Abstract
The rising prevalence of obesity in the last few decades has been accompanied by an increase in nonalcoholic fatty liver disease (NAFLD). NAFLD encompasses a spectrum of liver pathology from isolated hepatic steatosis to steatohepatitis and advanced fibrosis. Dietary habits characterized by consumption of high-caloric, lipid-rich diets play a major role in the development of NAFLD. Recent studies have uncovered the importance of certain components of the diet. In this review, we will focus on the growing evidence for a central role of n-6 polyunsaturated fatty acids. We will discuss novel findings linking oxidative stress and increased production of reactive oxygen species in the liver to oxidation of n-6 polyunsaturated fatty acids and production of specific lipid oxidation metabolites. In particular, we will highlight the potential role of these metabolites as noninvasive markers to diagnose and monitor the extent of liver damage in patients with NAFLD.
Keywords: n-3 polyunsaturated fatty acids, n-6 polyunsaturated fatty acids, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, oxidized linoleic acid metabolites
Paralleling the worldwide epidemic in childhood obesity, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of liver disease in pediatrics [1]. It is defined by the presence of macrovescicular steatosis in more than 5% of the hepatocytes in the absence of use of medications, alcohol abuse and other determinants that may result in fatty liver [2]. NAFLD encompasses a range of disease severities, spanning from the simple steatosis to nonalcoholic steatohepatitis (NASH), which in turn can progress to cirrhosis [3]. In an article using 742 autopsy specimens from children in San Diego County (CA, USA), 13% of subjects were found to have NAFLD, with the highest rate found in Hispanics (11.8%) and the lowest in African–Americans (1.5%) [4]. Although the natural history of NAFLD in the pediatric population is not clearly understood because of the lack of prospective studies evaluating children over time, adult data indicate that a third of patients with NASH will progress to cirrhosis in 10–15 years [5–7]. In addition, the metabolic complications of NAFLD have been well defined. In fact, recent studies in obese adolescents demonstrated that increased alanine aminotransferase levels are associated with deterioration in insulin sensitivity and glucose tolerance, as well as increasing free fatty acid and triglyceride (TG) levels [8]. Further studies demonstrated that the prevalence of metabolic syndrome and prediabetes increases with the rise in hepatic fat content in a cohort of obese adolescents [9], that the pronounced dyslipidemic profile is characterized by large VLDL, small dense LDL and decreased large HDL concentrations [10], and that fatty liver, independent of visceral and intramyocellular lipid content, plays a central role in the impairment of liver, muscle and adipose insulin sensitivity in obese adolescents [11]. Thus, fatty liver disease should be considered the hepatic component of the metabolic syndrome and a strong cardiovascular risk factor [1]. This data clearly suggests that although fatty liver accumulation per se does not affect liver histology, it represents a strong adverse metabolic risk factor. This evidence challenges the concept that fatty liver accumulation is a benign condition because it might be benign in the sense that it protects the liver from developing steatohepatits by buffering the free fatty acids but, in the meantime, it is responsible for the development of an adverse cardiovascular profile [12,13].
n-6 & n-3 fatty acid ratio in the development of NAFLD
Dietary habits have dramatically changed over the last 15–20 years in western countries. These changes have contributed not only to the development of obesity, but also to the accumulation of excess fat stores into ectopic tissues and organs, such as the liver. Thus, while overnutrition is fundamental to the development of NAFLD, the excessive ingestion or deficiency of particular dietary components may significantly contribute to its pathogenesis, as well as its progression. Hepatic steatosis, in fact, arises from an imbalance between TG acquisition and removal in the liver [14]. The free fatty acids used for TG formation derive from the diet, hepatic de novo lipogenesis and adipose tissue lipolysis. In animal models, approximately 20% of the dietary fat is delivered to the liver [15]; extrapolating from these experiments, a typical American diet (containing approximately 100 g of fat per day) furnishes the liver with nearly 20 g of fat each day, equivalent to half of the total TG content of an average liver [14]. A large body of evidence suggests that not only the amount, but also the quality of dietary fat plays a part in the development of NAFLD [16]. In particular, recently published literature provides clues that the dietary imbalance between n-6 and n-3 polyunsaturated fatty acids (PUFAs) leads to the development of an adverse cardiovascular and metabolic profile, thus contributing to the pathogenesis of NAFLD [17]. In fact, it has been demonstrated that individuals with NAFLD have a lower dietary intake of n-3 PUFAs than healthy controls, leading to an increase in the n-6:n-3 PUFA ratio consumed in the diet [18,19]. Consistent with these data, lipidomic studies have shown that the intrahepatic fat in subjects with steatohepatitis is composed of an excess of n-6 PUFA [20]. In particular, studying three groups of subjects – NAFLD, NASH and healthy controls – the authors observed a progressive increase in the n-6:n-3 ratio from controls to NASH subjects [20].
What is the biological meaning of the dietary imbalance between n-6/n-3 fatty acids?
Although some studies indicate that the optimal n-6:n-3 ratio is 1:1, in the western diet the ratio is approximately 15:1 or even 20:1, whereas during the paleolithic period when the human genetic profile was established, there was a balance between n-6 and n-3 PUFAs [21]. Therefore, humans are now living in a nutritional environment that differs from that for which our genetic constitution was selected [21]. This phenomenon has mostly occurred in the past 100–150 years and is totally new in human evolution [21–23]. n-6 and n-3 PUFAs are essential fatty acids; this means that they are not synthesized by the human body. n-6 species are mainly represented by linoleic acid (LA; 18:3 n-6), while n-3 are represented by α-linolenic acid (18:3 n-3), mainly found in plants and limited sets of seeds and nuts [24]. These two classes of PUFA are not interconvertible and are metabolically and functionally distinct [24]. However, in contrast to n-3, n-6 is readily converted by the body into other species, such as n-9, and so incorporated into TGs, or converted into arachidonic acid, which is the parent molecule of the main regulators of the inflammatory response including prostaglandins (COX pathway), leukotrienes (lipoxygenase pathway) and thromboxane [24]. Instead, the dietary n-3 PUFAs are:
Incorporated into the cellular membranes where they partially replace the n-6-derived fatty acids [24];
Converted to active metabolites, in particular, molecules known as resolvins and protectins, which are thought to mediate, at least in part, the anti-inflammatory effect of n-3 PUFA [25].
More importantly, several studies have demonstrated that n-3 PUFAs modulate multiple molecular pathways that altogether contribute to their physiological effects:
Reduction of the inflammatory phenomenon;
Diminution of hepatic de novo lipogenesis;
Reduction of plasma TG levels;
Improvement of insulin sensitivity [26].
Detailed animal studies have, in fact, demonstrated that n-3 PUFAs downregulate the expression of the SREBP-1 transcription factor, which regulates the expression of lipogenic genes [27] and promote the expression of peroxisome proliferator-activated receptor-α [28], which, in turn, stimulates haptic fatty acid oxidation [29] and increases transcription of fatty acid degradation genes [28,29].
Some investigators have tried to translate this evidence by studying whether supplementation with n-3 PUFAs might be beneficial for hepatic fat accumulation. In particular, a randomized crossover study sought to examine the effect of n-3 fatty acids on liver fat in women with polycystic ovary syndrome [30]. The authors supplemented 25 women with marine-derived n-3 fatty acids (4 g/day) versus placebo for 8 weeks. n-3 supplementation decreased liver fat content compared with placebo by 18% (10.2% [1.1%] vs 8.4% [0.9%]). There was also a reduction in TGs and systolic and diastolic blood pressure [30]. More recently, Nobili et al. performed a randomized controlled trial of docosahexaenoic acid (DHA) supplementation (250 and 500 mg/day) versus placebo in 60 children with biopsy-proven NAFLD (20 children per group) [31]. The authors demonstrated that DHA significantly increased in children supplemented with DHA and that the odds of more versus less severe liver steatosis after treatment was lower in children treated with DHA 250 mg/day (odds ratio: 0.01; 95% CI: 0.002–0.11; p < 0.001) and DHA 500 mg/day (odds ratio: 0.04; 95% CI: 0.002–0.46; p = 0.01) compared with placebo, but there was no difference between the DHA groups (p = 0.4) [31]. These data have been further strengthened by a recent meta-analysis evaluating nine studies, which included a total of 335 subjects [32]. Beneficial changes in liver fat were obtained by using n-3 PUFA supplementation (effect size = −0.97; 95% CI: −0.58 to −1.35; p < 0.001). A benefit of n-3 PUFA supplementation versus control was also observed for aspartate aminotransferase (AST; effect size = −0.97; 95% CI: −0.13 to −1.82; p = 0.02). Also, the subanalyses of only randomized control trials demonstrated a significant benefit of n-3 PUFA supplementation versus control on liver fat (effect size = −0.96; 95% CI: −0.43 to −1.48; p < 0.001) [32].
Role of oxidative stress in liver injury & NAFLD progression
Oxidative stress is one of the major contributors to the pathogenesis and progression of NAFLD [33]. In fact, oxidative and mitochondrial stress are associated with the increased production of reactive oxygen species (ROS) and proinflammatory cytokines related to NAFLD [34], as there is an association between the severity of NASH and the degree of oxidative stress [34–36]. In general, the term ROS describes molecules and free radicals deriving from oxygen such as singlet oxygen, hydroxyl and superoxide, among others. One of the effects of ROS is to cause lipid peroxidation of PUFAs within the cells generating metabolites (e.g., LA oxidized metabolites) that are deleterious for the hepatocyte [37]. Most oxidative reactions occur in the mitochondria and the primary factor governing ROS production is the redox state of the respiratory chain because oxidative reactions are associated with the conversion of oxidized factors (e.g., NAD and FAD) into reduced factors (NADH and FADH2). The lipid peroxidation leads to the activation of Kupffer cells with the production of inflammatory cytokines, such as TNF-α, and to the activation of stellate cells, which, in turn, will favor neutrophil chemotaxis, as well as hepatocyte apoptosis and liver fibrosis [37–39].
LA-oxidized metabolites as biomarkers of disease severity in NAFLD
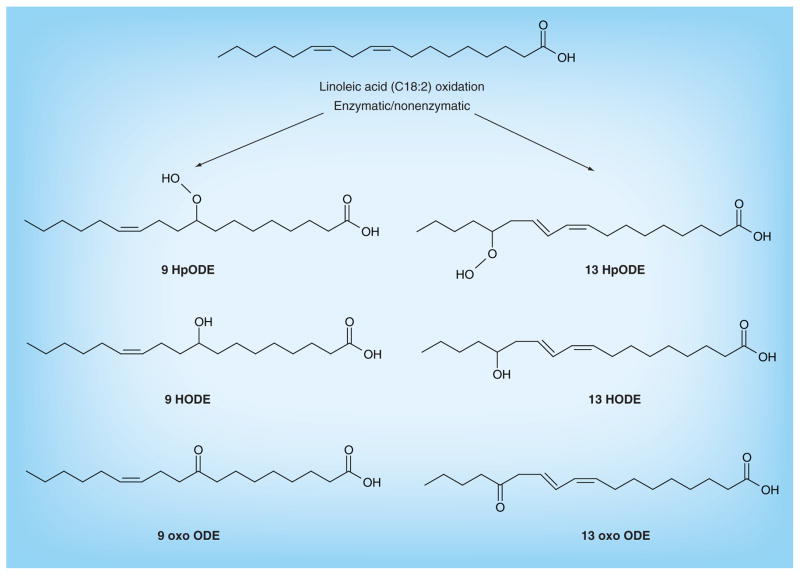
LA is converted to arachidonic acid, but part of it can be also oxidized. LA oxidation can proceed enzymatically via the actions of 12–15 lipoxygenase, COX or the CYP450 enzyme family [40,41], or nonenzymatically by the oxidative effect of free radicals (Figure 1) [42]. The oxidized derivatives of LA (oxidized LA metabolites; OXLAMs) are mainly represented by 9- and 13-hydroxyl-octadecadienoic acid (HODE) and by the 9- and 13-oxo-octadecadienoic acid. These compounds have been demonstrated to be a major component of the oxidized [43–45] and atherosclerotic plaques [46,47]; they also play a pivotal role in the formation of foam cells, as well as in the pathogenesis of atherosclerosis [48–50]. In a recent study, OXLAM levels, in particular, 9- and 13-hydroxy-eicosatetraenoic acids, and 9- and 13-oxo-octadecadienoic acids, have been shown to be associated with NASH (but not in simple steatosis) [51]. Moreover, a risk score for histopathologic diagnosis of NASH (oxNASH) was generated by multivariable modeling demonstrating the best prediction for NASH [51]. The oxNASH was calculated from the ratio of 13-HODE:LA, age, BMI and AST; in this model, a 0.05 mmol/mol increase of 13-HODE:LA was associated with a 3.8-fold increase in the likelihood of having NASH [51]. In addition, the area under the receiver operating characteristic curve for oxNASH was higher than the area under the curve for alanine aminotransferase and AST [51], and a cut-off value of 55 for oxNASH was able to exclude the presence of NASH, with a sensitivity of 81%, while a cut-off value of 73 was able to detect NASH, with a specificity of 97% [51]. Overall, these data suggest that bioactive lipids derived from oxidized LA are major factors in the progression of steatosis to steatohepatitis, and that they might be helpful in the diagnosis and prognosis of NASH [51]. Although the cellular mechanisms by which OXLAMs cause liver injury are only partially known, they involve Kupffer cell activation. In fact, animal studies have demonstrated that peroxidized forms of LA cause an increase of TNF-α, a strong proinflammatory signal, in Kupffer cells [52]. In vitro studies have also demonstrated that LA promotes endoplasmic reticulum stress and liver cell apoptosis through the modulation of cytochrome C expression [53].
Figure 1. Oxidized linoleic acid metabolite synthesis.
The synthesis of the oxidized fatty acids derived from linoleic acid is mediated by enzymatic and nonenzymatic reactions.
HODE: Hydroxyl-octadecadienoic acid; HpODE: Hydroperoxy-octadecadienoic acid; oxo ODE: Oxo-octadecadienoic acid.
Novel therapeutic strategies
These observations suggest novel therapeutic strategies. In fact, because LA cannot be synthesized de novo in humans, dietary LA is the sole source of LA for the human body, therefore also being the sole source for OXLAM synthesis. Lowering LA in the diet might help to reduce OXLAMs in the blood and other tissues such as the liver, leading to an improvement of liver histology. Recently, a study was designed to evaluate the effect of low dietary LA on plasma and erythrocyte fatty acids, and it has demonstrated that a 12-week diet low in n-6 PUFAs significantly reduced the plasma levels of OXLAMs and reduced the LA content of several circulating lipid fractions that may serve as precursors of endogenous OXLAM synthesis [54]. LA reductions were more pronounced in phospholipids and TGs, indicating that these fractions may be more responsive to dietary modifications [51]. These data clearly suggest that if the same reduction of LA and OXLAMs may be achieved in the liver, it may help to reduce or even cure NASH.
A reduction in the tissues of OXLAMs can also be obtained by using compounds with antioxidant activity. Recent studies have demonstrated how compounds with antioxidant properties may ameliorate NASH features [55,56]. In particular, in a recent randomized controlled trial, it has been shown that 1-year therapy with pentoxifylline (PTX), a methylxanthine derivative specifically inhibiting lipid peroxidation, improves liver histology in patients with NASH [55]. More recently, Zein et al. have demonstrated in a randomized controlled trial that PTX therapy compared with placebo is associated with a significant reduction of OXLAMs and with a contemporary improvement of liver histology, supporting the notion that PTX decreases the oxidative stress-related lipid peroxidation, probably through its hydroxyl radical scavenging properties [56].
Role of PNPLA3 rs738409 in the pathogenesis of NAFLD/NASH
Recently, a nonsynonymous single nucleotide polymorphism (rs738409), characterized by a C to G substitution encoding an isoleucine to methionine substitution at amino acid position 148 in the PNPLA3 gene, has been found to be associated with hepatic steatosis in a multiethnic cohort of adults [57], as well as in children [58–60]. Moreover, it has been demonstrated that this variant interacts with environmental stressors such as obesity and alcohol consumption, which induce fatty liver [61,62]. Indeed, these stressors seem to uncover the association between the rs738409 minor allele (G) and hepatic injury in populations in whom it is otherwise covert. Interestingly, the same process occurs with some nutrients; the association between the PNPLA3 variant, in fact, seems to be exacerbated by n-6:n-3 PUFA ratio intake [63], although this variant is not associated with the n-6:n-3 PUFA ratio per se [63].
The PNPLA3 gene encodes for a protein called adiponutrin, which is expressed in the liver and adipose tissue, and is nutrionally regulated. In particular, its expression is low during fasting and increases after meals [64]. In the liver, adiponutrin forms part of the lipid droplets, wherein it seems to have both lipolytic and lipogenic activity [64]. In vitro and animal studies have demonstrated that the rs738409 variant in PNPLA3 leads to hepatic steatosis, both by impairing the lipolytic activity and enhancing the lipogenic activity of PNPLA3 in the liver [65–68]. In addition, metabolic studies in transgenic mice recapitulating the phenotype of the PNPLA3 rs738409 single nucleotide polymorphism in humans provided evidence for three distinct alterations in hepatic triacylglyceride (TAG) metabolism:
Increased formation of fatty acids and TAG;
Impaired hydrolysis of TAG;
Relative depletion of TAG long-chain PUFAs.
These findings suggest that PNPLA3 plays a role in remodeling TAG in lipid droplets, as they accumulate in response to food intake, and that the increase in hepatic TAG levels associated with I148M substitution results from multiple changes in hepatic TAG metabolism [69].
Conclusion & future perspective
These recent studies have provided novel insights into the pathogenesis of NASH, suggesting new diagnostic and therapeutic approaches to this disease (Figure 2). Further knowledge will probably be gained by studying the genetic background underlying the pathways modulating n-3 and n-6 PUFA metabolism. In fact, as stated above, the common variant in the PNPLA3 gene (rs738409), known to be associated with fatty liver and liver damage in children and adults [57,60], has recently been shown to play its role in the development of fatty liver by possibly interacting with the dietary intake of n-6:n-3 PUFA ratio [63]. This observation raises further questions:
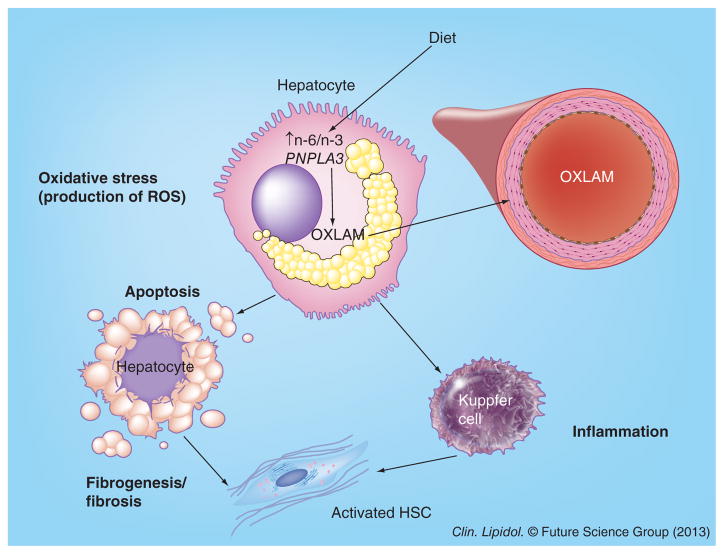
Figure 2. Oxidized linoleic acid metabolites as biomarkers for monitoring liver damage in nonalcoholic fatty liver disease.
The excess n-6 from dietary and other sources in an environment characterized by increased oxidative stress will lead to the formation of oxidized linoleic acid metabolites. The lipid peroxidation leads to the activation of Kupffer cells, with the production of inflammatory cytokines such as TNF-α and to the activation of stellate cells, which is a key event leading to liver fibrosis. The presence of a predisposing genotype (the G allele of the PNPLA3 rs738409 single nucleotide polymorphism) will favor and enhance this process.
HSC: Hepatic stellate cell; OXLAM: Oxidized linoleic acid metabolite; ROS: Reactive oxygen species.
Is there an interaction between the PNPLA3 rs738409 and OXLAMs?
Is it possible to mitigate or reverse the phenotype in subjects carrying the risk allele by changing dietary composition?
Are there other common variants associated with fatty liver whose association with disease is modulated by dietary PUFA?
Surely the study of gene–nutrient interaction in the near future will help uncover novel mechanisms leading to the development of fatty liver disease and its complications, and will also provide key information required to develop more personalized therapies.
Executive summary.
Nonalcoholic fatty liver disease (NAFLD) has emerged as one of the most common complications of childhood obesity.
An increased ratio between dietary n-6 and n-3 polyunsaturated fatty acids has been associated with the development of NAFLD.
Linoleic acid, which represents the most common n-6 polyunsaturated fatty acid in the diet, is an essential fatty acid and is oxidized through enzymatic and nonenzymatic mechanisms.
The oxidized compounds derived from linoleic acid have been associated with NAFLD and its progression towards nonalcoholic steatohepatitis.
A gene variant may interact with dietary fatty acids in modulating liver fat deposition and injury.
Future therapeutic strategies for NAFLD will need to take into account dietary habits, as well as the genetic background.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
AE Feldstein is listed as coinventor on pending and issued patents filed by the Cleveland Clinic and University of California, San Diego that refer to the use of biomarkers in fatty liver disorders. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of considerable interest
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:e462–e468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 3.Mencin AA, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Pediatr Clin North Am. 2011;201(58):1375–1392. doi: 10.1016/j.pcl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 5.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 7.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 8.Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–4294. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 9.Cali AM, De Oliveira AM, Kim H, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896–1903. doi: 10.1002/hep.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cali AM, Zern TL, Taksali SE, et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care. 2007;30:3093–3098. doi: 10.2337/dc07-1088. [DOI] [PubMed] [Google Scholar]
- 11▪▪.D’Adamo E, Cali AM, Weiss R, et al. The central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817–1822. doi: 10.2337/dc10-0284. Shows that the effect of fatty liver on insulin resistance is independent of visceral and intramuscular fat accumulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fierbinteanu-Braticevici C, Negreanu L, Tarantino G. Is fatty liver always benign and should not consequently be treated? J Physiol Pharmacol. 2013;64(1):3–9. [PubMed] [Google Scholar]
- 13.Tarantino G, Conca P, Riccio A, et al. Enhanced serum concentrations of transforming growth factor-beta1 in simple fatty liver: is it really benign? J Transl Med. 2008;6:72. doi: 10.1186/1479-5876-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redgrave TG. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970;49:465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortez-Pinto H, Jesus L, Barros H, Lopes C, Moura MC, Camilo ME. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin Nutr. 2006;25:816–823. doi: 10.1016/j.clnu.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Toshimitsu K, Matsuura B, Ohkubo I, et al. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46–52. doi: 10.1016/j.nut.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Chen D. The optimal dose of omega-3 supplementation for non-alcoholic fatty liver disease. J Hepatol. 2012;57:468–469. doi: 10.1016/j.jhep.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci. 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 20.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 21.Eaton SB, Eaton SB, 3rd, Sinclair AJ, Cordain L, Mann NJ. Dietary intake of long-chain polyunsaturated fatty acids during the paleolithic. World Rev Nutr Diet. 1998;83:12–23. doi: 10.1159/000059672. [DOI] [PubMed] [Google Scholar]
- 22.Simopoulos AP. Overview of evolutionary aspects of omega-3 fatty acids in the diet. World Rev Nutr Diet. 1998;83:1–11. doi: 10.1159/000059674. [DOI] [PubMed] [Google Scholar]
- 23.Sanders TA. Polyunsaturated fatty acids in the food chain in Europe. Am J Clin Nutr. 2000;71(Suppl 1):S176–S178. doi: 10.1093/ajcn/71.1.176s. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 25.González-Périz A, Horrillo R, Ferré N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masterton GS, Plevris JN, Hayes PC. Review article: omega-3 fatty acids – a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31:679–692. doi: 10.1111/j.1365-2036.2010.04230.x. [DOI] [PubMed] [Google Scholar]
- 27.Sekiya M, Yahagi N, Matsuzaka T, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–1539. doi: 10.1016/j.hep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Levy JR, Clore JN, Stevens W. Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology. 2004;39:608–616. doi: 10.1002/hep.20093. [DOI] [PubMed] [Google Scholar]
- 29.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab. 2009;94:3842–3848. doi: 10.1210/jc.2009-0870. [DOI] [PubMed] [Google Scholar]
- 31.Nobili V, Bedogni G, Alisi A, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 32.Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Narasimhan S, Gokulakrishnan K, Sampathkumar R, et al. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without Type 2 diabetes. Clin Biochem. 2010;43:815–821. doi: 10.1016/j.clinbiochem.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Byrne CD. Fatty liver: role of inflammation and fatty acid nutrition. Prostaglandins Leukot Essent Fatty Acids. 2010;82:265–271. doi: 10.1016/j.plefa.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 36.Yesilova Z, Yaman H, Oktenli C, et al. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:850–855. doi: 10.1111/j.1572-0241.2005.41500.x. [DOI] [PubMed] [Google Scholar]
- 37.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatoshepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Tarantino G, Finelli C, Colao A, Capone D, Tarantino M, Grimaldi E, et al. Are hepatic steatosis and carotid intima media thickness associated in obese patients with normal or slightly elevated gamma-glutamyl-transferase? J Transl Med. 2012;10:50. doi: 10.1186/1479-5876-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarantino G, Scopacasa F, Colao A, et al. Serum Bcl-2 concentrations in overweight-obese subjects with nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:5280–5288. doi: 10.3748/wjg.v17.i48.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reinaud O, Delaforge M, Boucher JL, Rocchiccioli F, Mansuy D. Oxidative metabolism of linoleic acid by human leukocytes. Biochem Biophys Res Commun. 1989;161:883–891. doi: 10.1016/0006-291x(89)92682-x. [DOI] [PubMed] [Google Scholar]
- 41.Engels F, Willems H, Nijkamp FP. Cyclooxygenase-catalyzed formation of 9-hydroxylinoleic acid by guinea pig alveolar macrophages under non-stimulated conditions. FEBS Lett. 1986;209:249–253. doi: 10.1016/0014-5793(86)81121-8. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Yin H, Akazawa YO, Yoshida Y, Niki E, Porter NA. Ex vivo oxidation in tissue and plasma assays of hydroxyoctadecadienoates: Z,E/E,E stereoisomer ratios. Chem Res Toxicol. 2010;23:986–995. doi: 10.1021/tx1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiteller G. Linoleic acid peroxidation – the dominant lipid peroxidation process in low density lipoprotein – and its relationship to chronic diseases. Chem Phys Lipids. 1998;95:105–162. doi: 10.1016/s0009-3084(98)00091-7. [DOI] [PubMed] [Google Scholar]
- 44.Barlic J, Murphy PM. An oxidized lipid-peroxisome proliferator-activated receptor gamma-chemokine pathway in the regulation of macrophage–vascular smooth muscle cell adhesion. Trends Cardiovasc Med. 2007;17:269–274. doi: 10.1016/j.tcm.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belkner J, Wiesner R, Rathman J, Barnett J, Sigal E, Kühn H. Oxygenation of lipoproteins by mammalian lipoxygenases. Eur J Biochem. 1993;213:251–261. doi: 10.1111/j.1432-1033.1993.tb17755.x. [DOI] [PubMed] [Google Scholar]
- 46.Brooks CJ, Harland WA, Steel G, Gilbert JD. Lipids of human atheroma: isolation of hydroxyoctadecadienoic acids from advanced aortal lesions. Biochim Biophys Acta. 1970;202:563–566. doi: 10.1016/0005-2760(70)90131-1. [DOI] [PubMed] [Google Scholar]
- 47.Shibata N, Toi S, Shibata T, et al. Immunohistochemical detection of 13(R) hydroxyoctadecadienoic acid in atherosclerotic plaques of human carotid arteries using a novel specific antibody. Acta Histochem Cytochem. 2009;42:197–203. doi: 10.1267/ahc.09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPAR gamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 49.Jira W, Spiteller G, Carson W, Schramm A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids. 1998;91:1–11. doi: 10.1016/s0009-3084(97)00095-9. [DOI] [PubMed] [Google Scholar]
- 50.Kuhn H, Heydeck D, Hugou I, Gniwotta C. In vivo action of 15-lipoxygenase in early stages of human atherogenesis. J Clin Invest. 1997;99:888–893. doi: 10.1172/JCI119253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51▪▪.Feldstein AE, Lopez R, Tamimi TA, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. Provides evidence for an association between oxidized linoleic acid metabolite and nonalcoholic steatohepatitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Böhm T, Berger H, Nejabat M, et al. Food-derived peroxidized fatty acids may trigger hepatic inflammation: a novel hypothesis to explain steatohepatitis. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.04.025. pii: S0168-8278(13)00280-8. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Xue R, Zhang Z, Yang X, Shi H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis. 2012;11:1. doi: 10.1186/1476-511X-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramsden CE, Ringel A, Feldstein AE, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87:135–141. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪▪.Zein CO, Lopez R, Fu X, et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291–1299. doi: 10.1002/hep.25778. Provides evidence of the efficacy of pentoxifylline in lowering oxidized fatty acids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57▪▪.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. First genome-wide association study for hepatic steatosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santoro N, Kursawe R, D’Adamo E, et al. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;52:1281–1290. doi: 10.1002/hep.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santoro N, Zhang CK, Zhao H, et al. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. 2012;55:781–789. doi: 10.1002/hep.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santoro N, Feldstein AE, Enoksson E, et al. The association between hepatic fat content and liver injury in obese children and adolescents: effects of ethnicity, insulin resistance, and common gene variants. Diabetes Care. 2013;36:1353–1360. doi: 10.2337/dc12-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giudice EM, Grandone A, Cirillo G, et al. The association of PNPLA3 variants with liver enzymes in childhood obesity is driven by the interaction with abdominal fat. PLoS ONE. 2011;6(11):e27933. doi: 10.1371/journal.pone.0027933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 63▪▪.Santoro N, Savoye M, Kim G, et al. Hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS ONE. 2012;7(5):e37827. doi: 10.1371/journal.pone.0037827. Shows the interaction between PNPLA3 rs738409 and the dietary n-6:n-3 polyunsaturated fatty acids ratio in modulating hepatic fat content and liver injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chamoun Z, Vacca F, Parton RG, Gruenberg J. PNPLA3/adiponutrin functions in lipid droplet formation. Biol Cell. 2013;105:219–233. doi: 10.1111/boc.201200036. [DOI] [PubMed] [Google Scholar]
- 65.He S, McPhaul C, Li JZ, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Y, He S, Li JZ, et al. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci USA. 2010;107:7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Y, Cohen JC, Hobbs HH. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J Biol Chem. 2011;286:37085–37093. doi: 10.1074/jbc.M111.290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumari M, Schoiswohl G, Chitraju C, et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012;15:691–702. doi: 10.1016/j.cmet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69▪▪.Li JZ, Huang Y, Karaman R, et al. Chronic overexpression of PNPLA3 I148M in mouse liver causes hepatic steatosis. J Clin Invest. 2012;122:4130–4144. doi: 10.1172/JCI65179. Shows an animal model recapitulating the human PNPLA3 rs738409 phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]