of EGFR, leading to increased cell proliferation, invasion, and suppression of apoptosis. EGFR overexpression has been identified in breast cancer, inversely correlated with hormone receptor status, and may be associated with disease progression and an inferior prognosis.[1] Specific inhibition of EGFR activation is thus an attractive target for control of cancer cell growth.[2]
Multiple agents have been developed to target EGFR, including monoclonal antibodies and small molecule tyrosine kinase inhibitors, such as gefitinib and erlotinib. Preclinical studies of gefitinib demonstrated potent inhibition of multiple human cancer cell lines, including breast cancer cell lines overexpressing EGFR or HER2, [3–6]. Specific activity has been seen in cell lines with acquired endocrine resistance, thought to be dependent on EGFR signaling for growth.[7–10] Initial studies of gefitinib in breast cancer demonstrated the agent to be well tolerated, with rash and diarrhea as common toxicities, and prolonged stabilization of disease observed in some patients.[11–13] Phase II studies of gefitinib monotherapy in pretreated advanced breast cancer have typically demonstrated minimal activity, with few objective responses, though a modest fraction of treated patients have had prolonged stable disease.[14–17]
Capecitabine is an oral fluoropyrimidine carbamate prodrug of 5-fluorouracil, active in advanced breast cancer with response rates of 20–35% in the pretreated metastatic setting,[18–21] and 30% as first line therapy.[22] Common adverse events include diarrhea, palmar plantar erythrodysesthesia (PPE), and fatigue.
Pre-clinical studies have suggested that the combination of capecitabine and an EGFR inhibitor may achieve synergistic interaction through alteration in the ratios of enzymes required for oral fluoropyrimidine activation, potentially increasing both the efficacy (and the toxicity of) capecitabine.[23, 24] Based on this promising preclinical data, the convenience of oral administration, and the non-overlapping toxicity profiles, this phase I study was initiated to characterize the safety, toxicity, and adherence to oral therapy with gefitinib and capecitabine for the treatment of advanced breast cancer.
Patients and Methods
Study Design
This phase I, open-label, dose escalation study (reference number IRUSIRES0245) was conducted through the Dana-Farber/Harvard Cancer Center, Boston, MA. The protocol was approved by the institutional review board at the Dana Farber/Harvard Cancer Center. All patients provided written informed consent. The study was conducted in compliance with Good Clinical Practice guidelines (sixth International Conference on Harmonization and the Declaration of Helsinki).
Patient Population
Patients age 18 years or older with histologically or cytologically confirmed locally advanced or metastatic breast cancer (MBC) were eligible for study. Patients had an Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2; normal renal, hepatic, and hematologic function on prestudy evaluation.
Patients were ineligible if they had more than 4 prior chemotherapeutic regimens for MBC, prior progression of disease on capecitabine treatment, or if they had HER2-overexpressing tumors (positive by fluorescent in situ hybridization or 3+ by immunohistochemistry) and had not received trastuzumab.
Safety Assessments
Patients underwent a physical examination, vital signs measurement, ECOG performance status, and laboratory evaluations 7 days prior to the first dose of study drug. A 12-lead EKG and appropriate radiologic evaluation were performed within 14 days prior to the first dose. Physical examination, vital signs, and safety laboratory evaluations were repeated weekly for the first cycle, and then on day 1 of each subsequent cycle. Adverse events (AEs) were graded using the National Cancer Institute Common Toxicity Criteria version 3.0.
Treatment
Patients received oral capecitabine in one of three sequential escalating cohorts: 1500 mg/m2/day, 1750 mg/m2/day, and 2000 mg/m2/day, in divided doses for 14 days, followed by 1 week off; 500 mg tablets were used in this study and the dose of capecitabine was rounded to the nearest 500 mg. Gefitinib 250 mg was administered orally on a continuous daily basis. Cycles were repeated every 21 days. An additional 10 patients were enrolled in a validation cohort at the maximum tolerated dose (MTD). Dose limiting toxicity (DLT) was defined during the first cycle of treatment as any grade 3/4 toxicity, (including grade 3 acneiform rash not resolving to grade 1 after 5 days off therapy or recurring with rechallenge) or a delay > 3 weeks due to unresolved toxicity. The protocol specified that if 0/3 patients had DLT in the first cycle then escalation would occur; if 1/3 had DLT than another 3 patients were to be added to that dose level, and if ≥2/3 or ≥2/6 had DLT then the next lowest dose would be the MTD. In subsequent cycles, capecitabine dose was held for ≥ grade 2 non-hematologic toxicity or ≥ grade 3 hematologic toxicity, and was resumed with resolution to ≤ grade 1, and with dose reduction for any grade 3 toxicity. Gefitinib was held for any ≥ grade 3 non-hematologic toxicity or ≥ grade 2 diarrhea and was resumed with resolution to ≤ grade 1, without dose reduction. Gefitinib dose was not adjusted based on hematologic toxicity. Patients whose disease responded (complete or partial response) or those with stable disease were treated until disease progression, intolerable toxicity, or withdrawal of consent.
Evaluation of Clinical Activity
Patients were assessed for response according to RECIST criteria at baseline and after every 3 cycles (9 weeks). For patients who remained on treatment for ≥18 weeks, assessments were performed every 4 cycles (12 weeks).
Evaluation of Adherence to Oral Therapy
At time of study enrollment, subjects were offered optional participation in the adherence monitoring section of the study. For participating subjects, adherence to oral therapy was measured by two methods. First, patient self-report was assessed at each visit using a daily drug diary completed by each patient. Second, both gefitinib and capecitabine were dispensed in bottles with microelectronic monitoring system (MEMS) caps for the first two complete cycles of therapy. The MEMS system consists of an “intelligent” cap that records time and date of each removal. The data can then be processed to generate a list of times and dates of bottle openings, a graph of number of doses taken each day, number of missed or extra doses, and dosing intervals. Patients were instructed to take medication at the same time every day, to open the vial only when a dose is being taken, not to switch the lids on medication vials, and not to transfer medications to another container. All patients were aware of the methods being used to monitor adherence to oral therapy.
Evaluation of Serum EGFR
Serum EGFR/ECD was assessed by Oncogene Science EGFR Microtiter ELISA®, a sandwich immunoassay which uses a mouse monoclonal capture antibody and an alkaline phosphatase labeled mouse monoclonal as detector. Both capture and detector reagents specifically recognize the extracellular domain of EGFR, with the capture antibody immobilized on the interior surface of the microtiter plate wells. To perform the test, an appropriate volume of serum was incubated in the wells to allow binding of the antigen by the capture antibody. The immobilized antigen was then exposed to the alkaline phosphatase labeled detector antibody. Addition of substrate to the wells allows the catalysis of a chromogen, para-nitrophenylphosphate into a colored product, the intensity of which is proportional to the amount of EGFR which has been bound to the plate. After correction for dilution factor, samples were assigned a quantitative value of EGFR in nanograms per mL of serum.
Results
Patient Demographics
Twenty patients were enrolled to this study from November 2004 through September 2005; one did not receive protocol therapy, leaving 19 patients included in this report. All patients met eligibility criteria. Demographic data are displayed in Table 1. The median age was 47 years old (range 34–63), 53% of tumors were hormone receptor positive, 42% were “triple negative,” and 11% were HER2 positive (and had prior trastuzumab). The median number of prior MBC chemotherapy regimens was 1 (range 0–4) and 74% had visceral spread of disease.
Table 1.
Patient Characteristics (n = 19)
| Characteristic | Number |
|---|---|
|
| |
| Median age, years (range) | 47 (34–63) |
|
| |
| Performance Status | |
| 0 | 16 (84%) |
| 1 | 3 (16%) |
| 2 | 0 |
|
| |
| ER positive | 10 (53%) |
|
| |
| HER2 positive | 2 (11%) |
|
| |
| Previous Therapy | |
| Adjuvant Setting | |
| Chemotherapy | 16 (84%) |
| Endocrine therapy | 8 (42%) |
| Metastatic Setting | |
| Chemotherapy | |
| No. of prior regimens | |
| 0 | 6 (32%) |
| 1 | 7 (37%) |
| 2–4 | 6 (32%) |
| Endocrine therapy | |
| No. of prior regimens | |
| 0 | 10 (53%) |
| 1 | 3 (16%) |
| 2–3 | 6 (32%) |
|
| |
| Visceral metastatic disease present | 14 (74%) |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor-2
Duration of Therapy and Safety
The median number of completed cycles of therapy was 5, with a range of 1–13, as shown in Table 2. Nine patients (47%) completed ≥ 18 weeks on therapy, with 5 pts (26%) treated for ≥ 24 weeks. No patients in dose escalation cohorts experienced DLT during the first cycle of therapy, so each of the dose escalation cohorts consisted of 3 patients rather than 6 patients. In the dose escalation cohorts, 3 of the 9 patients experienced 5 episodes of grade 3 toxicity after completion of cycle 1 (cycle 2: 1, cycle 3: 1, cycle 7: 1, cycle 8: 2). In the 10 patient validation cohort at the capecitabine MTD of 2000 mg/m2, a total of 4 patients experienced treatment-related grade 3 toxicity in any cycle starting in cycles 2 to 13. The frequencies of all toxicities grade 2 and above for the entire study population for all cycles of therapy are listed in Table 3; the most common included grade 2/3 PPE (8, 42%), grade 2 fatigue (5, 26%), grade 3 diarrhea (4, 21%), and grade 3 mucositis (2, 11%). Gefitinib-associated acneiform rash was common, but mild, with 8 patients (42%) from all cohorts experiencing grade 1 toxicity. Of the 13 patients at the MTD dose, 6 (46%) required a capecitabine dose reduction, with 1 patient requiring 2 reductions. Reasons for dose reduction included diarrhea (1), weight loss (2), PPE (1), fatigue (1), abdominal cramps (1), and mucositis (1). Three patients came off study for toxicity (PPE, 2; pancreatitis, 1); all began at the MTD level and required subsequent dose reductions. No grade 4 toxicity was observed during protocol therapy, and there were no deaths on study.
Table 2.
Cycles of therapy completed
| Cycles of therapy1 cycle = 3 weeks | Number of patients completing |
|---|---|
| 1 | 1 |
| 2 | - |
| 3 | 4 |
| 4 | 3 |
| 5 | 2 |
| 6 | 4 |
| 7 | - |
| 8 | 1 |
| 9 | 3 |
| 10 | - |
| 11 | - |
| 12 | - |
| 13 | 1 |
Table 3.
Drug-related Adverse Events in Any Cycle
| Capecitabine Dose (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adverse event | 1000mg/m2 (n = 3) | 1500mg/m2 (n = 3) | 2000mg/m2 (n = 3) | 2000mg/m2validation (n = 10) | ||||
| CTCAE event | G2 | G3 | G2 | G3 | G2 | G3 | G2 | G3 |
| N | N | N | N | N | N | N | N | |
| Diarrhea | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 3 |
| PPE | 1 | 0 | 1 | 0 | 0 | 1 | 5 | 0 |
| Fatigue | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 0 |
| Nausea/Vomiting | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 1 |
| Constipation | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Joint Pain | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 |
| Dyspnea | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mucositis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Pancreatitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hematologic | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; G, grade; N, number; PPE, palmar-plantar erythrodysesthesia
Efficacy
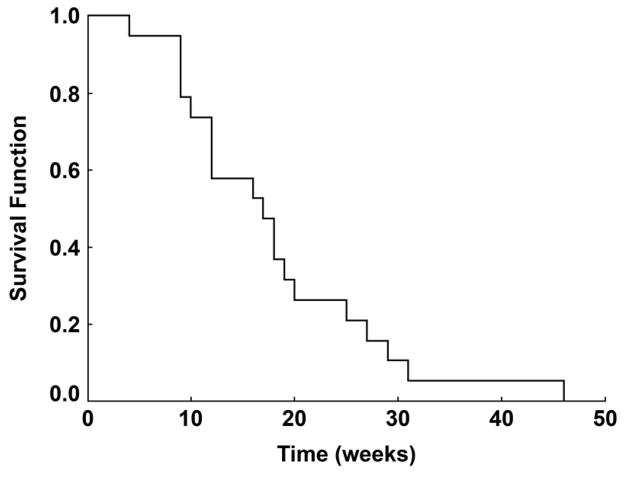
All patients underwent radiologic evaluation of disease status. Of the 19 patients, 12 had RECIST measurable disease at time of study entry. In this group, one patient (8%) demonstrated a partial response (PR) lasting 12 weeks (95% CI of 0.3% to 53%). 12 (63%) of the 19 patients had stable disease as their best response, with 5 (26%) patients demonstrating stable disease for ≥ 24 weeks. 16 patients came off study for progressive disease; 3 patients were removed for excessive toxicity. Time to disease progression (defined as the time from protocol registration to the first of: new metastatic lesions, increase in size of old lesions over smallest size since registration (either a 20% increase in the sum of longest diameters of measurable lesions or clear increase in non-measurable lesion size), second cancer of any type, or death) is represented in Figure 1.
Figure 1.
Time to Progression for the Study Cohort
Adherence
A total of 18 patients participated in the adherence monitoring section of the protocol. Twelve patients completed a daily drug diary for at least two cycles, with a range of 2–9 cycles observed. MEMs caps were used by 13 patients, with a range of 1–4 cycles observed. Eight patients contributed data using both methods of adherence monitoring. Adherence rates were calculated for each individual patient as “expected/observed” doses; a rate of adherence of > 80% observed/expected was used to define acceptable adherence.[25] Days when therapy was held for toxicity were not categorized as “expected” treatment days and thus not included in the calculations. Additionally, extra MEMs cap openings on clinic visit days when medications were refilled were not included in observed data.
Adherence rates reflecting all monitored cycles are presented in Table 4. In general, patients were extremely adherent with the oral therapy, with mean and median values for adherence >95% regardless of the method of observation. All individual patients were found to have acceptable adherence detected by either self report or MEMs caps, except for one patient with suboptimal capecitabine adherence (75%) detected by MEMs cap only. However, MEMs and drug diary found a similar percent of patients who had at least one medication error. Adherence rates were also calculated for just the first 2 cycles of therapy to reduce over-representation of data from long-term adherent individuals; no differences were noted when compared to the entire course of therapy (data not shown).
Table 4.
Adherence rates for all monitored cycles
| Number of Patients | Method of Observation | Gefitinib Adherence Rate (%) | Capecitabine Adherence Rate (%) | ||
|---|---|---|---|---|---|
| 12 | Diary | median | 96 | median | 97 |
| mean | 98 | mean | 99 | ||
| range | 93–113 | range | 89–100 | ||
| 13 | MEMS | median | 99 | median | 96 |
| mean | 100 | mean | 96 | ||
| range | 86–107 | range | 75–100 | ||
Caption: Adherence rates were calculated as a percentage of observed/expected doses for all monitored cycles for each individual patient. The mean and median adherence rates are presented for each agent by both methods of monitoring. A subset of the study population was monitored concurrently by both methods, with 8 patients contributing data from both diary and MEMs monitoring.
Close analysis of patient materials disclosed unexpected patterns of patients taking more medications than directed. As summarized in Table 5, 8 patients (44% of cohort contributing adherence information) experienced at least one “over-medication” error, detected by either MEMs or drug diary. Three primary types of errors were noted: patients taking extra days of medication beyond planned cycle, patients taking extra doses per day and occasionally holding drug the following day, and patients missing a day of dosing and “compensating” by taking extra the following day. One patient, despite extensive teaching prior to initiation of therapy, took 4.5g gefitinib over a 4-day period of time (450% recommended dose); drug was held when the error was recognized and no adverse effects were observed.
Table 5.
Observed Patient Medication Errors
| Events for each of the eight patients who demonstrated overadherence | Detection Method |
|---|---|
| Patient took 2 extra doses gefitinib | MEMs |
| Patient took 5 extra doses gefitinib, also missed a dose of capecitabine, then took extra the following day | MEMs |
| Took extra dose gefitinib, held drug the next day | MEMs |
| Patient missed a dose of capecitabine, then took extra dose the next day | MEMs |
| Patient took multiple extra doses of gefitinib, also had 2 episodes of missing a dose of gefitinib, then taking two the following day. | Both |
| Patient took 3 extra days of capecitabine, took double dose gefitinib once | Diary |
| Patient took extra dose gefitinib in one day, held drug the next day | Diary |
| Patient took 1 extra dose of gefitinib | Diary |
Caption: Description of the 8 different patients who demonstrated medication errors as part of adherence monitoring. Type of error is described, along with method of detection. Overall adherence rates may not reflect instances of overadherence, as ratios may have been balanced out by missed doses at other times.
Correlative Studies
Of the 19 patients, 13 contributed paired samples for serum EGFR analysis at baseline and at time of first restaging. Mean serum EGFR at baseline was 52.4 ng/mL (range 36.5–70.7), and mean value at first restaging was 48.0 ng/mL (range 37.3–61.1). Median value at baseline was 51 ng/mL, and median value at time of first restaging was 51 ng/mL.
Discussion
In this phase I dose escalation study of gefitinib and capecitabine in patients with advanced breast cancer, combination therapy led to significant toxicity, with expected side effects of diarrhea, PPE, mucositis, and fatigue. Rates of grade 3 toxicity were somewhat greater than those reported with either gefitinib or capecitabine monotherapy.[14, 18] Toxicity occurred in all three dose levels with the described capecitabine 14 day on/7 day off dosing schedule; use of the recently described 7 day on/7 day off schedule might decrease the incidence of adverse side effects with this combination regimen.[26]An objective response rate of 8% was observed, with one quarter of patients demonstrating prolonged stable disease.
Combining gefitinib with chemotherapy or hormonal therapy has been explored in multiple trials in advanced breast cancer. In a phase I study of gefitinib with epirubicin in the second-line setting, 2 of 14 patients were found to have a PR (14.3%), with an additional 7 (50%) having prolonged stable disease.[27] Combination therapy with paclitaxel, carboplatin, and gefitinib in a phase I/II trial in the first-line setting produced a response rate of 57.3%.[28] Phase II studies of gefitinib and docetaxel in the first-line setting in predominantly taxane-naïve patients have demonstrated response rates of 39–54%.[29, 30] In the absence of randomized trials, it is unclear if these results indicate superior benefit over that expected with chemotherapy alone. The combination of anti-EGFR therapy with an endocrine agent has been explored in a phase 2 study randomizing patients to anastrozole and placebo vs anastrozole and gefitinib; results demonstrated an improvement in progression-free survival in the gefitinib arm.[31]
Although evaluation of efficacy was not a primary goal of this study, additive activity from combination therapy was not observed in this limited experience. There are several possible explanations for this finding, beyond those related to the inclusion of a small, unselected patient population. It is possible that the 250 mg dose level of gefitinib selected was too low, although other breast cancer studies have not confirmed an advantage for the higher 500 mg/day dose. It is also possible that alternative inhibitors of EGFR may have more activity in breast cancer than gefitinib, although studies with erlotinib have not supported this hypothesis.[32, 33] Furthermore, the activity in this study appears inferior to that of capecitabine monotherapy for breast cancer in a similar clinical setting, where response rates of 30–35% have been observed.[18–21] Randomized trials of EGFR inhibitors and platinum chemotherapy in non-small cell lung cancer (NSCLC) have suggested a potential antagonistic effect from the addition of gefitinib to doublet chemotherapy;[34–37] it is not known if the low response rate observed in this study could reflect a negative in vivo interaction.
As demonstrated by the development of other targeted therapies, identification and evaluation of patients possessing the target of interest is crucial for measuring activity of a drug. The population under study in this trial was not specifically selected for EGFR overexpression, as an association between EGFR overexpression and responsiveness to gefitinib has been inconsistently characterized.[28, 38, 39] Serum EGFR was analyzed as part of a correlative analysis, and while there was a wide range of EGFR serological measurements at baseline (range 36.5 – 70.7 ng/mL), there was only one clinical response, and hence no evidence that higher serologic EGFR values might be associated with a larger response rate. “Triple negative” breast cancers have a high rate of EGFR overexpression, however clinically they have not demonstrated specific sensitivity to EGFR inhibitors.[40] It is possible that enrichment with specific tumor subsets, including selection for EGFR-dependent tumors, or patients with fewer prior therapies, may have changed the observed clinical activity in this trial. Given the complexity and redundancy of signal transduction in breast cancer cells, blockade of activation of several receptors, including ER, EGFR, and HER2, may be necessary to achieve appropriate growth suppression.[41] Therefore, a role may still exist for gefitinib in the treatment of breast cancer, although the appropriate patient population and drug combination has not yet been identified. At present, owing to issues of toxicity and efficacy, no further evaluation of this combination regimen is planned. The success of future breast cancer studies using novel biologic therapies will likely depend on improved selection of study participants to allow appropriate “tailoring” of drug and tumor target.
This study was also designed to intensively investigate patients’ adherence to an all-oral treatment regimen. Few studies have evaluated adherence to oral antineoplastic agents, and limited available evidence suggests that adherence is quite variable.[42] Prior studies of endocrine therapies suggest despite adequate initial adherence, rates drop off over time, and patients report frequent non-adherence with medication, which could potentially impact efficacy of chemotherapeutic, hormonal, and novel agents. [25, 43, 44] Nonadherence to oral antineoplastic agents and divergence from the prescribed dosing schedule may not only expose a patient to increased toxicity from the drug, but also potentially compromise response.
Adherence was measured in this study by two validated methods; overall, patients in this highly controlled clinical trial exhibited excellent adherence with oral medication. As seen in other studies using the MEMs monitoring system, MEMs results suggest somewhat lower levels of adherence compared to patient diary records, implying patient self-reporting methods may over-estimate rates of adherence when compared to MEMs.[25] Both forms of monitoring detected a tendency for “overadherence;” despite extensive medication teaching and assistance, some patients took extra doses or days of medication, or missed doses and took double the next day. About 40% of the small cohort in this study demonstrated a medication error on at least one day, although these errors did not appear to impact the observed safety and activity of the medications under study.
The finding of overadherence in a clinical research population is of interest and the prevalence of this practice is not known. Non-adherence by participants in clinical trials – both under, over, and incorrect drug dosing – might affect study findings with respect to both efficacy and toxicity[45, 46] Additionally, it has been observed that patients participating in clinical trials differ from a general patient population, and tend to be highly motivated, possibly leading to substantially greater adherence to treatment protocols.[47] Thus, adherence measurements in the context of a clinical trial may not accurately capture the true adherence, whether under-adherence or over-adherence, experienced by patients in general practice receiving oral anti-neoplastic therapy.
Observing overadherence among clinical trial participants underscores the need for careful patient education into the mechanics of oral treatment administration, the proper management of missed/skipped doses, and the underlying rationale for safe use of oral therapy. Validated methods to reliably monitor patient adherence in “real time” are needed. The recent emergence of multiple active anti-neoplastic oral agents highlights the importance of evaluating and improving patient adherence in an effort to enhance patient safety and clinical outcomes.
Acknowledgments
We thank AstraZeneca for providing gefitinib and support for the clinical trial.
References
- 1.Klijn JG, Berns PM, Schmitz PI, Foekens JA. The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev. 1992;13:3–17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Busse D, Yakes FM, Lenferink AE, Arteaga CL. Tyrosine kinase inhibitors: rationale, mechanisms of action, and implications for drug resistance. Semin Oncol. 2001;28:47–55. doi: 10.1016/s0093-7754(01)90282-9. [DOI] [PubMed] [Google Scholar]
- 3.Ciardiello F, Caputo R, Bianco R, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–2063. [PubMed] [Google Scholar]
- 4.Moulder SL, Yakes FM, Muthuswamy SK, et al. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–8895. [PubMed] [Google Scholar]
- 5.Anderson NG, Ahmad T, Chan K, et al. ZD1839 (Iressa), a novel epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the growth of EGFR-positive cancer cell lines with or without erbB2 overexpression. Int J Cancer. 2001;94:774–782. doi: 10.1002/ijc.1557. [DOI] [PubMed] [Google Scholar]
- 6.Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 ("Iressa") inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- 7.Nicholson RI, Hutcheson IR, Harper ME, et al. Modulation of epidermal growth factor receptor in endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr Relat Cancer. 2001;8:175–182. doi: 10.1677/erc.0.0080175. [DOI] [PubMed] [Google Scholar]
- 8.Knowlden JM, Hutcheson IR, Jones HE, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 9.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 10.Lu C, Speers C, Zhang Y, et al. Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2003;95:1825–1833. doi: 10.1093/jnci/djg117. [DOI] [PubMed] [Google Scholar]
- 11.Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–4302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Maddox AM, Rothenberg ML, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002;20:3815–3825. doi: 10.1200/JCO.2002.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002;20:2240–2250. doi: 10.1200/JCO.2002.10.112. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Albanell J, Ruiz A, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–5333. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 15.Albain KS, Elledge R, Gradishar WJ, et al. Open-label, phase II, multicenter trial of ZD1839 (’Iressa’) in patients with advanced breast cancer. Breast Cancer Res Treat. 2002;76(A20):S33. [Google Scholar]
- 16.Robertson JFR, Gutteridge E, Cheung KL, et al. Gefitinib (ZD1839) is active in acquired tamoxifen (TAM)-resistant oestrogen receptor (ER)-positive and ER-negative breast cancer: Results from a phase II study. Journal of Clinical Oncology, 2003 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2003;22:A23. [Google Scholar]
- 17.von Minckwitz G, Jonat W, Fasching P, et al. A multicentre phase II study on gefitinib in taxane- and anthracycline-pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005;89:165–172. doi: 10.1007/s10549-004-1720-2. [DOI] [PubMed] [Google Scholar]
- 18.Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92:1759–1768. doi: 10.1002/1097-0142(20011001)92:7<1759::aid-cncr1691>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 20.Talbot DC, Moiseyenko V, Van Belle S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer. 2002;86:1367–1372. doi: 10.1038/sj.bjc.6600261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–542. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 22.O’Shaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–1254. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 23.Magne N, Fischel JL, Dubreuil A, et al. ZD1839 (Iressa) modifies the activity of key enzymes linked to fluoropyrimidine activity: rational basis for a new combination therapy with capecitabine. Clin Cancer Res. 2003;9:4735–4742. [PubMed] [Google Scholar]
- 24.Ouchi KF, Yanagisawa M, Sekiguchi F, Tanaka Y. Antitumor activity of erlotinib in combination with capecitabine in human tumor xenograft models. Cancer Chemother Pharmacol. 2006;57:693–702. doi: 10.1007/s00280-005-0079-3. [DOI] [PubMed] [Google Scholar]
- 25.Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 26.Traina TA, Theodoulou M, Feigin K, et al. Phase I study of a novel capecitabine schedule based on the Norton-Simon mathematical model in patients with metastatic breast cancer. J Clin Oncol. 2008;26:1797–1802. doi: 10.1200/JCO.2007.13.8388. [DOI] [PubMed] [Google Scholar]
- 27.Gasparini G, Sarmiento R, Amici S, et al. Gefitinib (ZD1839) combined with weekly epirubicin in patients with metastatic breast cancer: a phase I study with biological correlate. Ann Oncol. 2005;16:1867–1873. doi: 10.1093/annonc/mdi393. [DOI] [PubMed] [Google Scholar]
- 28.Fountzilas G, Pectasides D, Kalogera-Fountzila A, et al. Paclitaxel and carboplatin as first-line chemotherapy combined with gefitinib (IRESSA) in patients with advanced breast cancer: a phase I/II study conducted by the Hellenic Cooperative Oncology Group. Breast Cancer Res Treat. 2005;92:1–9. doi: 10.1007/s10549-005-0322-y. [DOI] [PubMed] [Google Scholar]
- 29.Ciardiello F, Troiani T, Caputo F, et al. Phase II study of gefitinib in combination with docetaxel as first-line therapy in metastatic breast cancer. Br J Cancer. 2006;94:1604–1609. doi: 10.1038/sj.bjc.6603141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennison SK, Jacobs SA, Wilson JW, et al. A phase II clinical trial of ZD1839 (Iressa) in combination with docetaxel as first-line treatment in patients with advanced breast cancer. Invest New Drugs. 2007;25:545–551. doi: 10.1007/s10637-007-9055-6. [DOI] [PubMed] [Google Scholar]
- 31.Cristofanilli M, Valero V, Mangalik A, et al. A phase II multicenter, double-blind, randomized trial to compare anastrozole plus gefinitib with anastrozole plus placebo in postmenopausal women with hormone receptor-positive (HR+) metastatic breast cancer (MBC) J Clin Oncol. 2008;26:A1012. [Google Scholar]
- 32.Winer E, Cobleigh M, Dickler M, et al. Phase II multicenter study to evaluate the efficacy and safety of Tarceva ™ (erlotinib, OSI-774) in women with previously treated locally advanced or metastatic breast cancer. Breast Cancer Res Treat, 2002 San Antonio Breast Cancer Symposium Proceedings (Post-Meeting Edition) 2002;76:A445. [Google Scholar]
- 33.Tan AR, Yang X, Hewitt SM, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol. 2004;22:3080–3090. doi: 10.1200/JCO.2004.08.189. [DOI] [PubMed] [Google Scholar]
- 34.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 36.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 37.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 38.Bailey LR, Janas M, Schmidt K, et al. Evaluation of epidermal growth factor receptor (EGFR) as a predictive marker in patients with non-small-cell lung cancer (NSCLC) receiving first-line gefitinib combined with platinum-based chemotherapy. Journal of Clinical Oncology, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004;22:A7013. [Google Scholar]
- 39.Polychronis A, Sinnett HD, Hadjiminas D, et al. Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: a double-blind placebo-controlled phase II randomised trial. Lancet Oncol. 2005;6:383–391. doi: 10.1016/S1470-2045(05)70176-5. [DOI] [PubMed] [Google Scholar]
- 40.Carey L, Rugo H, Marcom P, et al. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer. J Clin Oncol. 2008;26:A1009. [Google Scholar]
- 41.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 43.Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer. 2006;42:2271–2276. doi: 10.1016/j.ejca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 45.Urquhart J. Compliance and clinical trials. Lancet. 1991;337:1224–1225. doi: 10.1016/0140-6736(91)92896-a. [DOI] [PubMed] [Google Scholar]
- 46.Lasagna L, Hutt P. Heath care, research and regulatory impact of noncompliance. In: Cramer JA, Spikler BE, editors. Patient compliance in medical practice and clinical trials. New York: Raven Press; 1991. pp. 393–403. [Google Scholar]
- 47.Leventhal H, Nerenz DR, Leventhal EA, et al. The behavioral dynamics of clinical trials. Prev Med. 1991;20:132–146. doi: 10.1016/0091-7435(91)90014-u. [DOI] [PubMed] [Google Scholar]