Abstract
BACKGROUND
l-Threo-3,4-dihydroxyphenylserine (L-DOPS, droxidopa) is a norepinephrine (NE) prodrug under development to treat orthostatic hypotension. 3,4-Dihydroxyphenylacetaldehyde (DOPAL), an endogenous catecholaldehyde produced by enzymatic oxidative deamination of dopamine, is toxic to catecholaminergic neurons. Based on the observation of increasing plasma DOPAL after oral administration of L-DOPS to a patient, we examined whether other subjects also had DOPAL in their plasma after droxidopa administration, and whether droxidopa is contaminated with DOPAL.
METHODS
Thirteen subjects took 400 mg droxidopa orally. We sampled venous blood at baseline and 1, 2, 3, 6, 24, and 48 h after drug administration and assayed L-DOPS, NE, and DOPAL by use of liquid chromatography with electrochemical detection (LC-ED). Droxidopa in acidic solution (20:80 mixture of 0.04 mol/L phosphoric acid:0.20 mol/L acetic acid) was vacuum centrifuged for 1 h at 30 °C and then assayed by LC-ED.
RESULTS
Droxidopa contained 0.01% DOPAL. At 6 h after droxidopa, all subjects had detectable DOPAL in plasma (1.89 nmol/L, P = 0.0001). Across the sampling times, plasma DOPAL correlated with plasma L-DOPS (r = 0.996). The mean increment in plasma DOPAL was more than 4 times that in plasma NE (0.39 nmol/L). In 2 patients with Parkinson disease and orthostatic hypotension, DOPAL was detected in plasma at baseline (0.12 nmol/L) and increased by about 70-fold after droxidopa. Vacuum concentration of droxidopa in the acid solution converted L-DOPS to DOPAL completely.
CONCLUSIONS
Droxidopa is contaminated with DOPAL. After oral droxidopa administration, DOPAL is detected in plasma of humans. Droxidopa is susceptible to extensive nonenzymatic conversion to DOPAL.
l-Threo-3,4-dihydroxyphenylserine (droxidopa, L-DOPS)3 is a norepinephrine (NE) prodrug (1, 2). Just as L-3,4-dihydroxyphenylalanine (levodopa) is converted to dopamine via l-aromatic-amino-acid decarboxylase, droxidopa is converted to NE. After oral droxidopa ingestion, circulating L-DOPS is taken up into cells via the neutral amino acid transporter and decarboxylated enzymatically by l-aromatic-amino-acid decarboxylase, which is abundant in parenchymal cells of organs such as the liver, kidneys, and gut. Since 1989, droxidopa has been marketed in Japan for treatment of Parkinson disease and orthostatic hypotension. For the latter indication, the drug is currently being developed for marketing in the US.
3,4-Dihydroxyphenylacetaldehyde (DOPAL) is an endogenous catecholaldehyde produced by oxidative deamination of dopamine. DOPAL is toxic to catecholaminergic neurons and has been proposed to play a pathogenetic role in Parkinson disease (3, 4).
As part of a clinical research study about hemodynamic, neurochemical, and neurobehavioral effects of droxidopa, we noted that after oral droxidopa administration a patient with Parkinson disease and orthostatic hypotension had progressively increasing plasma DOPAL concentrations, with the DOPAL concentration directly related to the L-DOPS concentration. This led us to examine whether other subjects also had DOPAL in their plasma after droxidopa administration, whether droxidopa is contaminated with DOPAL, and whether droxidopa can be converted nonenzymatically to DOPAL.
Materials and Methods
SUBJECTS
We studied 13 subjects (11 patients, 2 healthy volunteers, mean age 65 years, range 52–83 years, 10 men) in the NIH Clinical Center after they provided written informed consent to a research protocol approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board of intramural NIH. Of the 11 patients, 7 had pure autonomic failure (PAF), 3 Parkinson disease (2 with persistent, consistent orthostatic hypotension), and 1 multiple system atrophy (MSA).
The Parkinson disease patients were not on levodopa at the time of L-DOPS administration. Levodopa had been discontinued at least 3 days beforehand, and baseline plasma dihydroxyphenylalanine (DOPA) concentrations were within normal limits. Levodopa treatment is associated with plasma DOPA concentrations about 1000 times those in untreated patients. Levodopa and drugs that inhibit l-aromatic-amino-acid decarboxylase or catechol-O-methyltransferase were withdrawn throughout the study. For parkinsonism and anxiety related to withdrawal of levodopa, alternative drugs such as serotonin reuptake blockers, antianxiety agents, or dopamine receptor agonists were used at constant doses if needed.
DRUG SOURCES
Avance GmbH provided the droxidopa used in this study under a cooperative research and development agreement. Avance GmbH did not design the study, collect or analyze the data, or write any part of this report. Avance GmbH did provide financial support for neurochemical assays. The provided droxidopa was in capsules (100 mg per capsule) manufactured by Dainippon-Sumitomo Pharma. The active pharmaceutical ingredient was obtained from Patheon.
DOPAL standard was synthesized at the NIH by J. Heredia-Moya in the laboratory of Kenneth Kirk. The amount of synthesized DOPAL could not be quantified precisely, because the standard consisted of a brownish coating on the side of the preparative glass vial rather than crystals or dry powder, and the mass of DOPAL was calculated from the difference in weight between the preweighed empty glass vial and the vial with DOPAL product.
Identity and purity of the DOPAL standard was tested by mass spectroscopy and nuclear magnetic resonance (NMR) at the time of synthesis and confirmed by liquid chromatography with electrochemical detection (LC-ED) and LC-TOF-MS as part of the present study. There were no impurities by LC-ED or NMR. Small additional peaks by LC-TOF-MS probably corresponded to normal, nondestructive, and reversible reaction of an aldehyde. The DOPAL standard was estimated to be at least 95% pure.
STUDY DESIGN
With the subject supine with head on a pillow, an intravenous (IV) catheter was inserted in an arm vein for drawing blood samples. At least 15 min after IV catheter insertion, a 5 mL blood sample was drawn through the IV and transferred to a heparinized glass tube and placed on ice. At about 1000, the patient took droxidopa (4 capsules containing 100 mg each of droxidopa) orally with water. Blood was sampled again at 1, 2, 3, 6, 24, and 48 h after administration of droxidopa. Between evaluation points on the day of drug administration, the IV was usually kept in place, and the subject was allowed to ambulate and to take a hospital diet; however, the subject lay supine with head on pillow for at least 15 min before blood sampling.
NEUROCHEMICAL ASSAYS
We assayed plasma concentrations of catechols by alumina extraction followed by HPLC with electrochemical detection; coefficients of correlation for the assay have been published (5, 6). Intraassay CVs for L-DOPS ranged from 1%–4%, with a lower limit of detection of about 1 nmol/L.
DROXIDOPA CONVERSION TO DOPAL
We dissolved droxidopa standard in a 20:80 (vol:vol) solution of 0.04 mol/L phosphoric acid:0.2 mol/L acetic acid. We placed aliquots of 2 ng in 20 μL in 1.5-mL plastic sample tubes and concentrated them to dryness in a vacuum centrifuge (Vacufuge™ 5301, Eppendorf North America) for 30 min or 1 h at 30 °C. We compared the chromatographic results to those of droxidopa standard that had not been incubated or evacuated to dryness. This experiment was done twice on separate days. Droxidopa (2 ng in 20 μL) in the same solution was also incubated in a water bath at 37 °C for 30 min without vacuum centrifugation.
DATA ANALYSIS AND STATISTICS
We graphed trends over time in plasma concentrations of catechols were graphed and assessed relationships between neurochemical measures by linear regression using Kaleidagraph 4.01 (Synergy Software), with mean (SE) values expressed. Although we expected that droxidopa would increase plasma NE concentrations, we used 2-tailed statistical tests. A P value <0.05 defined statistical significance. We obtained mass spectrometry data using a Waters LCT Premier TOF system in the negative ionization mode. Flow injections were into a 50/50 mix acetonitrile/water/0.5% acetic acid at 200 μL/min.
Results
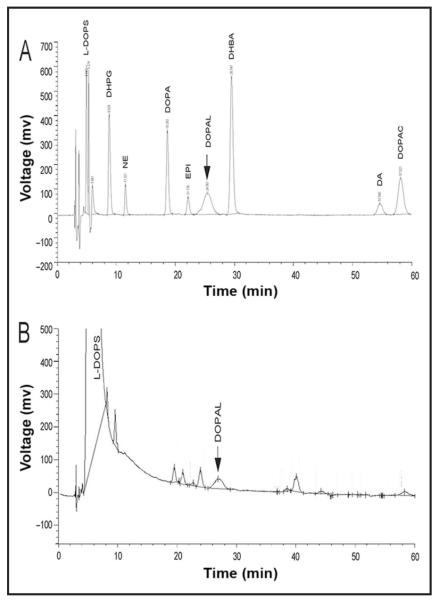
In a mixture of catechol standards, DOPAL was resolved chromatographically from other catechols (Fig. 1A). The DOPAL peak had an atypical but characteristic broad, short appearance compared to other peaks.
Fig. 1. (A), Chromatographic recording after injection of standards for dihydroxyphenylglycol (DHPG, 1 ng), NE (250 pg), DOPA (1 ng), epinephrine (EPI, 250 pg), DOPAL (approximately 1 ng), dihydroxybenzylamine (DHBA, an internal standard), dopamine (DA, 250 pg), and dihydroxyphenylacetic acid (DOPAC, 1 ng).
Note characteristically short, broad peak corresponding to DOPAL. (B), Chromatographic recording after direct injection of L-DOPS (10 μg). Note small peak corresponding to DOPAL.
Droxidopa injected directly into the LC-ED system contained DOPAL (Fig. 1B). The amount of contamination was 0.01% (100 pmol DOPAL per μmol L-DOPS). Other small contaminating peaks were also noted. Contamination by DOPAL was confirmed on direct injection of the active pharmaceutical ingredient (L-DOPS, Patheon). The same amount of contamination was noted whether the L-DOPS was dissolved in the 20:80 mixture of 0.04 mol/L phosphoric acid:0.20 mol/L acetic acid or in acetic acid alone. Neither diluent contained any DOPAL.
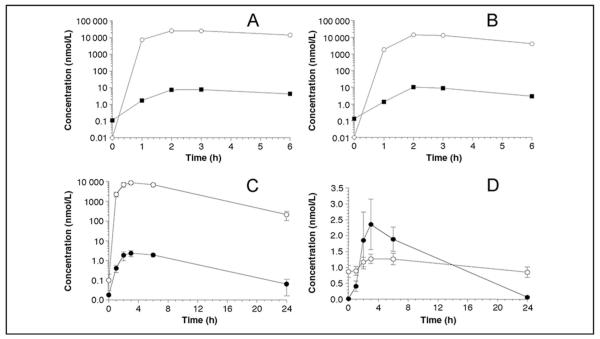
DOPAL was detected in all 13 subjects at 6 h after droxidopa administration, at plasma concentrations ranging from 0.26 to 4.24 nmol/L (P = 0.0001 by sign test) (Table 1). In the 2 healthy volunteers, DOPAL was detected at only the 6-h time point. In all 7 PAF patients, DOPAL was detected in plasma at both 3 and 6 h after droxidopa administration but not at baseline. In both patients with Parkinson disease and persistent consistent orthostatic hypotension, DOPAL was detected in plasma at baseline and increased by about 70-fold after droxidopa administration (Fig. 2A, B).
Table 1.
Plasma concentrations (nmol/L) of L-DOPS, NE, and DOPAL at baseline and after 400 mg oral droxidopa.a
| n | Baseline | 1 h | 2 h | 3 h | 6 h | 24 h | 48 h | |
|---|---|---|---|---|---|---|---|---|
| L-DOPS | ||||||||
| Healthy volunteer | 2 | N/A | 3696 (2011) | 6764 (699) | 7578 (1315) | 2930 (611) | 16 (3) | 1 (0) |
| Pure autonomic failure | 7 | N/A | 1762 (550) | 4433 (1227) | 6195 (950) | 7793 (1303) | 368 (210) | 14 (9) |
| Multiple system atrophy | 1 | N/A | 1169 | 3858 | 4852 | 7131 | 246 | 4 |
| Parkinson disease and orthostatic hypotensionb |
3 | N/A | 3205 (2171) | 13 955 (6739) | 14 025 (6233) | 6681 (3846) | 32 (9) | 3 (2) |
| NE | ||||||||
| Healthy volunteer | 2 | 1.21 (0.10) | 1.02 (0.40) | 1.17 (0.46) | 1.22 (0.40) | 0.63 (0.02) | 0.88 (0.31) | 0.90 (0.11) |
| Pure autonomic failure | 7 | 0.66 (0.25) | 0.73 (0.29) | 1.02 (0.24) | 1.07 (0.20) | 1.25 (0.24) | 0.64 (0.19) | 0.54 (0.17) |
| Multiple system atrophy | 1 | 0.44 | 0.60 | 0.91 | 1.29 | 1.55 | 0.50 | 0.53 |
| Parkinson disease and orthostatic hypotension |
3 | 1.30 (0.57) | 1.29 (0.25) | 1.56 (0.14) | 1.77 (0.27) | 1.63 (0.45) | 1.43 (0.44) | 0.98 (0.42) |
| DOPAL | ||||||||
| Healthy volunteer | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.62 (0.29) | 0.00 | 0.00 |
| Pure autonomic failure | 7 | 0.00 | 0.33 (0.15) | 0.80 (0.33) | 1.77 (0.42) | 2.16 (0.44) | 0.09 (0.09) | 0.11 (0.11) |
| Multiple system atrophy | 1 | 0.00 | 0.00 | 0.26 | 0.50 | 0.74 | 0.00 | 0.00 |
| Parkinson disease and orthostatic hypotension |
3 | 0.08 (0.04) | 1.00 (0.51) | 6.07 (2.87) | 5.91 (2.39) | 2.48 (1.17) | 0.08 (0.08) | 0.00 |
Data are mean (SE).
One patient had a history of Parkinson disease and orthostatic hypotension but did not have orthostatic hypotension at the time of the testing reported here.
Fig. 2. Plasma concentrations of L-DOPS (○) and DOPAL (∎) as a function of time after oral administration of droxidopa (400 mg) in 2 patients (A and B) with Parkinson disease and orthostatic hypotension.
(C), Plasma mean (SE) concentrations of L-DOPS (○) and DOPAL (●) as a function of time after oral administration of droxidopa (400 mg). (D), Mean (SE) plasma concentrations of NE (○) and DOPAL (●) as a function of time after oral administration of droxidopa (400 mg).
Across all subjects, the time course of plasma DOPAL concentrations closely paralleled that of L-DOPS (Fig. 2C). By the 24 h sample, DOPAL concentrations were below the detection limit of the assay (5 ng/L, or about 30 pmol/L) in most subjects. Across the 7 sampling times, plasma DOPAL correlated positively with plasma L-DOPS (r = 0.996).
After droxidopa administration, plasma NE concentrations increased slightly but significantly (Table 1, Fig. 2D). Across all subjects, the mean increment in plasma DOPAL at 6 h [1.87 (0.37) nmol/L] was more than 4 times the increment in plasma NE [0.39 (0.15) nmol/L, P = 0.001 by 2-tailed dependent-means t-test].
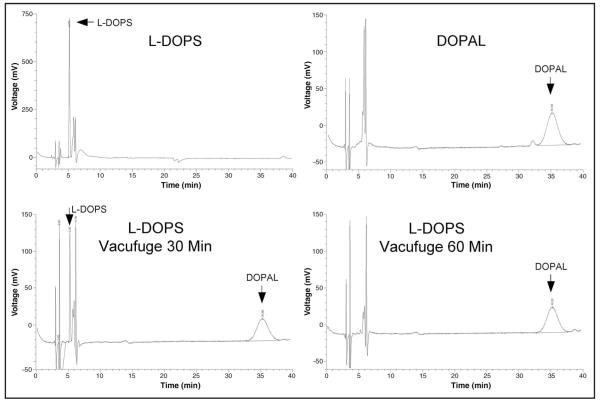
Vacuum centrifugation of droxidopa in acidic solution at 30 °C for 30 min resulted in a substantial decrease in the peak height of L-DOPS and appearance of a DOPAL peak (Fig. 3), demonstrating nonenzymatic conversion. After vacuum centrifugation for 1 h, there was no detectable L-DOPS, and there was a larger DOPAL peak.
Fig. 3.
Chromatographic recordings after direct injection of L-DOPS standard (1 ng), DOPAL standard (1 ng), and L-DOPS after 30 or 60 min of vacuum centrifugation at 30 °C in a 20:80 mixture of 0.04 mol/L phosphoric acid: 0.20 mol/L acetic acid.
From ratios of peak heights and peak areas, 0.73 ng (4.8 pmol) of DOPAL was generated from 1.0 ng (4.7 pmol) of L-DOPS—i.e., approximately all the droxidopa was converted to DOPAL. These findings were confirmed in the same experiment conducted on the active pharmaceutical ingredient (L-DOPS) obtained from a different source. Incubation of droxidopa at 37 °C in a water bath for 30 min decreased the L-DOPS peak height but without production of DOPAL.
Under the same conditions, catecholamines were also found to be susceptible to nonenzymatic conversion to DOPAL. The conversion was far more extensive for norepinephrine and epinephrine, which contain a β-hydroxyl group, than for dopamine, which does not. Analogously, L-DOPS, which contains a β-hydroxyl group, was more susceptible to conversion to DOPAL than was levodopa, which does not. When catecholamines were dissolved in acetic acid without phosphoric acid and subjected to vacuum concentration at 30 °C, there was little or no conversion to DOPAL.
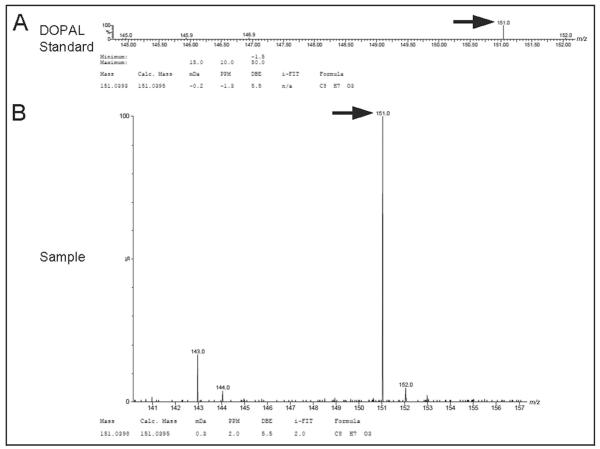
The identity of DOPAL as the standard and as the compound produced in the vacuum concentration experiment was confirmed by LC-TOF-MS (Fig. 4). The upper spectrum is that of the DOPAL standard, confirming unequivocally that the compound was indeed DOPAL. The lower spectrum is that of L-DOPS after vacuum concentration in the acid solution for 1 h, demonstrating that the vacuum concentration of L-DOPS led to formation of a compound identical in molecular weight and chemical composition to DOPAL standard.
Fig. 4. TOF-MS spectra for DOPAL standard (A) and DOPAL (B) produced after vacuum centrifugation of L-DOPS in a 20:80 mixture of 0.04 mol/L phosphoric acid:0.10 mol/L acetic acid for 60 min at 30 °C.
Calc., calculated; mDa, millidaltons; PPM, parts per million; DBE, double bond equivalents. i-Fit™ is an embedded software algorithm of Waters Corp., for isotope prediction applied to elemental composition analysis.
Discussion
Droxidopa is under development as a norepinephrine prodrug to treat orthostatic hypotension. Here we report that droxidopa contains a trace amount of DOPAL, that in humans DOPAL is detected consistently in plasma at 6 h after oral droxidopa administration, and that droxidopa in an acidic solution can be extensively converted to DOPAL nonenzymatically.
The contamination of droxidopa by DOPAL was not because of an atypical batch of drug. Review of chromatographic recordings from assays several years ago of plasma from patients who had received 1600 mg droxidopa (1) also revealed peaks resembling DOPAL (unpublished observations). Moreover, contamination by DOPAL was confirmed on direct injection of the active pharmaceutical ingredient obtained from a different source several months after the clinical study.
Catecholamines in the neuronal cytoplasm normally undergo enzymatic oxidative deamination catalyzed by monoamine oxidase to form catecholaldehydes, which are cytotoxic, as predicted by Blaschko more than a half century ago (7). Because catecholamines leak continuously from storage vesicles into the cytoplasm, endogenous catecholaldehydes are produced continuously during life. According to the “catecholaldehyde hypothesis,” processes that build up cytoplasmic dopamine or that interfere with DOPAL detoxification by aldehyde dehydrogenase result in DOPAL-mediated cell death.
That DOPAL is toxic has been documented in several studies. Incubation of PC-12 rat pheochromocytoma cells with DOPAL produces several signs of cytotoxicity (8, 9). Local administration of DOPAL destroys substantia nigra neurons (4); however, subacute neuropharmacologic manipulations expected to increase striatal DOPAL generation do not affect local dopaminergic innervation (10). In SH-SY5Y neuroblastoma cells, exposure to exogenous dopamine generates DOPAL (11), and ALDH inhibition by disulfiram augments dopamine-related cytotoxicity in this setting (11).
The plasma DOPAL concentration required to exert clinical toxicity is unknown. A search of the literature via PubMed culled only 84 citations about DOPAL or dihydroxyphenylacetaldehyde, only a handful of studies related to DOPAL toxicity, and none about clinical toxicology of this catecholaldehyde. In PC-12 cells, DOPAL at micromolar concentrations (about 500 times the peak concentrations in the present study) causes degeneration of neuritic processes, evokes nonexocytotic dopamine release, and reduces the percent of viable cells (8, 9, 12). In silico DOPAL at similar concentrations oligomerizes α-synuclein (13). DOPAL injected into the substantia nigra of rats at a dose of 1 μg of evokes α-synuclein oligomerization, and local microinjection of DOPAL at 0.2 μg (about 1 nmol) decreases tyrosine hydroxylase immunoreactivity in the substantia nigra (13). In rats, a combination of levodopa, benserazide, and disulfiram injected intraperitoneally increases striatal contents of DOPAL to about 2 ng/mg protein (about 13 pmol/mg), without a semi-chronic effect on striatal dopamine contents after repeated injections (10).
After vacuum concentration of droxidopa in a 20:80 mixture of 0.04 mol/L phosphoric acid:0.20 mol/L acetic acid at 30 °C for 1 h, no detectable L-DOPS remained. The L-DOPS had been converted completely to DOPAL, as confirmed by LC-TOF-MS. The same experiment conducted on the active pharmaceutical ingredient also showed loss of L-DOPS and conversion to DOPAL. We infer that droxidopa is unstable and can be broken down readily to DOPAL when in this acidic solution at low ambient oxygen concentration and tepid temperature. Based on the findings from vacuum concentration of catecholamines, it appears that the conversion of L-DOPS to DOPAL involves an interaction between phosphoric acid and the β-hydroxyl group.
Contamination of droxidopa by DOPAL should be preventable. Because DOPAL is retained much longer on a reversed-phase chromatographic column than is L-DOPS, collection of the early-retaining L-DOPS peak from a preparative chromatographic column should eliminate the contamination. Given the results of the vacuum concentration experiment, however, it is possible that even purified droxidopa may be susceptible to nonenzymatic or enzymatic breakdown after oral ingestion.
Both of the patients with Parkinson disease and orthostatic hypotension in the clinical study had detectable DOPAL in plasma before droxidopa administration. In these patients, plasma DOPAL increased substantially after droxidopa administration. The findings lead us to speculate that such patients may have decreased aldehyde dehydrogenase activity, resulting in DOPAL buildup after taking droxidopa.
Acknowledgments
Role of Sponsor: The funding organizations played a direct role in the final approval of manuscript.
Footnotes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: No authors declared any potential conflicts of interest.
Nonstandard abbreviations: L-DOPS, l-threo-3,4-dihydroxyphenylserine; NE, norepinephrine; DOPAL, dihydroxyphenylacetaldehyde; PAD, pure autonomic failure; MSA, multiple system atrophy; DOPA, dihydroxyphenylalanine; NMR, nuclear magnetic resonance; LC-ED, liquid chromatography with electrochemical detection; IV, intravenous.
References
- 1.Kaufmann H, Saadia D, Voustianiouk A, Goldstein DS, Holmes C, Yahr MD, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108:724–8. doi: 10.1161/01.CIR.0000083721.49847.D7. [DOI] [PubMed] [Google Scholar]
- 2.Blaschko H, Burn JH, Langemann H. The formation of noradrenaline from dihydroxyphenylserine. Br J Pharmacol Chemother. 1950;5:431–7. doi: 10.1111/j.1476-5381.1950.tb00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology. 2004;25:101–15. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 4.Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain Res. 2003;989:205–13. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DS, Holmes C, Kaufmann H, Freeman R. Clinical pharmacokinetics of the norepinephrine precursor l-threo-DOPS in primary chronic autonomic failure. Clin Auton Res. 2004;14:363–8. doi: 10.1007/s10286-004-0221-z. [DOI] [PubMed] [Google Scholar]
- 6.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatog B Biomed Applic. 1994;653:131–8. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 7.Blaschko H. Amine oxidase and amine metabolism. Pharmacol Rev. 1952;4:415–58. [PubMed] [Google Scholar]
- 8.Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R. An endogenous dopaminergic neurotoxin: implication for Parkinson’s disease. Neurodegeneration. 1995;4:271–81. doi: 10.1016/1055-8330(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 9.Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, Burke WJ. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Biol Med. 2001;30:924–31. doi: 10.1016/s0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 10.Legros H, Janin F, Dourmap N, Bonnet JJ, Costentin J. Semi-chronic increase in striatal level of 3,4-dihydroxyphenylacetaldehyde does not result in alteration of nigrostriatal dopaminergic neurones. J Neurosci Res. 2004;75:429–35. doi: 10.1002/jnr.10880. [DOI] [PubMed] [Google Scholar]
- 11.Legros H, Dingeval MG, Janin F, Costentin J, Bonnet JJ. Toxicity of a treatment associating dopamine and disulfiram for catecholaminergic neuroblastoma SH-SY5Y cells: relationships with 3,4-dihydroxyphenylacetaldehyde formation. Neurotoxicology. 2004;25:365–75. doi: 10.1016/S0161-813X(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Yabe-Nishimura C. Oxidative metabolite of dopamine, 3,4-dihydroxyphenylacetaldehyde, induces dopamine release from PC12 cells by a Ca2+-independent mechanism. Brain Res. 2002;931:96–9. doi: 10.1016/s0006-8993(02)02233-3. [DOI] [PubMed] [Google Scholar]
- 13.Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]