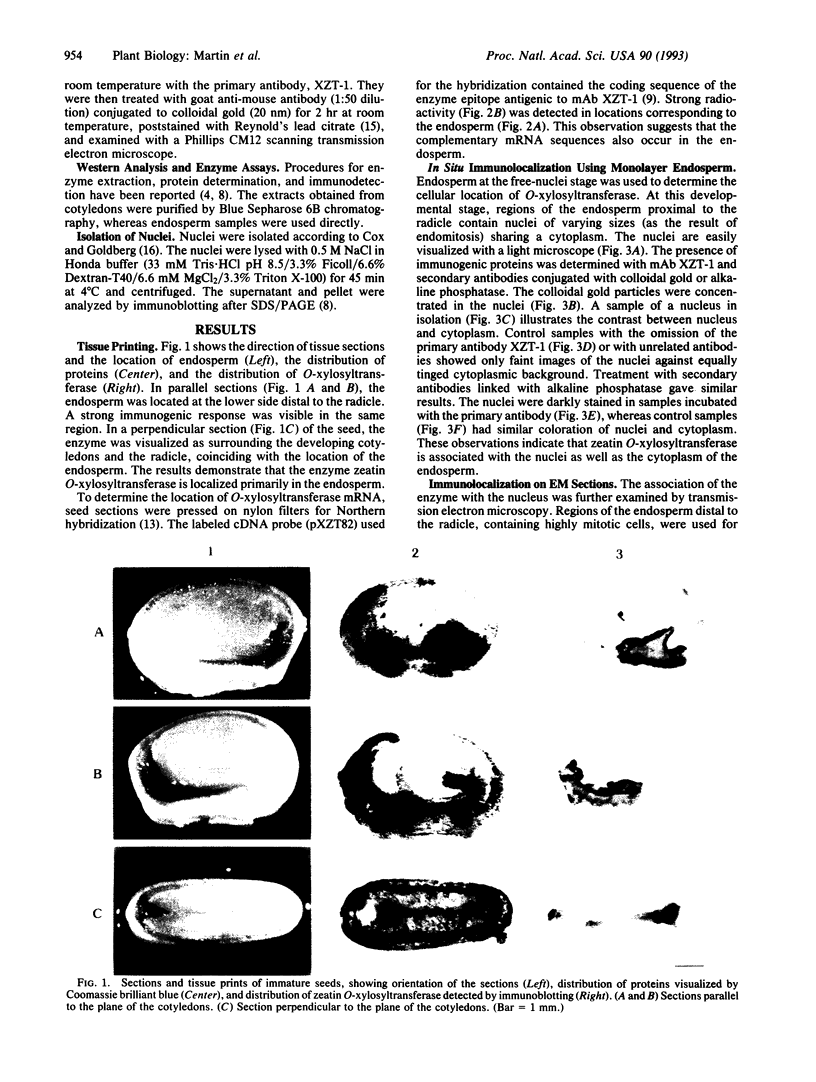
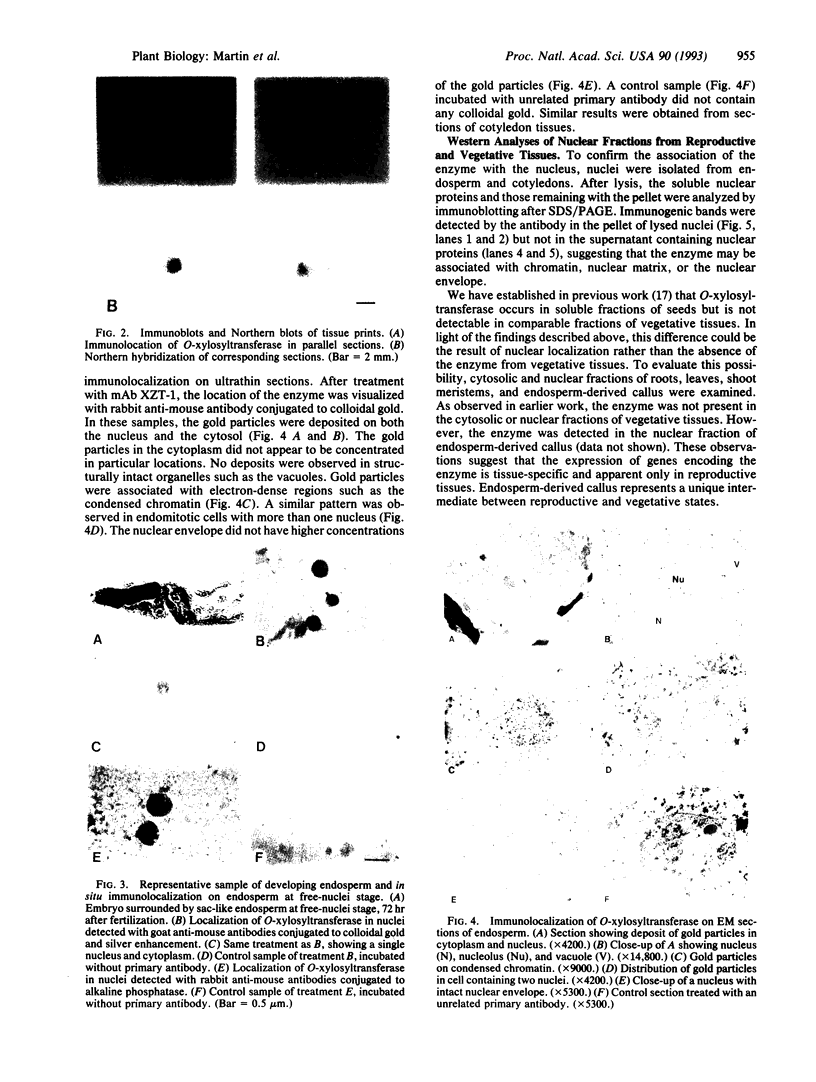
Abstract
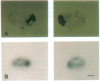
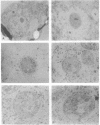
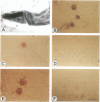
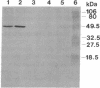
Zeatin O-xylosyltransferase (EC 2.4.2.-) mediates the formation of O-xylosylzeatin from trans-zeatin and UDP-xylose in immature seeds of Phaseolus vulgaris. Tissue printing with a monoclonal antibody specific for the enzyme and a cDNA probe demonstrated that the enzyme was primarily localized and synthesized in the endosperm. Immunolocalization performed on monolayer endosperm at the free-nuclei stage and on EM sections demonstrated that the enzyme was associated with the nucleus as well as with the cytoplasm. Immunoanalysis of nuclear fractions revealed that the enzyme was retained in the nuclear pellet. Western analysis also showed that the enzyme was present in the nuclei of cotyledons and endosperm callus. The findings suggest that the enzyme may be involved in the nuclear-cytoplasmic transport of cytokinins and related molecules or, possibly, with chromatin of rapidly dividing cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Klee H., Amasino R. M., Nester E. W., Gordon M. P. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G. F., Rogers S. G., Fraley R. T., Brand L. Identification of a cloned cytokinin biosynthetic gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F. R., Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Thissen J. A., Moomaw J. F. Enzymatic modification of proteins with a geranylgeranyl isoprenoid. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8631–8635. doi: 10.1073/pnas.88.19.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassab G. I., Varner J. E. Immunocytolocalization of extensin in developing soybean seed coats by immunogold-silver staining and by tissue printing on nitrocellulose paper. J Cell Biol. 1987 Dec;105(6 Pt 1):2581–2588. doi: 10.1083/jcb.105.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Dixon S. C., Martin R. C., Mok M. C., Shaw G., Mok D. W. Zeatin Glycosylation Enzymes in Phaseolus: Isolation of O-Glucosyltransferase from P. lunatus and Comparison to O-Xylosyltransferase from P. vulgaris. Plant Physiol. 1989 Aug;90(4):1316–1321. doi: 10.1104/pp.90.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T., Peter M., Nurse P., Nigg E. A. p34cdc2 acts as a lamin kinase in fission yeast. J Cell Biol. 1991 Mar;112(5):797–807. doi: 10.1083/jcb.112.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust J. R., Dice J. F. Evidence for isopentenyladenine modification on a cell cycle-regulated protein. J Biol Chem. 1991 May 25;266(15):9961–9970. [PubMed] [Google Scholar]
- Goldfarb D. S. Nuclear transport. Curr Opin Cell Biol. 1989 Jun;1(3):441–446. doi: 10.1016/0955-0674(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella A., Van Montagu M., Wang K. A bacterial peptide acting as a plant nuclear targeting signal: the amino-terminal portion of Agrobacterium VirD2 protein directs a beta-galactosidase fusion protein into tobacco nuclei. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9534–9537. doi: 10.1073/pnas.87.24.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., Lorch Y. Irresistible force meets immovable object: transcription and the nucleosome. Cell. 1991 Nov 29;67(5):833–836. doi: 10.1016/0092-8674(91)90354-2. [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Mok M. C., Mok D. W., Griffin D. A., Shaw G. Cytokinin metabolism in phaseolus embryos : genetic difference and the occurrence of novel zeatin metabolites. Plant Physiol. 1985 Mar;77(3):635–641. doi: 10.1104/pp.77.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. C., Martin R. R., Mok M. C., Mok D. W. A monoclonal antibody specific to zeatin o-glycosyltransferases of phaseolus. Plant Physiol. 1990 Nov;94(3):1290–1294. doi: 10.1104/pp.94.3.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. C., Mok M. C., Shaw G., Mok D. W. An enzyme mediating the conversion of zeatin to dihydrozeatin in phaseolus embryos. Plant Physiol. 1989 Aug;90(4):1630–1635. doi: 10.1104/pp.90.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisterernst M., Roeder R. G. Family of proteins that interact with TFIID and regulate promoter activity. Cell. 1991 Nov 1;67(3):557–567. doi: 10.1016/0092-8674(91)90530-c. [DOI] [PubMed] [Google Scholar]
- Mok M. C., Mok D. W., Armstrong D. J. Differential cytokinin structure-activity relationships in phaseolus. Plant Physiol. 1978 Jan;61(1):72–75. doi: 10.1104/pp.61.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Baeuerle P. A., Lührmann R. Nuclear import-export: in search of signals and mechanisms. Cell. 1991 Jul 12;66(1):15–22. doi: 10.1016/0092-8674(91)90135-l. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Okazaki H., Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989 Oct;109(4 Pt 1):1389–1397. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad P. V., Jones A. M. Putative receptor for the plant growth hormone auxin identified and characterized by anti-idiotypic antibodies. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5479–5483. doi: 10.1073/pnas.88.13.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Turner J. E., Mok D. W., Mok M. C., Shaw G. Isolation and partial purification of an enzyme catalyzing the formation of O-xylosylzeatin in Phaseolus vulgaris embryos. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3714–3717. doi: 10.1073/pnas.84.11.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagona M. J., Schmidt R. J., Raikhel N. V. Monocot regulatory protein Opaque-2 is localized in the nucleus of maize endosperm and transformed tobacco plants. Plant Cell. 1991 Feb;3(2):105–113. doi: 10.1105/tpc.3.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Chua N. H. The basic domain of plant B-ZIP proteins facilitates import of a reporter protein into plant nuclei. Plant Cell. 1991 Jul;3(7):667–675. doi: 10.1105/tpc.3.7.667. [DOI] [PMC free article] [PubMed] [Google Scholar]