Abstract
A new peptide modification strategy was recently developed to replace the i to i+4 hydrogen bond of the main chain of an a-helix with a carbon-carbon covalent bond to afford highly stable constrained α-helices, termed Hydrogen Bond Surrogate (HBS) helices. HBS helices that mimic the Bak BH3 domains were experimentally demonstrated to target protein Bcl-xL with high affinity. In this study, molecular dynamics (MD) simulation is used to understand how the covalent modification of the natural Bak sequence affects the binding to Bcl-xL at molecular levels. The binding mechanism of HBS helix to Bcl-xL and the effect of synthesized cyclic structures are analyzed by molecular dynamics and MM-PBSA calculations for comparison with the native binding of Bak-Bcl-xL. The present MD result shows that the entropy of the HBS structure is considerably reduced and the presence of the N-terminal HBS macrocycle impacts residues at the C-terminus of the helix. But the conformation of the corresponding binding structures is not significantly changed. Our analysis shows that substitution of an aspartic acid residue – a helix breaker – with a hydrophobic residue not only enhances the helicity of the peptide but also stabilizes the structure of the binding complex. The present computational result is consistent with the experimental observation and provides explanations for the altered binding properties of the artificial Bak a-helix. Our study underscores the importance of dynamical effect in protein-peptide interaction in which entropic effect plays a major role.
Keywords: hydrogen bond, bcl-xL, dynamical binding, helix, entropic effect
1. Introduction
The α-helix is a ubiquitous structural motif in proteins and is frequently involved in protein-protein interactions that dominate many biological pathways. In theory, peptides that mimic the helical domains at protein interfaces are ideal ligands for selective targeting of proteins and modulation of their biological functions [1, 2]. However, short peptide domains do not remain structured when taken out from their original biological environments. The in vivo efficacy of peptides is compromised by their loss of secondary structure, susceptibility to proteolytic degradation, and difficulty in penetrating intact cell membranes.
Recently a hydrogen-bond surrogate (HBS) strategy was developed to stabilize peptide secondary structures, specifically α-helices (Figure 1a) [3]. In this approach, an N-terminal main-chain i to i+4 hydrogen bond is replaced by a carbon-carbon bond to nucleate the helical conformation [4-5]. In theory, constraining flexible molecules into conformations that mimic their bound structures is expected to lower the entropic cost of the binding processes. Anti-apoptotic protein Bcl-xL and its natural peptide ligand Bak BH3 domain provide a good system to test the effectiveness of this method (Figure 1b). Arora and coworkers designed two HBS helices that mimic the Bak BH3 domains (Figure 1c-d). One of these helices, HBS 2a, is a direct mimic of the natural Bak BH3 sequence. HBS 2b was designed to further improve the helicity of the Bak helix. The Bak peptide 1 contains a GDD tripeptide residue (residues 582-584 in the Bak BH3 domain) in the middle of the Bak peptide sequence may limit the propagation of the helix and lower the overall helical content of 2a, since glycine is known to be a potent “helix breaker” and aspartic acid has been implicated as a helix stop signal. [6-8] Gly-582 and Asp-583 residues cannot be substituted with alanine without sacrificing binding affinity for the protein; however, Asp-584 may be replaced without any deleterious effects [9]. To test the effect of replacing Asp-84 on the helicity of Bak peptide, Arora et al. prepared HBS α-helix 2b in which Asp-584 is substituted with a side chain acetylated-lysine (LysAc) (Figure 1d). [10] This single substitution provided a significant boost in α-helicity; HBS helix 2b is roughly 140% more helical than 2a. Binding assays showed that both HBS peptides bound Bcl-xL with high affinities, with 2b binding with a higher affinity in agreement with its enhanced helicity.[11]. We were motivated to investigate the detailed binding mechanisms of these HBS peptides and evaluate their biological effects by using computational modeling techniques. In particular, we expect that dynamical effect such as entropic effect may play an important role in protein-peptide binding due to much increased flexibility of the peptide relative to rigid small molecular binders.
Figure 1.
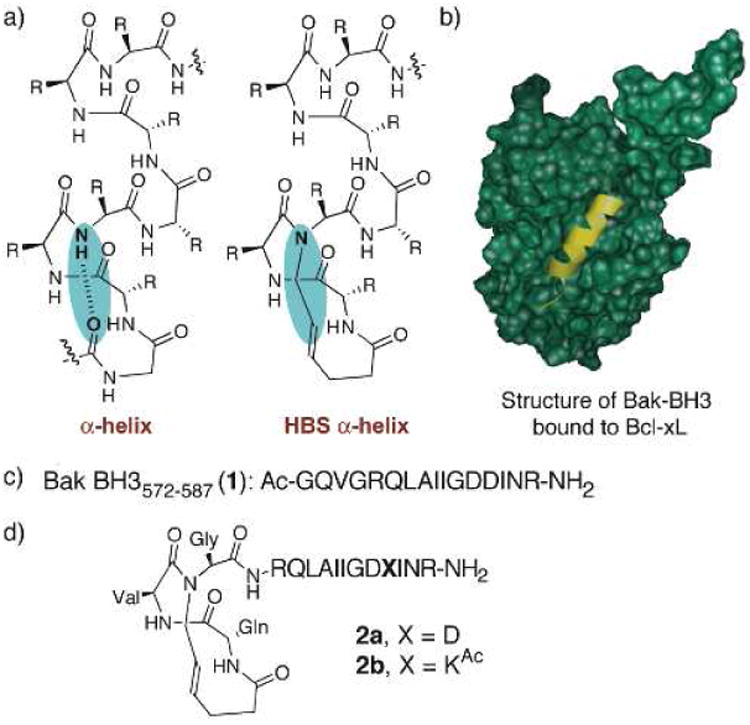
a) Formation of hydrogen-bond surrogate (HBS) derived α helices by replacement of a main-chain hydrogen bond with a carbon–carbon bond. b) HBS a helix and recognition of Bcl-xL (green) with Bak BH3 a helix (yellow); PDB code: 1BXL. c) Sequence of the unconstrained Bak BH3 peptide 1. d) Structures of HBS Bak a helices 2a and 2b.
In this paper, we present MD simulation studies for three peptide-Bcl-xL systems. The binding free energies are calculated from the MD trajectories, and the roles of different interactions and their effects are analyzed. The results reveal that although the HBS macrocycle doesn't directly impact its binding configuration, it does have the influence on configurations of residues in distance; the hydrophobic side chain at the site of Asp584 not only stabilizes the helical structure of the peptide but also increases the hydrophobic interactions with the Bcl-xL.
2. Computational Methods
The starting structure of the Bak-Bcl-xL complex was obtained from the Protein Data Bank (PDB) [12] with PDB entry 1BXL (Figure 1b) [9]. The HBS helices and KAC residue were constructed by InsightII, and optimized at the B3LYP/6-31G* level by Gaussian03 [13]. The partial charges of HBS helix and KAC were generated by using RESP [14] program, based on the ESP potentials generated by the ab initio calculation at the B3LYP/6-31G* level by Gausian03. AMBER9 [15] package was used to carry out molecular dynamics simulations with ff03 version of Amber force field [16]. Counterions were placed by Leap to neutralize the charges of three systems. A rectangular-shaped box of water was constructed using the TIP3P water model [17], with the buffering distance set to 10 Å. For each system, 500 steps of energy minimization constraining the solute were carried out, followed by 1000 steps of energy minimization without any constraints. After energy minimization, each system was heated to 300K by weak coupling algorithm in 50ps, and equilibrated in the following 2ns. 10ns of product MD calculations were carried out for each system under periodic boundary conditions. The cutoff was set to 8 Å. The long-range electrostatic interactions were calculated by the particle-mesh Ewald algorithm [18]. The time step of the MD simulations was set to 2.0fs, and the SHAKE algorithm was used to constrain hydrogen bond lengths at their equilibrium values. The system pressure was maintained at 1atm and coordinates were saved for analysis every 1ps.
Binding free energy of Bcl-xL to each peptide was calculated by using the MM-PBSA protocol, 400 snapshots of each model were taken at the time intervals of 5 ps from the last 2 ns production runs. The dielectric constants inside and outside the molecule were set as 1.0 and 78.0 respectively. The solvent accessible surface area (SASA) was computed with the molsurf module in AMBER9, using a probe radius of 1.4 Å. The surface tension proportionality constant and the free energy of nonpolar solvation of a point solute were set to 0.00542 kcal/mol/Å and 0.92 kcal/mol, respectively [19], at this stage, the configurational entropies were calculated by nmode module in AMBER9. Single trajectory method was used in MM-PBSA binding free energy calculations. Although MM-PBSA is empirical in nature, when the sampling is appropriate and parameters are properly chosen, it can produce reasonably accurate results for biomolecular systems.
In order to investigate detailed differences of entropic changes in three peptide-protein systems, Schlitter's formula [20] was later used for the analysis of configurational entropy, which yields an upper bound to the true entropy Strue,
| (1) |
where kB is the Boltzmann constant, T is the absolute temperature, e is Euler's number, ħ is the Plank's constant divided by 2π, M is the mass matrix that holds on the diagonal the masses belonging to the atomic Cartesian degrees of freedom, and σ is the covariance matrix of atom-positional fluctuations [20-22]. The elements of the covariance matrix are given by
| (2) |
where xi are the Cartesian coordinates after least-square superposition of the trajectory configurations with respect to a particular subset of atoms by using Kabsch's algorithm [23]. In our simulation, all the backbone atoms are included for covariance matrix and entropy calculations.
The entropy change on receptor-peptide binding is given by
| (3) |
where SC, SR, and SP is the entropy of the binding complex, receptor and the peptide, respectively. Similarly, we can evaluate the entropy change for each molecule (receptor and peptide) between bound and free states
| (4) |
where SB and SF is, respectively, the entropy of the molecule after and before the binding.
Direct calculation of the absolute entropy of a molecule requires a complete sampling of all the translational and vibrational freedoms of the system. For complex systems such as proteins, this is not practical because the limitation in computational cost. Estimating configurational entropy from MD trajectory was first proposed by Karplus [21] by using the formula ΔS = 0.5kB ln(det δa / det δb) where kB is the Boltzmann constant, δa and δb are covariance matrices of atomic positional fluctuations of configurations a and b. Later a heuristic formula based on Cartesian coordinates was introduced by Schlitter [20]. This formula computes an upper bound to the absolute entropy from a MD trajectory. Strictly speaking, the entropy calculated from this method is only approximate, yet it provides a practical and computationally feasible method in studying entropic effects in complex systems of many degrees of freedoms. Although decomposing the entropic contributions into subset of atoms neglects the correlation of motions between this subset and the rest of the system, nonetheless this method could be considered as a reasonable first order approximation of entropy for complex systems in which exact inclusion of all degrees of freedoms for entropy calculation is currently impossible. By this analysis we can quantitatively investigate the contributions of different subsets of the system to the overall entropy, which can provide additional insight information on dynamical properties of proteins.
3. Results and Analysis
a) RMSD of peptide-Bcl-xL Complexes and unliganded Bcl-xL
We carried out MD simulations for three binding complexes, the natural helix binding complex (Bak-Bcl-xL), HBS1 binding complex (HBS2a-Bcl-xL), and HBS2 binding complex (HBS2b-Bcl-xL). Bak is a multidomain BH1-3 proapoptotic protein required for outer mitochondrial membrane permeabilization (OMMP) and apoptosis induced by many types of cell death stimulation. [24-25] Bcl-xL, an antiapoptotic member of Bcl-2 family, inhibits Bak activation by binding to BH3 α helix domain of Bak protein [26-28]. BH3 α helix from Bak protein can bind to and antagonize Bcl-xL and thus activate apoptosis [29-30]. BH3 α helix from Bak protein is used to construct HBS proteins, the first hydrogen bonding at the N terminal of Bak peptide is covalently connected by replacing the H-O hydrogen bond with a C-C covalent bond. Asp584 is replaced by an Acetyl-Lysine residue in order to increase the stability of the helix in HBS2b peptide [11]. The root mean square deviations (RMSD) of Cα atoms from the initial structures of each complex and free Bcl-xL are shown in Fig. 2. The RMSD of each system is stabilized around 1 ns. The average RMSDs and corresponding standard deviations from 2 ns to 10 ns are: 1.66±0.38 Å (Bak-Bcl-xL), 3.38±0.35 Å (HBS 2a-Bcl-xL), 2.64±0.42Å (HBS ab-Bcl-xL), 2.99±0.37Å (free Bcl-xL), respectively. These results suggest that there is no significant structural drift in each system during the MD simulations, and there is no significant structural change when Bcl-xL is unliganded, which is in agreement with the crystal structure of Bcl-xL [31].
Figure 2.

Time evolutions of the RMSD of Ca atoms during 10 ns MD simulations of three peptide-Bcl-xL complexes and unliganded Bcl-xL.
b) Binding free energies and comparison with experimental data
Table I shows the binding free energy components of three complexes from the MM-PBSA calculation. All three complexes have large negative enthalpies, large negative electrostatic energies and van der Waals energies. There are important interaction energy components in determining the binding affinities of peptide-Bcl-xL system, indicating the binding processes are energetically favorable. HBS2b peptide overall has the lowest binding free energy, and the lowest van der Waals and electrostatic interaction energies. HBS2a peptide also shows more negative Van Der Waals interaction energy comparing with the natural peptide Bak (around 11.5 kcal/mol lower), this is probably due to the fact that both HBS peptides are more stable in bound states, thus results in better contacts with the Bcl-xL. The electrostatic interaction energy of HBS2a is about 20 kcal/mol higher than Bak and HBS 2b peptides as shown in Table 1.
Table 1.
Components of binding free energy for three binding complexes (all the units are in kcal/mol). (The standard deviations of TΔS for three systems are 10.92 kcal/mol, 12.35 kcal/mol and 8.93 kcal/mol, respectively, ΔEgas=ΔEele+ΔEvdw)
| Bak-Bcl-xL | HBS2a-Bcl-xL | HBS2b-Bcl-xL | |
|---|---|---|---|
| ΔEele | -152 | -132 | -153 |
| ΔEvdw | -89 | -100 | -100 |
| ΔEgas | -241 | -232 | -253 |
| ΔGSA | -14 | -14 | -14 |
| ΔGPB | 190 | 166 | 187 |
| ΔGEtot | -65 | -80 | -80 |
| TΔS | -43 | -72 | -40 |
| ΔΔGBinding | -22 | -8 | -40 |
In general, both HBS peptides exhibit more favorable enthalpy changes upon binding relative to the natural Bak peptide. However, when conformational entropic effects are included, the order of binding affinities could be changed. Considering the entropic changes from normal mode calculations, the dominant free energy increase comes from HBS2a system, which gives a large entropic change (TΔS=-72kcal/mol), and consequently, a relatively small binding free energy (-8kcal/mol). This suggests that HBS2a peptide is less effective in binding comparing with the natural Bak peptide and HBS2b and the system adopts a different binding mode in some sense. Combining the solvated free energies with entropic changes from normal mode calculations, the order of affinities from our calculation are in good agreement with the experimental affinity data [11].
c) Flexibilities of peptide helicities of free and bound peptides
The minimal region of Bak protein required to bind to Bcl-xL is a 16-amino acid peptide derived from the BH3 region of Bak protein [29]. This peptide tends to form random coil in solution but again forms an α-helix when it binds to Bcl-xL. The Ramachandran plots of the backbone distributions of the representative residues in free Bak peptide and corresponding residues in HBS peptides are shown in Fig. 3. There are two glycine residues inside Bak peptide, since glycine is known as one of the helix-breakers due to small size of its side chain, the flexibilities of glycine residues play influential roles in helix forming and breaking. The phi-psi distribution of Gly75 falls into regions mostly representing random coil and a small portion of left-handed helix, while Gly82 has some conformations falling into α-helix region. All of glycine residues show considerable flexibilities and there is only a small possibility that the phi-psi distribution falls into the α--helix region, that's probably the main reason that this peptide is largely random coil in solution.
Figure 3.
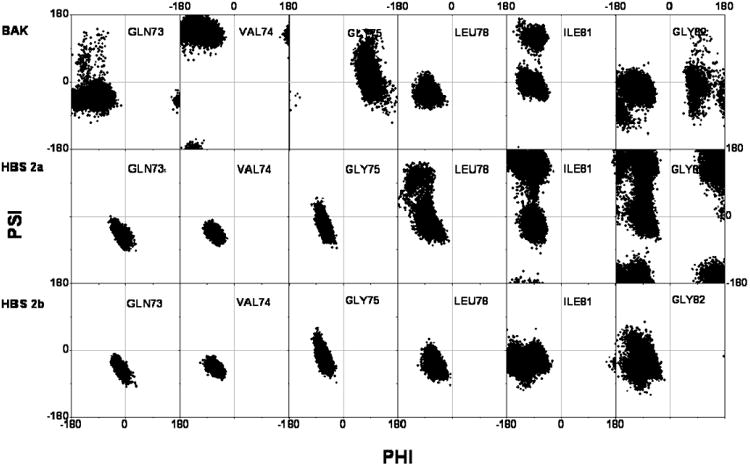
The Ramachandran plots of most flexible residues from Bak peptide and the comparisons with HBS peptides (Free states) A: Gln73, B: Val74, C: Gly75, D: Leu78, E: Ile81, F: Gly82.
Besides glycine residues, there are two other residues noteworthy to mention, Val74 and IIe81. Val74 takes the β-sheet backbone conformation in nearly all structures, and Ile81 also has considerable possibility to take the β-sheet backbone conformation.
When the Bak peptide is modified to HBS peptides, since the backbones of the residues which anticipate the formation of the HBS ring are covalently fixed, there is rarely possibility that they take the any other conformations but α--helix conformation. The Ramachandran plots of the backbones of these residues (Three are shown in Fig. 3) are not only inside the α-helix region but also restricted into very limited space, as expected.
Because of its rigidity and large spatial volume, the HBS ring has other impacts on the conformation of the peptide. The backbone distributions of Leu78, Ile81 and Gly82 are more diffused comparing with the natural Bak peptide. From the view of root-mean-square-fluctuation (RMSF) of each residue (Figure 5A), it is clear that the HBS ring induced consistently larger fluctuations.
Figure 5.
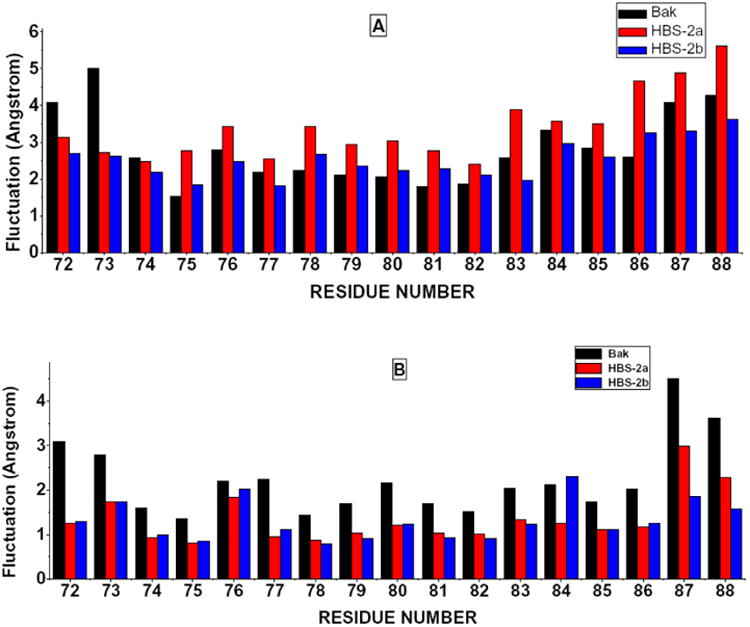
Residue Root Mean Square Fluctuation of Free and Bound States, A: Free States, B: Bound States.
The substitution of Asp84 by LysAC, however, provides additional side chain contacts with nearby residues and stabilized the secondary structure and minimized the fluctuations, because of the prolonged flexible hydrophobic side chain on KAC. This is proved by the distributions of Ramachandran plots and RMSF values as shown in Figs. 3-4. Experimentally determined helicities of three peptides are: Bak ≈20%, HBS helix 2a ≈45%, HBS helix 2b ≈65% [11], which are qualitatively in good agreement with our simulations.
Figure 4.
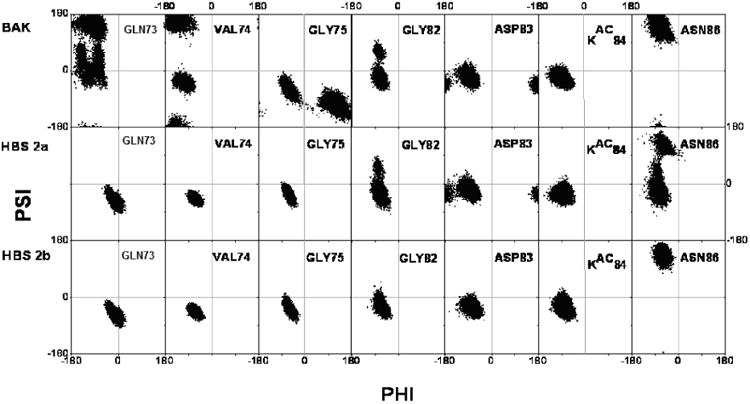
The Ramachandran plots of most flexible residues from Bak peptide and the comparisons with HBS peptides (Bound states).
After peptide-Bcl-xL complex formation, it is expected that the flexibilities of some residues will be restricted, Ramachandran plots of two glycine residues (shown in Fig. 4) indicate the α--helix-forming trends have been largely improved comparing with free states. On the other hand, the different position of Asn86 in bound HBS helix 2a implies that the binding mode of HBS helix 2a is not identical to the natural Bak peptide and HBS helix 2b. The overall residual fluctuations of the bound states have been considerably reduced (Fig. 5B), however, the natural Bak peptide is still quite flexible in bound state. Roughly speaking, in free states, the HBS helix 2a shows the largest fluctuation, natural Bak peptide is the next; in bound states, the residual fluctuation of HBS helix 2a is dramatically lowered, and natural Bak peptide is more flexible in this case. Finally, HBS helix 2b is the least fluctuated peptide in both cases.
d) Analysis of conformational entropies
The conformational entropic changes of backbone atom sets of free peptides and Bcl-xL and their complexes are shown in Fig. 6A. According to the recent protein-protein binding model proposed by Grunberg et al. [32], the protein-protein binding process follows a three-step mechanism of diffusion, free conformer selection, and refolding. The recognition step is an entropy loss process because only a subset of free conformations will be selected. After recognition, the complex is relaxed again, and it will regain some entropy. The entropies of three peptides begin to level off after ∼0.5 ns. For larger systems of Bcl-xL and three complexes, it takes around 2ns to level off. All systems show satisfactory convergences within the 10ns simulation time. However, since the absolute values of entropies are nearly 100 fold than ΔS values, it can still cause convergence problems. Fortunately, the ΔScomplex-receptor-peptide values for three systems converge well within 10ns. All systems undergo drastically entropic drops at the beginning, then gradually get back and level off. This process takes around 3ns for three systems, and the general entopic change picture is in agreement with the model proposed by Grunberg et al. [32]. Bak-Bcl-xL system has the lowest entropy loss, 99.09 cal/mol, the entropy loss of HBS helix 2b-Bcl-xL is 140.94 cal/mol, while HBS helix 2a-Bcl-xL system gives the largest entropy loss, 365.90 cal/mol, which is expected from the RMSFs of free and bound states.
Figure 6.
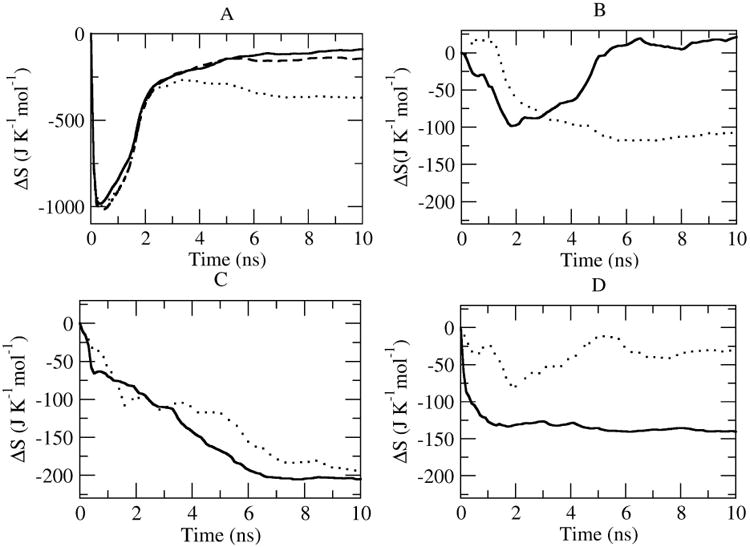
A) Entroy change ΔScomplex-receptor-peptide as a function of simulation time (solid line: Bak-Bcl-xL system; dots: HBS2a-Bcl-xL system; dashed line: HBS2b-Bcl-xL system;. B-D) Decompositions of entropy changes of inhibitory peptides and Bcl-xL (B: Bak-Bcl-xL, C: HBS helix 2a-Bcl-xL, D: HBS helix 2b-Bcl-xL (Solid line: Bcl-xL dots: peptides). The entropy at each time point is derived from the covariance matrix calculated as a time average taken over configurations in the trajectories.
Although the entropic difference is a relatively small value comparing to the absolute value of the individual systems and the sampling may not be converged yet because of the computational limitations, the order of entropic changes calculated from the triple trajectory method by covariance matrix scheme coincides with the result of normal mode method, and confirms that HBS2a peptide brings significant entropic loss when bound to Bcl-xL.
e) Decomposition of Entropic Changes by Peptides and Bcl-xL
Changes in conformational entropy upon binding can also be estimated from the contributions of the peptides and the receptor. For this purpose, the atom-positional least-square fitting of trajectory structures was performed by using backbone atoms of the individual peptides or receptor in order to exclude the entropy contributions of collective motions. Figure 6 B-D shows the entropic differences by the peptides and Bcl-xL, for the both cases of Bak and HBS helix 2b system, the entropic differences of peptides keep dropping and finally level off; on the other hand, the entropic differences of the Bcl-xL are all of the similar feature that the entropic differences drop down around 2ns then go up, after some fluctuations and finally level off, indicating that the Bcl-xL doesn't undergo large structural changes during the binding processes, which is in agreement with the structural determinations of free Bcl-xL and Bak-Bcl-xL complex. HBS helix 2a system, however, is different from Bak and HBS helix 2b systems. The entropic difference of Bcl-xL, keeps dropping and only starts to level off after 8ns, further proves the binding process of HBS helix 2a to Bcl-xL is in some sense different with Bak and HBS 2b, thus longer simulation time for entropic difference of Bcl-xL is required. Specific entropic values from covariance matrix method are listed in Table 2.
Table 2. Average values of entropy change TΔS from covariance matrix method.
| TΔS | |
|---|---|
| Bak-Bcl-xL c-l-r | -29.5 (0.9) |
| HBS 2a-Bcl-xLc-l-r | -109.1 (0.9) |
| HBS 2b-Bcl-xLc-l-r | -42.0 (0.8) |
| Bak in Bak-Bcl-xL b-f | -32.7 (0.6) |
| Bcl-xL in Bak-Bcl-xL b-f | 4.5 (1.3) |
| HBS helix 2a in Bak-Bcl-xL b-f | -60.66 (0.21) |
| Bcl-xL in Bak-Bcl-xL b-f | -55.98 (1.45) |
| HBS helix 2b in Bak-Bcl-xL b-f | -38.33 (0.49) |
| Bcl-xL in Bak-Bcl-xL b-f | -9.40 (0.42) |
(c: complex; l: ligand; r: receptor; b: bound state; f: free state all the units are kcal/mol, numbers in parentheses are standard deviations, all the values are averaged from the entropy values of last 2 ns of all trajectories)
f) Similarities and differences in three peptide-Bcl-xL complex systems
The residues are considered as contacting if the distance of one atom pair from two residues is within 4Å. Contacts are counted if the interaction ratios are over 60% during simulations, Table 3 lists all these residues for three systems.
Table 3.
Detailed residue-residue contacts in binding of Bcl-xL with Bak, HBS2a and HBS2b.
| Bcl-xL | Bak | HBS 2a | HBS 2b |
|---|---|---|---|
| Gly572 | - | Gln125, Gln129 | Gln125, Val126, Glu129 |
| Gln573 | Gln111 | Gln111 | Gln111 |
| Val574 | Gln111, Leu112, Ser122, Gln125, Val126, Glu129, Phe146 | Tyr101, Leu108, Gln111, Leu112, Val126, Phe146 | Tyr101, Gln111, Leu112, Val126, Phe146 |
| Gly575 | Val126, Glu129,Leu130 | Gln125, Val126, Glu129, Leu130 | Val126, Glu129, Leu130 |
| Arg576 | Glu129 | Glu129 | Glu129 |
| Gln577 | Tyr101, Phe105 | Tyr101, Phe105, Gln111 | Tyr101, Phe105, Gln111 |
| Leu578 | Phe97, Tyr101, Leu108, Leu126, Leu130, Ala142, Phe146 | Phe97, Tyr101, Leu108, Leu130, Ala142, Phe146 | Phe97, Tyr101, Leu108, Leu126, Leu130, Ala142, Phe146 |
| Ala579 | Glu129, Leu130, Arg139 | Glu129, Leu130, Arg139 | Glu129, Leu130, Arg139 |
| Ile580 | - | Phe105 | Phe105 |
| Ile581 | Phe97, Arg100, Tyr101 | Phe97, Arg100, Tyr101, Ala104, Phe105 | Phe97, Arg100, Tyr101, Ala104, Phe105 |
| Gly582 | Phe97, Gly138, Arg139 Ala142 | Phe97, Leu130, Gly138, Arg139, Ala142 | Phe97, Gly138, Arg139, Ala142 |
| Asp583 | Gly138, Arg139 | Asn136, Arg139 | Arg139, Arg204 |
| Asp584 (KAC for HBS2b) | Arg100, Arg204 | Arg100 | Arg100, Ala104, Arg204, His215 |
| Ile585 | Ala93, Glu96, Phe97, Arg100, Gly138, Val141, Ala200 | Ala93, Glu96, Phe97, Arg100, Gly138, Val141, Ala142, Tyr195 | Glu96, Phe97, Arg100, Gly138, Val141, Ala142, Tyr195, Ala200 |
| Asn586 | Trp137, Gly138 | Gly138, Leu194, Tyr195, Asn136, Trp137, Arg139 | Gly138, Leu194, Tyr195, Ala200, Arg204 |
| Arg587 | Ser203 | Leu194, His214, His215 | Leu194, Tyr195, Gln207 |
| NH2 terminal | Ala164, Ser167 | Leu158, His178, His179 | Tyr159, Ala164 |
According to the results of Alanine mutation experiments [9], the starting GQVGR sequence, Arg576, Leu578, Ile581, Asp583, Ile585 play important roles in stabilizing the complex, from Table 3 and Fig. 7, it is clear that the binding conformations of the peptides for the N-end to Asp583 in three systems are very close, shows that the synthesized cyclic structure does not interfere with peptide binding in this region significantly. However, this cyclic structure does have spatial impacts on binding structures of the region from Asp584 to the C-end. In the simulation of original Bak-Bcl-xL complex, Asp584 forms salt bridges with two Arg residues; comparing with two synthesized peptides, two residues at the C-end of Bak are quite flexible, few residues of Bcl-xL can form stable contacts with them. In HBS helix 2a system, Asp584 can only form salt bridge with Arg100, thus may largely weaken the binding affinity. In HBS helix 2b system, the extra long hydrophobic side chain of KAC is firmly anchored in the hydrophobic pocket comprised by Arg100, Ala104, Arg204 and His215 as shown in Fig. 8, and the C-end residue Arg587 is pointing to the region of Leu194, Tyr195 and Gln207, which can hardly be reached by Bak and HBS helix 2a. The binding conformation of KAC and the Asp to Ala mutation at this point both implies that although there are two Arg residues to form salt bridges with Asp584 of the peptide, the enhanced hydrophobic interactions are more effective in stabilizing the complex. This prediction is supported by the single residue modification of Asp584 to KAC at Bak peptide, which gives the highest binding affinity among the Bak and two HBS helix peptides (unpublished data).
Figure 7.
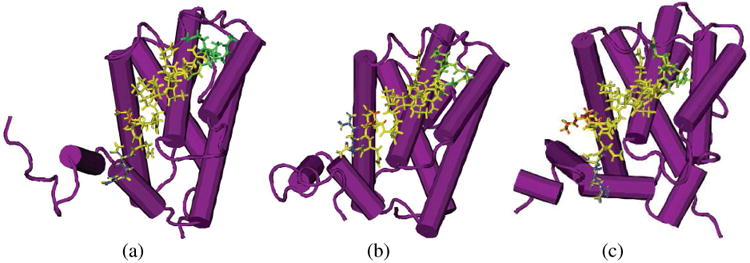
Structures of the Bcl-xL with three peptides for simulations at t=10 ns. (A: Bak-Bcl-xL system; B:HBS helix 2a-Bcl-xL system; C: HBS helix 2b-Bcl-xL system) Bcl-xL structures are shown in purple, residues in Bak from Gly572 to Gly575 and the corresponding cyclic rings in HBS helix 2a and HBS helix 2b are shown in green; residue Asp584 (KAC in HBS helix 2b) is shown in red, HBS helix 2b forms a Y shape and anchors at the hydrophobic surface of Bcl-xL. The rest Parts of the three inhibitive peptides are shown in yellow.
Figure 8.
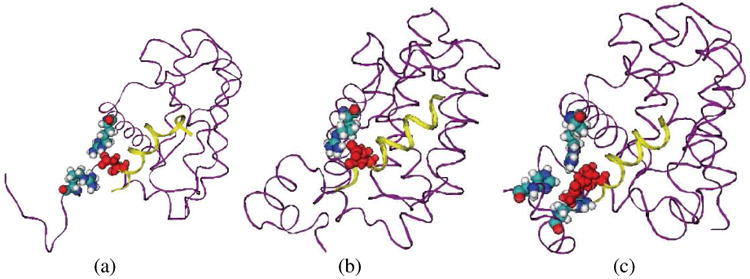
Detailed molecular interactions between Asp584 (KAC in HBS helix 2b) and Bcl-xL. (A. Bak-Bcl-xL system, residue Arg100 and Arg204 at Bcl-xL involved; B. HBS helix 2a-Bcl-xL system, Arg100 at Bcl-xL involved; C. HBS helix 2b-Bcl-xL system, Arg100, Ala104, Arg185, His215 at Bcl-xL involved) Bcl-xL is represented in purple ribbon, while the peptides are in yellow ribbon; Asp584/KAC584 residues are represented in red VDW form, and all residues interacting with Asp584/KAC584 are represented in VDW form with atom type color representation.
Conclusions
In the present study, the binding mechanisms of HBS helix peptides with Bcl-xL and the effects of macrocyclic regions within the synthetic helices are analyzed by molecular dynamics and MM-PBSA calculations for comparison with the native binding of Bak-Bcl-xL. Based on our results, the HBS structure largely decreases the entropy of the system but does not significantly change the conformation of the corresponding binding structures. It indirectly impacts the binding conformation at the C-end of the peptides. Our simulation shows that substitution of a hydrophobic side chain at Asp584 not only enhances the helicity of the peptide but also stabilizes the structure of the binding complex.
Acknowledgments
JZHZ is partially supported by the Petroleum Research Fund (44056-AC6), administered by the American Chemical Society. PSA gratefully acknowledges support from the NIH (GM073943) for this work.
References
- 1.Briz V, Poveda E, Soriano V. HIV entry inhibitors: mechanisms of action and resistance pathways Journal of Antimicrobial. Chemotherapy. 2006;57:619–627. doi: 10.1093/jac/dkl027. [DOI] [PubMed] [Google Scholar]
- 2.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of Apoptosis in Vivo by a Hydrocarbon-Stapled BH3 Helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jochim AL, Arora PS. A hydrogen Bond Surrogate approach for Stabilization of Short Peptide Sequences in Alpha-Helical Conformation. Acc Chem Res. 2008;41:1289–1300. doi: 10.1021/ar700264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman RN, Dimartino G, Arora PS. A Highly Stable Short -Helix Constrained by a Main-Chain Hydrogen-Bond Surrogate. J Am Chem Soc. 2004;126:12252–2253. doi: 10.1021/ja0466659. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Chen K, Kulp J, Arora PS. Evaluation of Biologically Relevant Short Alpha-Helices Stabilized by a Main-Chain Hydrogen-Bond Surrogate. J Am Chem Soc. 2006;128:9248–9256. doi: 10.1021/ja062710w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabartty A, Kortemme T, Baldwin RL. Protein Sci. 1994;3:843–852. doi: 10.1002/pro.5560030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson JW, Kallenbach NR. Biochemistry. 1989;28:5256–5261. doi: 10.1021/bi00438a050. [DOI] [PubMed] [Google Scholar]
- 8.OENeil KT, DeGrado WF. Science. 1990;250:646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- 9.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Nimmer P, Rosenberg SH, Ng SC, Joseph M. Anal Biochem. 2002;307:70–75. doi: 10.1016/s0003-2697(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Liao W, Arora PS. Enhanced Metabolic Stability and Protein-Binding Properties of Artificial Alpha-Helices Derived from a Hydrogen-Bond Surrogate: Application to Bcl-xL. Angew Chem Int Ed. 2005;44:6525–6529. doi: 10.1002/anie.200501603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berman HM, Battistuz T, Bhat TN, Bluhm WF, Bourne PE, Burkhardt K, Feng Z, Gilliland GL, Iype L, Jain S, Fagan P, Marvin J, Padilla D, Ravichandran V, Schneider B, Thanki N, Weissig H, Westbrook JD, Zardecki C. The Protein Data Bank. Acta Cryst. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- 13.Gaussian 03, Revision C.02, Frisch, M. J. ; Trucks, G. W. ; Schlegel, H. B. ; Scuseria, G. E. ; Robb, M. A. ; Cheeseman, J. R. ; Montgomery, J. A. ; Vreven, Jr., T. ; Kudin, K. N. ; Burant, J. C. ; Millam, J. M. ; Iyengar, S. S. ; Tomasi, J. ; Barone, V. ; Mennucci, B. ; Cossi, M. ; Scalmani, G. ; Rega, N. ; Petersson, G. A. ; Nakatsuji, H. ; Hada, M. ; Ehara, M. ; Toyota, K. ; Fukuda, R. ; Hasegawa, J. ; Ishida, M. ; Nakajima, T. ; Honda, Y. ; Kitao, O. ; Nakai, H. ; Klene, M. ; Li, X. ; Knox, J. E. ; Hratchian, H. P. ; Cross, J. B. ; Bakken, V. ; Adamo, C. ; Jaramillo, J. ; Gomperts, R. ; Stratmann, R. E. ; Yazyev, O. Austin, A. J. ; Cammi, R. ; Pomelli, C. ; Ochterski, J. W. ; Ayala, P. Y. ; Morokuma, K. ; Voth, G. A. ; Salvador, P. ; Dannenberg, J. J. ; Zakrzewski, V. G. ; Dapprich, S. ; Daniels, A. D. ; Strain, M. C. ; Farkas, O. ; Malick, D. K. ; Rabuck, A. D. ; Raghavachari, K. ; Foresman, J. B. ; Ortiz, J. V. ; Cui, Q. ; Baboul, A. G. ; Clifford, S. ; Cioslowski, J. ; Stefanov, B. B. ; Liu, G. ; Liashenko, A. ; Piskorz, P. ; Komaromi, I. ; Martin, R. L. ; Fox, D. J. ; Keith, T. ; Al-Laham, M. A. ; Peng, C. Y. ; Nanayakkara, A. ; Challacombe, M. ; Gill, P. M. W. ; Johnson, B. ; Chen, W. ; Wong, M. W. ; Gonzalez, C. ; Pople, J. A. Gaussian, Inc., Wallingford CT, 2004.
- 14.Bayley CI, Cieplak P, Cornell WD, Kollman PA. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges:The RESP method. J Phys Chem. 1993;97:10269–10280. [Google Scholar]
- 15.Case DA, Darden TA, Cheatham TE, III, Simmerling CL, Wang J, Duke RE, Luo R, Merz KM, Pearlman DA, Crowley M, Walker RC, Zhang W, Wang B, Hayik S, Roitberg A, Seabra G, Wong KF, Paesani F, Wu X, Brozell S, Tsui V, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Beroza P, Mathews DH, Schafmeister C, Ross WS, Kollman PA. AMBER 9. University of California; San Francisco: 2006. [Google Scholar]
- 16.Duan Y, Wu C, Chowdhury S, Lee MC, Xiong G, Zhang W, Yang R, Cieplak P, Luo R, Lee T, Caldwell J, Wang J, Kollman PA. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comp Chem. 2003;24:1999–2012. doi: 10.1002/jcc.10349. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 18.Zhou R, Harder E, Xu H, Berne BJ. Efficient multiple time step method for use with Ewald and Particle-Mesh Ewald for large biomolecular systems. J Chem Phys. 2001;115:2348–2358. [Google Scholar]
- 19.Sitkoff D, Sharp KA, Honig B. Accurate calculation of hydration free energies using macroscopic solvent models. J Phys Chem. 1984;98:1978–1988. [Google Scholar]
- 20.Schlitter J. Estimation of absolute and relative entropies of macromolecules using the covariance matrix. Chem Phys Lett. 1993;215:617–621. [Google Scholar]
- 21.Karplus M, Kushick JN. Methods of estimating the configurational entropy of macromolecules. Macromolecules. 1981;14:325–332. [Google Scholar]
- 22.Andricioaei I, Karplus M. On the calculation of entropy from covariance matrices of the atomic fluctuations. J Chem Phys. 2001;115:6289–6292. [Google Scholar]
- 23.Kabsch W. A solution for the best rotation to relate two sets of vectors. Acta Cryst. 1976;32:922–923. [Google Scholar]
- 24.Degenhardt K, Sundararajan R, Lindsten T, Thompson C, White E. Bax and Bak independently promote cytochrome C release from mitochondria. J Biol Chem. 2002;277:14127–14134. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
- 25.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, Mac Gregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–113. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, Fesik SW. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holinger EP, Chittenden T, Lutz RJ. Bak BH3 Peptides Antagonize Bcl-xL Function and Induce Apoptosis through Cytochrome c-independent Activation of Caspases. J Biol Chem. 1999;274:13298–13304. doi: 10.1074/jbc.274.19.13298. 1999. [DOI] [PubMed] [Google Scholar]
- 31.Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SC, Fesik SW. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 32.Grunberg R, Leckner J, Nilges M. Complementarity of structure ensembles in protein-protein binding. Structure. 2004;12:2125–2136. doi: 10.1016/j.str.2004.09.014. [DOI] [PubMed] [Google Scholar]


